A Study on the Safety and Effects of Amorpha fruticosa Fruit Extract on Spontaneously Hypertensive Rats with Induced Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis
2.3. Animals
2.4. Design of the Experiment
2.5. Acute Toxicity Tests
2.6. Blood Pressure Measurement
2.7. Induction of Type 2 Diabetes Mellitus in SHRs
2.8. Measurement of MDA Levels in Liver Homogenate
2.9. Measurement of GSH Levels in Liver Homogenate
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Acute Oral Toxicity of EAF
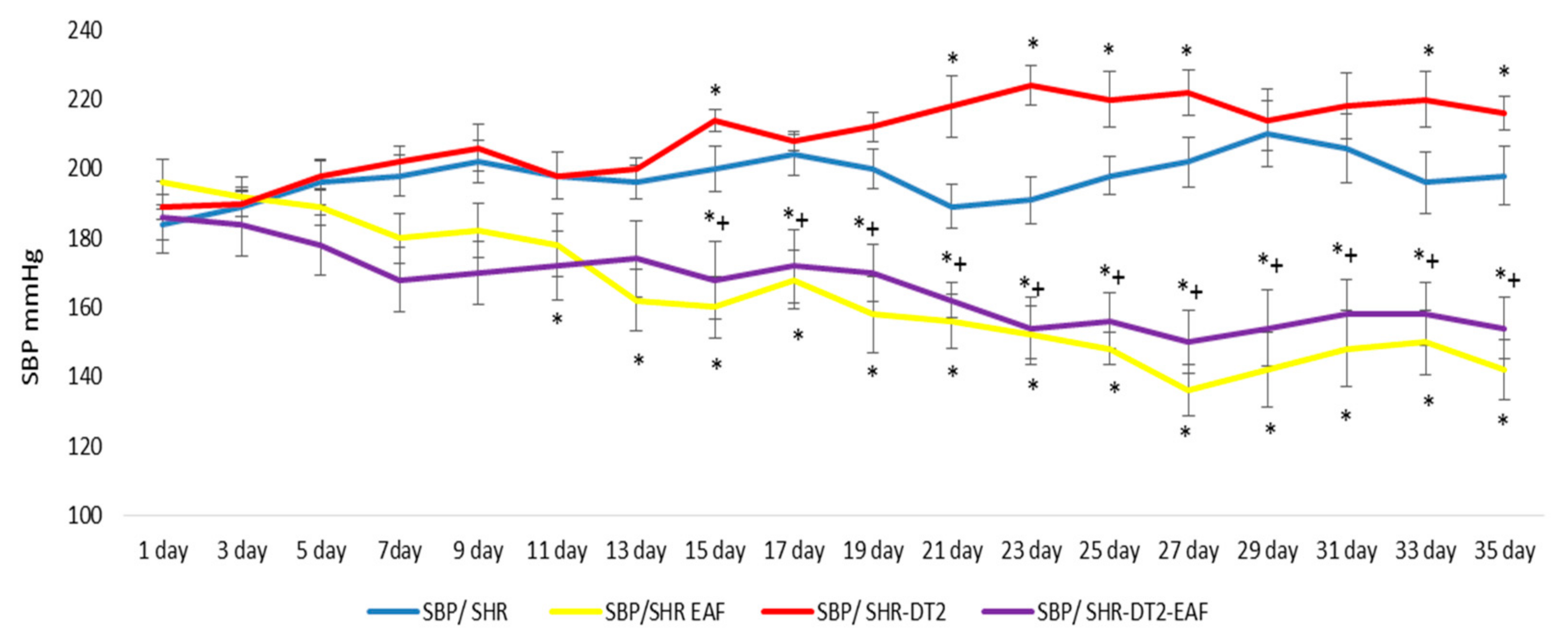
3.3. Changes in Systolic Blood Pressure (SBP)
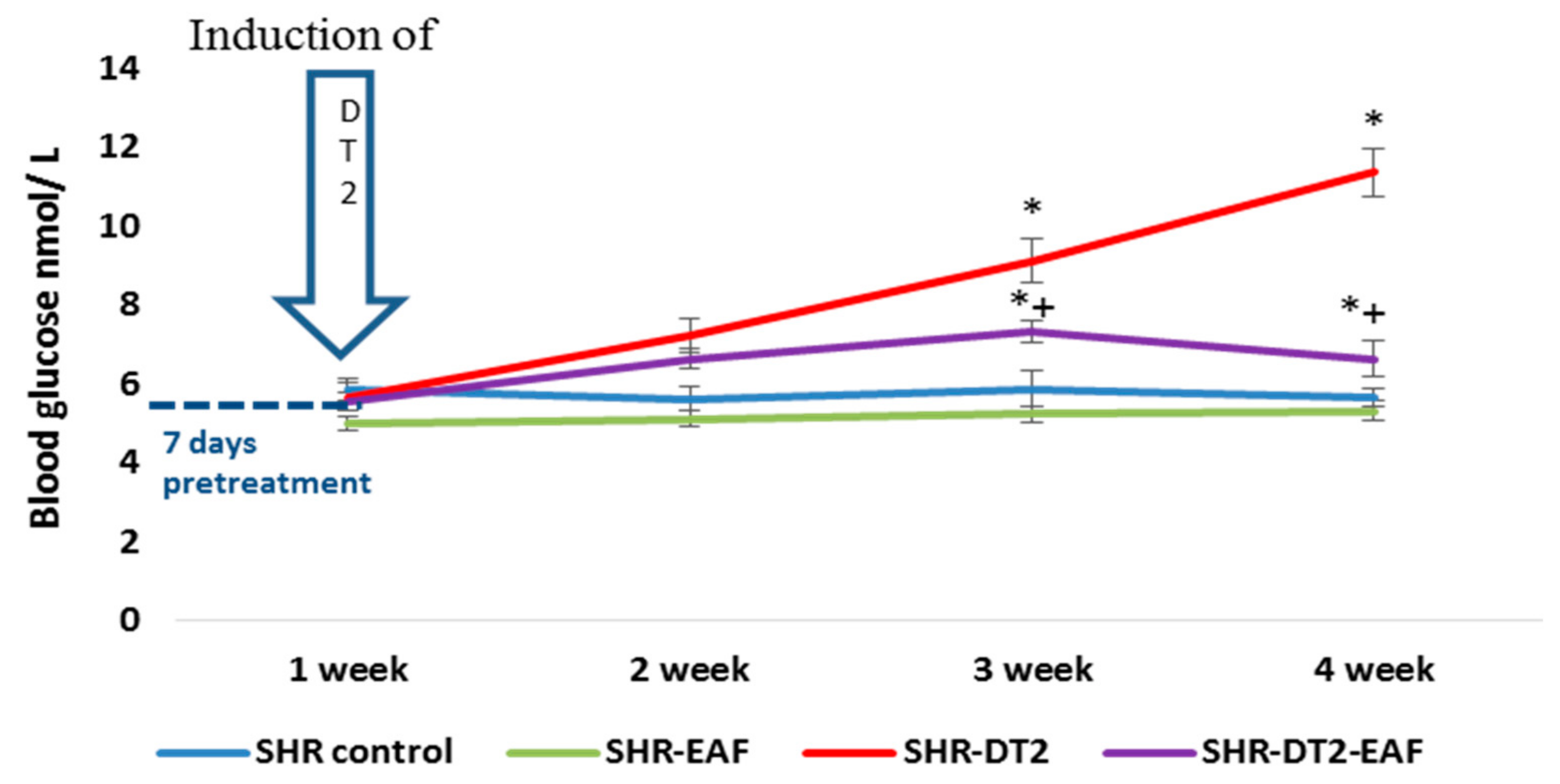
3.4. In Vivo Changes in Blood Glucose Level
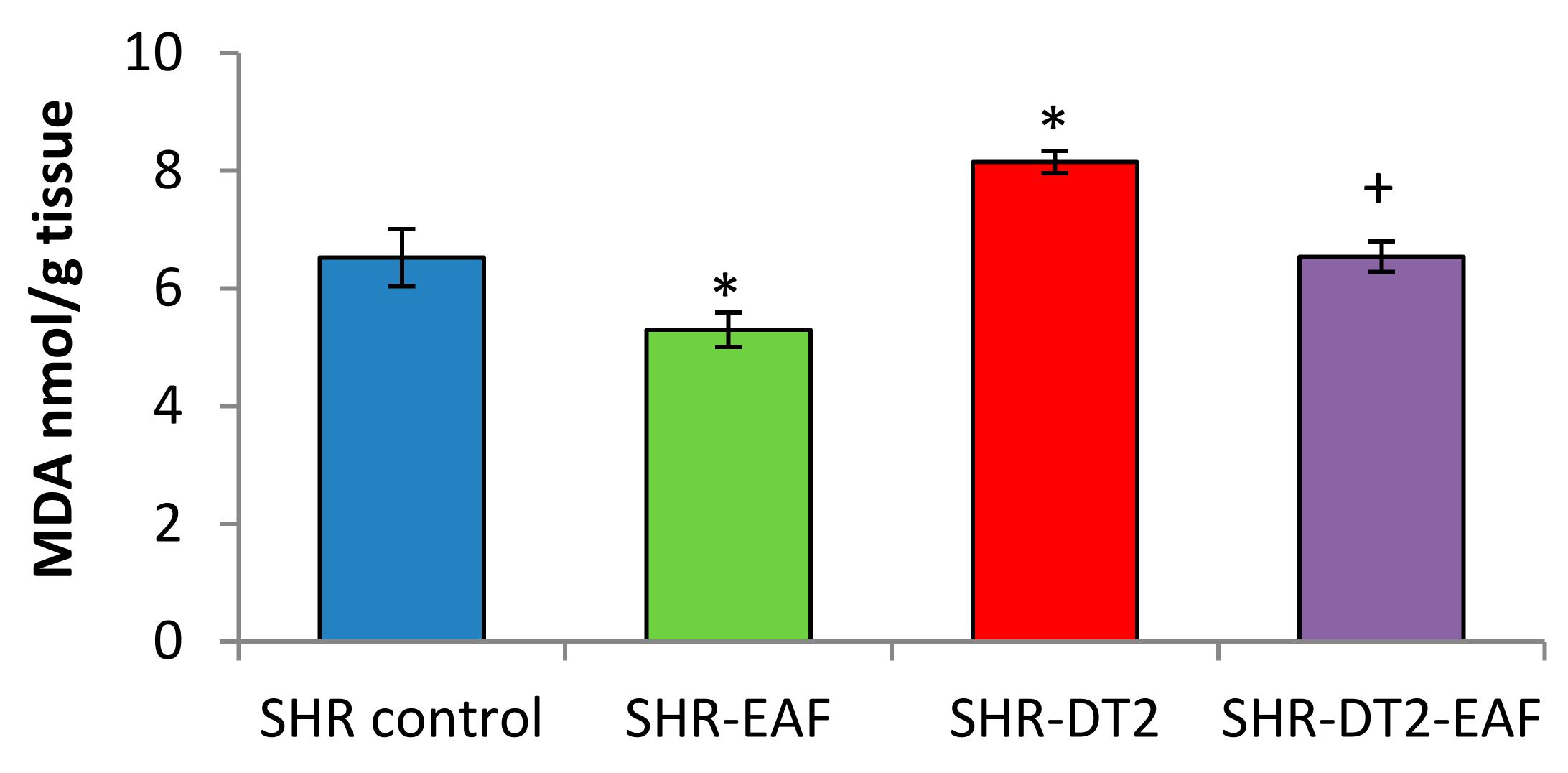
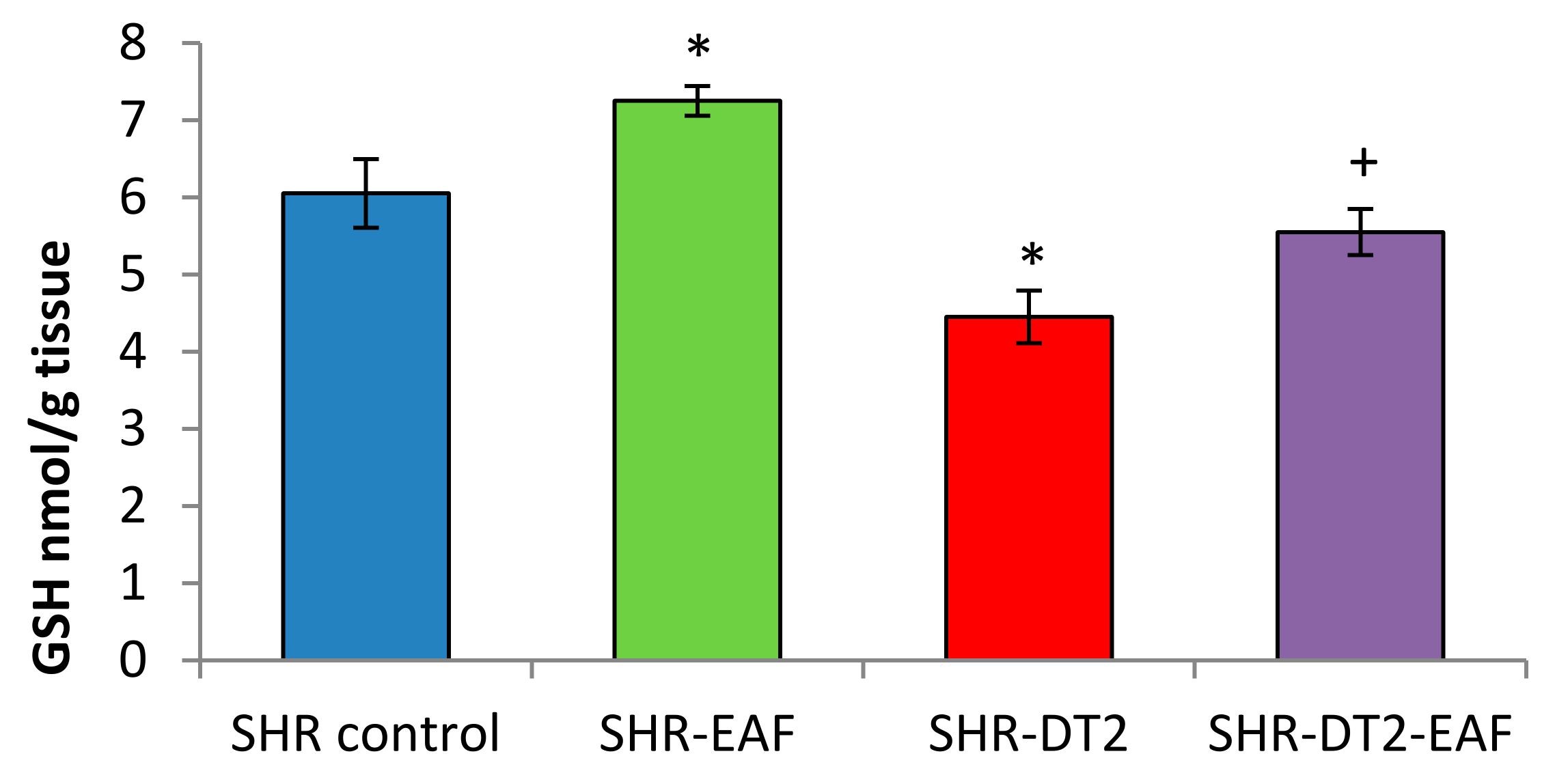
3.5. Oxidative Stress Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization. Global Diffusion of EHealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on EHealth; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive Effects and Mechanisms of Chlorogenic Acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, G.; Satya, S.; Khandelwal, R.K.; Naik, S.N. Commonly Consumed Indian Plant Food Materials in the Management of Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 21–40. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. Reactive Oxygen/Nitrogen Species and Their Functional Correlations in Neurodegenerative Diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef] [PubMed]
