Correlation between Cancer Stem Cells, Inflammation and Malignant Transformation in a DEN-Induced Model of Hepatic Carcinogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. DEN-Induced Liver Cancer Model
2.3. Histology Analysis
2.4. Reticulin Staining
2.5. Immunohistochemistry
2.6. Data Analyses
3. Results
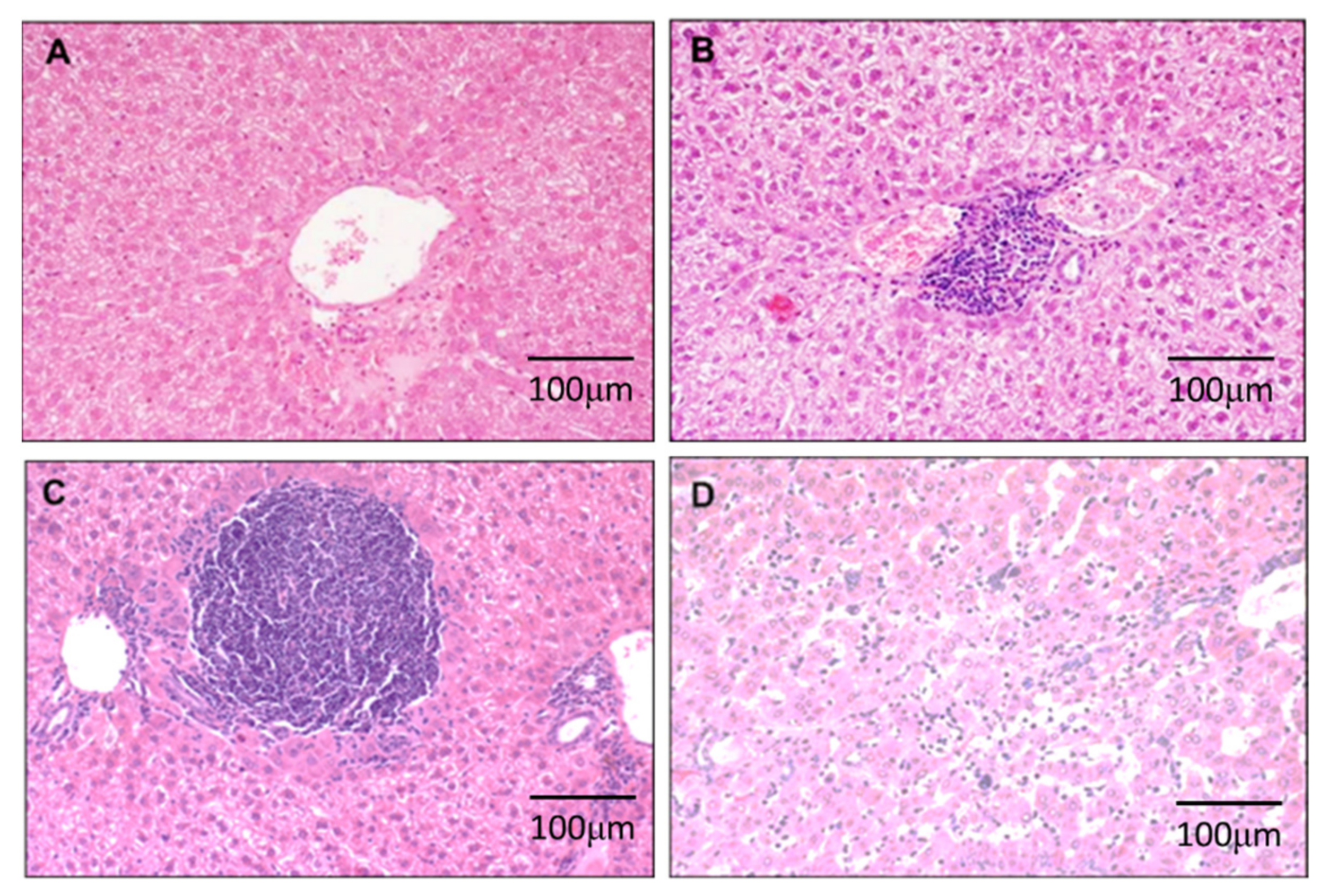
3.1. DEN-Induced Inflammation in Liver
3.2. DEN Induces Malignant Transformation in the Liver
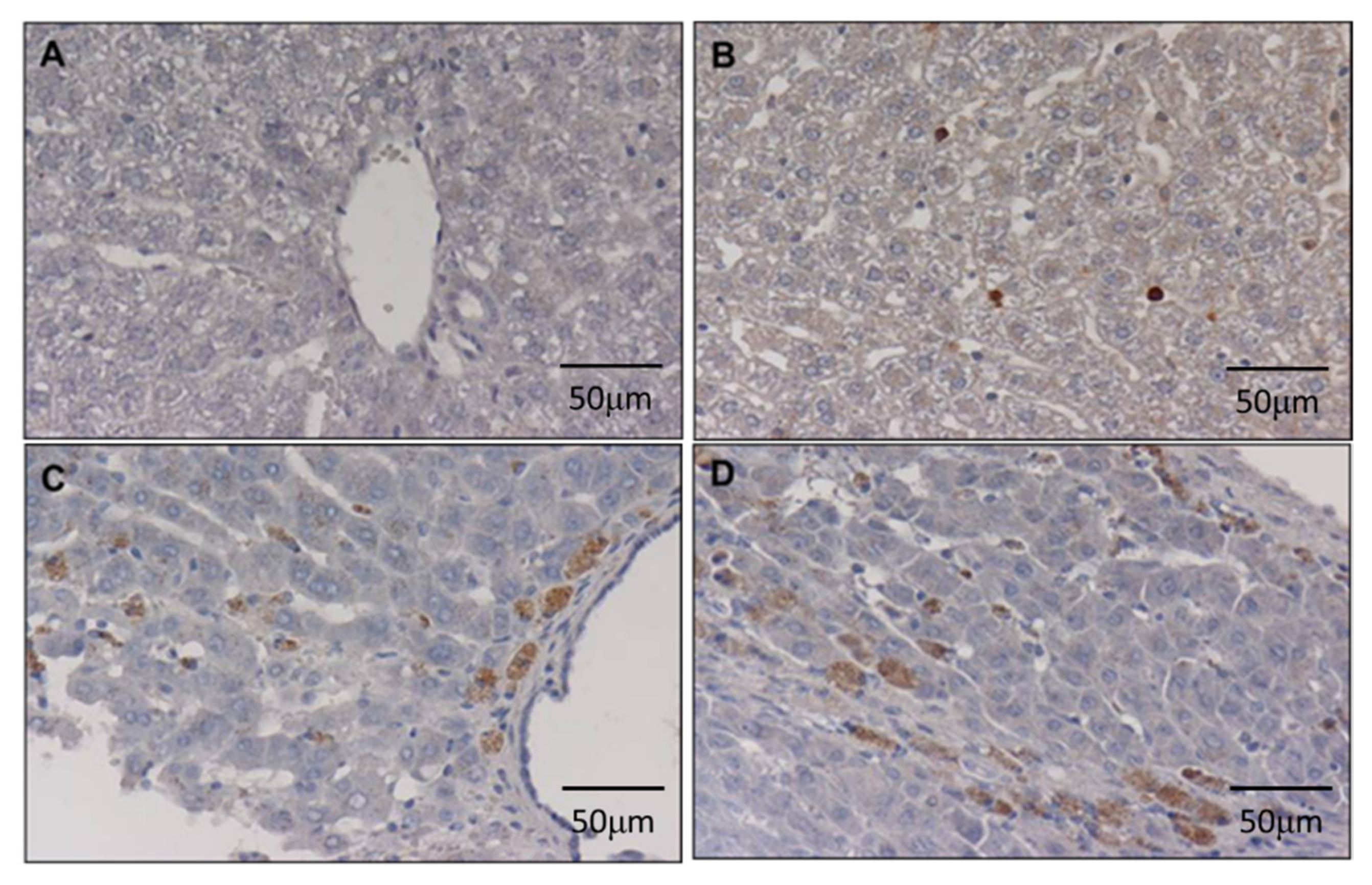
3.3. DEN Enhances Cancer Stem Cell Marker in the Liver
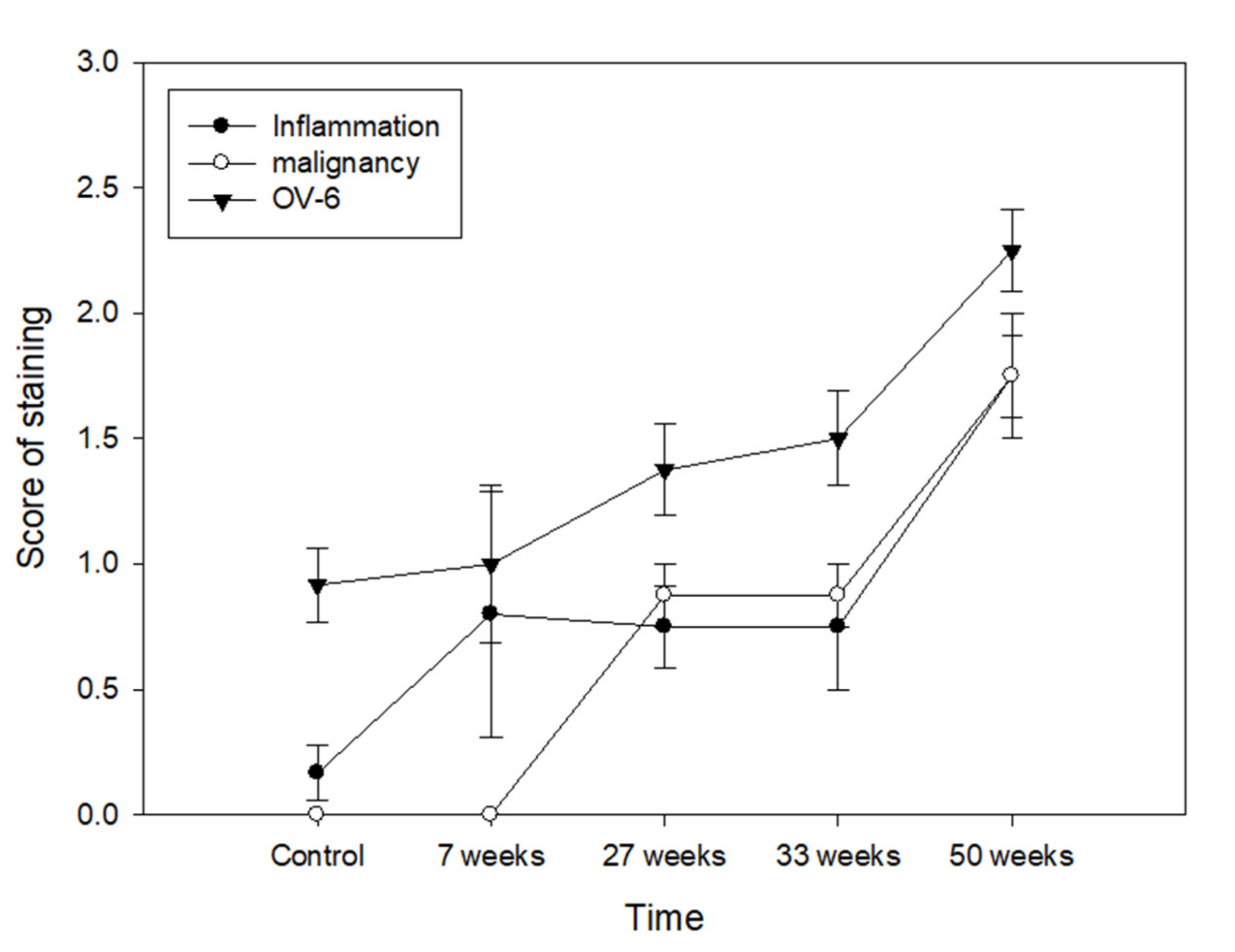
3.4. Relationship between Cancer Stem Cell, Inflammation and Malignant Transformation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Blum, H.E. Pathogenesis of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2005, 17, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.; Leffert, H.L. Liver cancer stem cells. J. Clin. Oncol. 2008, 26, 2800–2805. [Google Scholar] [CrossRef]
- Spee, B.; Carpino, G.; Schotanus, B.A.; Katoonizadeh, A.; Vander Borght, S.; Gaudio, E.; Roskams, T. Characterisation of the liver progenitor cell niche in liver diseases: Potential involvement of Wnt and Notch signalling. Gut 2010, 59, 247–257. [Google Scholar] [CrossRef]
- Beier, D.; Hau, P.; Proescholdt, M.; Lohmeier, A.; Wischhusen, J.; Oefner, P.J.; Aigner, L.; Brawanski, A.; Bogdahn, U.; Beier, C.P. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007, 67, 4010–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillmore, C.; Kuperwasser, C. Human breast cancer stem cell markers CD44 and CD24: Enriching for cells with functional properties in mice or in man? Breast Cancer Res. 2007, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, S.; Li, M.Y.; Hu, B.G.; Liu, L.P.; Yang, S.L.; Yang, S.; Gong, Z.; Lai, P.B.S.; Chen, G.G. Cancer stem cells in hepatocellular carcinoma: An overview and promising therapeutic strategies. Ther. Adv. Med. Oncol. 2018, 10, 1758835918816287. [Google Scholar] [CrossRef]
- Fausto, N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology 2004, 39, 1477–1487. [Google Scholar] [CrossRef]
- Li, Z.K.; Ma, Y.; Xu, C. Animal model and modified technique of orthotopic liver transplantation in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2004, 18, 34–36. [Google Scholar]
- Roskams, T.; De Vos, R.; Van Eyken, P.; Myazaki, H.; Van Damme, B.; Desmet, V. Hepatic OV-6 expression in human liver disease and rat experiments: Evidence for hepatic progenitor cells in man. J. Hepatol. 1998, 29, 455–463. [Google Scholar] [CrossRef]
- Ruck, P.; Xiao, J.C.; Pietsch, T.; Von Schweinitz, D.; Kaiserling, E. Hepatic stem-like cells in hepatoblastoma: Expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6. Histopathology 1997, 31, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Boivin, G.P.; Knudsen, E.S.; Nebert, D.W.; Xia, Y.; Puga, A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010, 70, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakami, Y.; Gottesman, M.E.; Blaner, W.S. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin:retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis 2011, 33, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nature reviews. Cancer 2006, 6, 674–687. [Google Scholar]