- May, L.D.; Lefkowitch, J.H.; Kram, M.T.; Rubin, D.E. Mixed Hepatocellular-Cholestatic Liver Injury after Pioglitazone Therapy. Ann. Intern. Med. 2002, 136, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the History of Diabetes Mellitus: The Main Contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sapra, A.; Bhandari, P. Diabetes Mellitus; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pardo-de-Santayana, M.; Quave, C.L.; Sõukand, R.; Pieroni, A. Medical Ethnobotany and Ethnopharmacology of Europe. Ethnopharmacology 2015, 29, 343–355. [Google Scholar]
- Asadi-Samani, M.; Moradi, M.-T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional Uses of Medicinal Plants to Prevent and Treat Diabetes; an Updated Review of Ethnobotanical Studies in Iran. J. Nephropathol. 2017, 6, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Polat, R.; Cakilcioglu, U.; Kaltalioğlu, K.; Ulusan, M.D.; Türkmen, Z. An Ethnobotanical Study on Medicinal Plants in Espiye and Its Surrounding (Giresun-Turkey). J. Ethnopharmacol. 2015, 163, 1–11. [Google Scholar] [CrossRef]
- Özdemir, E.; Alpınar, K. An Ethnobotanical Survey of Medicinal Plants in Western Part of Central Taurus Mountains: Aladaglar (Nigde–Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef]