- Hsia, C.C.; Axiotis, C.A.; Di Bisceglie, A.M.; Tabor, E. Transforming growth factor-alpha in human hepatocellular carcinoma and coexpression with hepatitis B surface antigen in adjacent liver. Cancer 1992, 70, 1049–1056. [Google Scholar] [CrossRef]
- Schaff, Z.; Hsia, C.C.; Sarosi, I.; Tabor, E. Overexpression of transforming growth factor-alpha in hepatocellular carcinoma and focal nodular hyperplasia from European patients. Hum. Pathol. 1994, 25, 644–651. [Google Scholar] [CrossRef]
- Legoix, P.; Bluteau, O.; Bayer, J.; Perret, C.; Balabaud, C.; Belghiti, J.; Franco, D.; Thomas, G.; Laurent-Puig, P.; Zucman-Rossi, J. Beta-catenin mutations in hepatocellular carcinoma correlate with a low rate of loss of heterozygosity. Oncogene 1999, 18, 4044–4046. [Google Scholar] [CrossRef] [Green Version]
- Weihrauch, M.; Benick, M.; Lehner, G.; Wittekind, M.; Bader, M.; Wrbitzk, R.; Tannapfel, A. High prevalence of K-ras-2 mutations in hepatocellular carcinomas in workers exposed to vinyl chloride. Int. Arch. Occup. Environ. Health 2001, 74, 405–410. [Google Scholar] [CrossRef]
- Levine, A.J.; Momand, J.; Finlay, C.A. The p53 tumour suppressor gene. Nature 1991, 351, 453–456. [Google Scholar] [CrossRef]
- Nigro, J.M.; Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Hostetter, R.; Cleary, K.; Bigner, S.H.; Davidson, N.; Baylin, S.; Devilee, P.; et al. Mutations in the p53 gene occur in diverse human tumour types. Nature 1989, 342, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C. Recent translational research: Stem cells as the roots of breast cancer. Breast Cancer Res. 2006, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Fitzpatrick, F.A. Inflammation, carcinogenesis and cancer. Int. Immunopharmacol. 2001, 1, 1651–1667. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benelli, R.; Lorusso, G.; Albini, A.; Noonan, D.M. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr. Pharm. Des. 2006, 12, 3101–3115. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Borm, P.J.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef]
- Lim, H.B.; Ichinose, T.; Miyabara, Y.; Takano, H.; Kumagai, Y.; Shimojyo, N.; Devalia, J.L.; Sagai, M. Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice. Free Radic. Biol. Med. 1998, 25, 635–644. [Google Scholar] [CrossRef]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Delladetsima, J.; Alexandrou, P.; Giaslakiotis, K.; Psichogiou, M.; Hatzis, G.; Sypsa, V.; Tiniakos, D. Hepatic progenitor cells in chronic hepatitis C: A phenomenon of older age and advanced liver disease. Virchows Arch. 2010, 457, 457–466. [Google Scholar] [CrossRef]
- Oliva, J.; French, B.A.; Qing, X.; French, S.W. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp. Mol. Pathol. 2010, 88, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Giron-Gonzalez, J.A.; Martinez-Sierra, C.; Rodriguez-Ramos, C.; Macias, M.A.; Rendon, P.; Diaz, F.; Fernandez-Gutierrez, C.; Martin-Herrera, L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004, 24, 437–445. [Google Scholar] [CrossRef]