- Skalli, S.; Hassikou, R.; Arahou, M. An Ethnobotanical Survey of Medicinal Plants Used for Diabetes Treatment in Rabat, Morocco. Heliyon 2019, 5, e01421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner, C.; de Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.-T.; Giegold, S.; et al. Amorfrutins Are Potent Antidiabetic Dietary Natural Products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, S. Amorfrutins: A Promising Class of Natural Products That Are Beneficial to Health. ChemBioChem 2014, 15, 1231–1238. [Google Scholar] [CrossRef]
- Han, J.; Heo, H.; Jeong, M.; Kim, H.; Jang, I. Review on Amorfrutin of Licorice for Type2 Diabetes Mellitus. J. Intern. Korean Med. 2020, 41, 1078–1088. [Google Scholar] [CrossRef]
- Austin, D.F. Florida Ethnobotany; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kozuharova, E.; Matkowski, A.; Woźniak, D.; Simeonova, R.; Naychov, Z.; Malainer, C.; Mocan, A.; Nabavi, S.M.; Atanasov, A.G. Amorpha Fruticosa—A Noxious Invasive Alien Plant in Europe or a Medicinal Plant against Metabolic Disease? Front. Pharmacol. 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wu, Y.; Du, L. Qualitative and Quantitative Analysis of Amorfrutins, Novel Antidiabetic Dietary Natural Products, by HPLC. Pharm. Biol. 2016, 54, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Zdraveva, P.; Popova, P.; Shkondrov, A.; Krasteva, I.; Ionkova, I. Investigation of in Vitro Cultures of Astragalus Monspessulanus L. Comptes Rendus Acad. Bulg. Sci. 2017, 70, 1131–1137. [Google Scholar]
- Cooper, M.E. Antihypertensive Therapy and Diabetic Microvascular Disease. Clin. Exp. Hypertens. 1997, 19, 769–778. [Google Scholar] [CrossRef]
- Lorke, D. A New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Pari, L.; Karthikesan, K.; Menon, V.P. Comparative and Combined Effect of Chlorogenic Acid and Tetrahydrocurcumin on Antioxidant Disparities in Chemical Induced Experimental Diabetes. Mol. Cell. Biochem. 2010, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Polizio, A.H.; Pena, C. Effects of Angiotensin II Type 1 Receptor Blockade on the Oxidative Stress in Spontaneously Hypertensive Rat Tissues. Regul. Pept. 2005, 128, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bump, E.A.; Taylor, Y.C.; Brown, J.M. Role of Glutathione in the Hypoxic Cell Cytotoxicity of Misonidazole. Cancer Res. 1983, 43, 997–1002. [Google Scholar] [PubMed]
- Chan, C.C.; Saraswat, P. Analytical Method Verification, Method Revalidation, and Method Transfer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Derelanko, M.J.; Hollinger, M.A. Handbook of Toxicology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Wu, L.-L.; Zheng, F.; Wu, N.; Chen, A.-D.; Zhou, H.; Chen, J.-Y.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; et al. MiR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression. Biomedicines 2021, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Yang, J.Y.; Park, C.H.; Son, K.H.; Byun, K. Dieckol Reduces Muscle Atrophy by Modulating Angiotensin Type II Type 1 Receptor and NADPH Oxidase in Spontaneously Hypertensive Rats. Antioxidants 2021, 10, 1561. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of Hypertension and Vascular Dysfunction by Hydroxyhydroquinone-Free Coffee in a Genetic Model of Hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Waltner-Law, M.E.; Wang, X.L.; Law, B.K.; Hall, R.K.; Nawano, M.; Granner, D.K. Epigallocatechin Gallate, a Constituent of Green Tea, Represses Hepatic Glucose Production*. J. Biol. Chem. 2002, 277, 34933–34940. [Google Scholar] [CrossRef] [Green Version]
- Panagiotakos, D.B.; Lionis, C.; Zeimbekis, A.; Gelastopoulou, K.; Papairakleous, N.; Das, U.N.; Polychronopoulos, E. Long-Term Tea Intake Is Associated with Reduced Prevalence of (Type 2) Diabetes Mellitus among Elderly People from Mediterranean Islands: MEDIS Epidemiological Study. YMJ 2009, 50, 31–38. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.Z. Antioxidant and Acetylcholinesterase Inhibition Properties of Amorpha Fruticosa L. and Phytolacca Americana L. Pharmacogn. Mag. 2013, 9, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Zhao, H.; Wang, Q.; Wu, F.; Cao, W. A Novel Chinese Honey from Amorpha Fruticosa L.: Nutritional Composition and Antioxidant Capacity In Vitro. Molecules 2020, 25, 5211. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antioxidants-Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2020.