- Plentz, R.R.; Caselitz, M.; Bleck, J.S.; Gebel, M.; Flemming, P.; Kubicka, S.; Manns, M.P.; Rudolph, K.L. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology 2004, 40, 80–86. [Google Scholar] [CrossRef]
- Fang, C.H.; Gong, J.Q.; Zhang, W. Function of oval cells in hepatocellular carcinoma in rats. World J. Gastroenterol. 2004, 10, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, C.; Lin, Y.; Liu, Q.; Yu, L.X.; Tang, L.; Yan, H.X.; Fu, J.; Chen, Y.; Zhang, H.L.; et al. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J. Hepatol. 2012, 57, 613–620. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Lin, C.-J.; Kuo, K.-K.; Chen, W.-T.; Ker, C.-G.; Chai, C.-Y.; Tsai, H.-P.; Yang, S.-F. Correlation between Cancer Stem Cells, Inflammation and Malignant Transformation in a DEN-Induced Model of Hepatic Carcinogenesis. Curr. Issues Mol. Biol. 2022, 44, 2879-2886. https://doi.org/10.3390/cimb44070198
Wu C-C, Lin C-J, Kuo K-K, Chen W-T, Ker C-G, Chai C-Y, Tsai H-P, Yang S-F. Correlation between Cancer Stem Cells, Inflammation and Malignant Transformation in a DEN-Induced Model of Hepatic Carcinogenesis. Current Issues in Molecular Biology. 2022; 44(7):2879-2886. https://doi.org/10.3390/cimb44070198
Chicago/Turabian StyleWu, Chun-Chieh, Chien-Ju Lin, Kong-Kai Kuo, Wan-Tzu Chen, Chen-Guo Ker, Chee-Yin Chai, Hung-Pei Tsai, and Sheau-Fang Yang. 2022. "Correlation between Cancer Stem Cells, Inflammation and Malignant Transformation in a DEN-Induced Model of Hepatic Carcinogenesis" Current Issues in Molecular Biology 44, no. 7: 2879-2886. https://doi.org/10.3390/cimb44070198