| SHR Group | Treatment |
|---|---|
| Group 1 (SHR C) | Treated for 35 days with saline vehicle administered via gavage at 5 mL/kg bw/day; on day 7 of the experiment, the animals received an i.p. injection with citrate buffer (pH 4.5) |
| Group 2 (SHR-EAF) | treated with EAF (100 mg/kg b.w./day, (1/20 of LD50 p.o.) oral-gavage for 35 days |
| Group 3 (SHR T2D) | Challenged on day 7 of the experiment with nicotinamide (230 mg/kg bw, i.p.) and 15 min after that, with streptozotocin (40 mg/kg b.w., i.p.) |
| Group 4 (SHR T2D + EAF) | Treated with EAF (100 mg/kg b.w./day, oral-gavage for 7 days); on day 7, the animals were challenged with nicotineamide–streptozotocine (230 mg/kg b.w./40 mg/kg b.w. i.p.) and were treated with EAF for additional 28 days |
| 1st Phase | 2nd Phase | ||
|---|---|---|---|
| Doses mg/kg p.o. | Mortality | Doses mg/kg p.o. | Mortality |
| 10 | 0/3 | 1500 | 0/3 |
| 100 | 0/3 | 3000 | 1/3 |
| 1000 | 0/3 | 5000 | 3/3 |
| 1st Phase | 2nd Phase | ||
|---|---|---|---|
| Doses mg/kg i.p. | Mortality | Doses mg/kg i.p. | Mortality |
| 10 | 0/3 | 1500 | 3/3 |
| 100 | 0/3 | 3000 | 3/3 |
| 1000 | 1/3 | 5000 | 3/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonova, R.; Shkondrov, A.; Kozuharova, E.; Ionkova, I.; Krasteva, I. A Study on the Safety and Effects of Amorpha fruticosa Fruit Extract on Spontaneously Hypertensive Rats with Induced Type 2 Diabetes. Curr. Issues Mol. Biol. 2022, 44, 2583-2592. https://doi.org/10.3390/cimb44060176
Simeonova R, Shkondrov A, Kozuharova E, Ionkova I, Krasteva I. A Study on the Safety and Effects of Amorpha fruticosa Fruit Extract on Spontaneously Hypertensive Rats with Induced Type 2 Diabetes. Current Issues in Molecular Biology. 2022; 44(6):2583-2592. https://doi.org/10.3390/cimb44060176
Chicago/Turabian StyleSimeonova, Rumyana, Aleksandar Shkondrov, Ekaterina Kozuharova, Iliana Ionkova, and Ilina Krasteva. 2022. "A Study on the Safety and Effects of Amorpha fruticosa Fruit Extract on Spontaneously Hypertensive Rats with Induced Type 2 Diabetes" Current Issues in Molecular Biology 44, no. 6: 2583-2592. https://doi.org/10.3390/cimb44060176
APA StyleSimeonova, R., Shkondrov, A., Kozuharova, E., Ionkova, I., & Krasteva, I. (2022). A Study on the Safety and Effects of Amorpha fruticosa Fruit Extract on Spontaneously Hypertensive Rats with Induced Type 2 Diabetes. Current Issues in Molecular Biology, 44(6), 2583-2592. https://doi.org/10.3390/cimb44060176









