Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review
Abstract
:1. Introduction
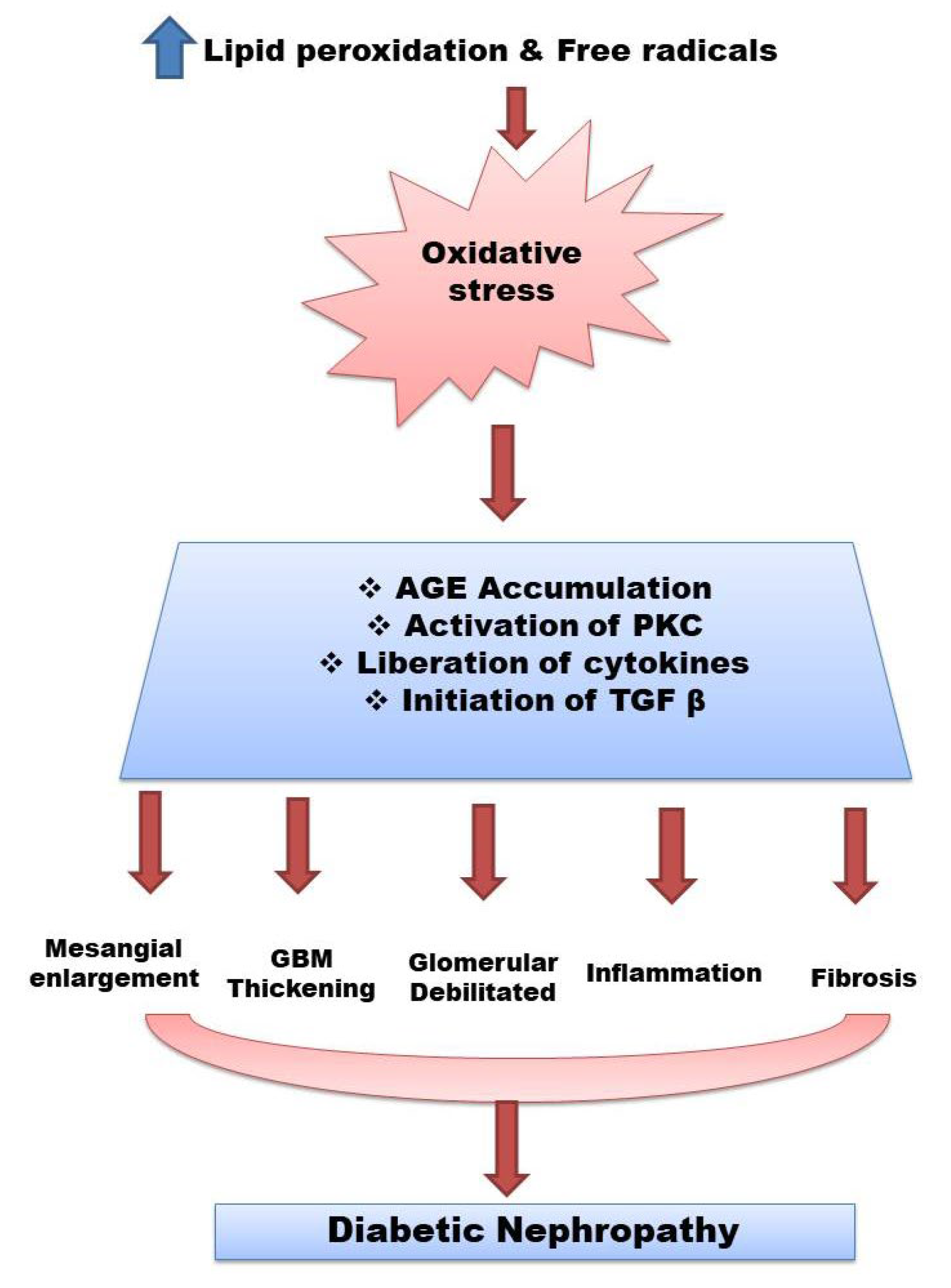
2. Pathogenesis of Diabetic Nephropathy
3. Medicinal Plants and Diabetic Nephropathy
3.1. Ginger
3.2. Garlic
3.3. Cinnamon
3.4. Clove
3.5. Turmeric
3.6. Green Tea
3.7. Guava
3.8. Fenugreek
3.9. Gooseberry
3.10. Oats
3.11. Pepper
3.12. Coriander
3.13. Silymarin
3.14. Tulasi
3.15. Red Sandal Wood
4. Clinical Trial Studies on Diabetic Nephropathy
5. Summary
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, Q.; Chen, L.; Chen, K. Studies of the Anti-Diabetic Mechanism of Puerarialobata Based on Metabolomics and Network Pharmacology. Processes 2021, 9, 1245. [Google Scholar] [CrossRef]
- Magee, C.; Grieve, D.J.; Watson, C.J.; Brazil, D.P. Diabetic nephropathy: A tangled web tounweave. Cardiovasc. Drugs Ther. 2017, 31, 579–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schena, F.P.; Gesualdo, L. Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16, S30–S33. [Google Scholar] [CrossRef]
- Vinod, P.B. Pathophysiology of diabetic nephropathy. Clin. Queries Nephrol. 2012, 1, 121–126. [Google Scholar] [CrossRef]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247. [Google Scholar] [CrossRef]
- Ayse, N.; Duygu, A.T.; Hakký, A.I.; Tansel, O.Y.; Ýsmet, D.G.; Ismail, K. Antimicrobial and cytotoxic activities of Zingiber officinalis extracts. FABAD J. Pharm. Sci. 2008, 33, 77–86. [Google Scholar]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycaemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef]
- Shiju, T.M.; Rajesh, N.G.; Viswanathan, P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013, 45, 18–23. [Google Scholar] [CrossRef]
- Muthenna, P.; Raghu, G.; Kumar, P.A.; Surekha, M.V.; Reddy, G.B. Effect of cinnamon and its procyanidin-B2 enriched fraction on diabetic nephropathy in rats. Chem. Biol. Interact. 2014, 222, 68–76. [Google Scholar] [CrossRef]
- Joshua, S.; Sweeten, O.; Ifunaya, C.C. Evaluation of Renal Function of Streptozotocin (STZ)-Induced Diabetic Albino Rats Treated with Syzygiumaromaticum (Clove). Int. J. Nephrol. 2021, 8, 17–26. [Google Scholar]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin ameliorates diabetic nephropathy by suppressing NLRP3 inflammasome signalling. BioMed. Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.Y.; Park, Y.H.; Kim, B.S.; Seo, S.Y.; Jeong, B.C.; Kim, J.I.; Kim, H.H. Preventive effects of green tea (Camellia sinensis var. assamica) on diabetic nephropathy. Yonsei Med. J. 2012, 53, 138–144. [Google Scholar] [PubMed] [Green Version]
- Lin, C.Y.; Yin, M.C. Renal Protective Effects of Extracts from Guava Fruit (Psidium guajava L.) in Diabetic Mice. Plant Foods Hum. Nutr. 2012, 67, 303–308. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, Y.; Zou, Y.; Miao, C.; Sun, B.; Li, C. Fenugreek prevents the development of STZ-induced diabetic nephropathy in a rat model of diabetes. Evid. Based Complement. Altern. Med. 2014, 2014, 259368. [Google Scholar] [CrossRef] [Green Version]
- Chandak, P.G.; Gaikwad, A.B.; Tikoo, K. Gallotannin ameliorates the development of streptozotocin-induced diabetic nephropathy by preventing the activation of PARP. Phytother. Res. 2009, 23, 72–77. [Google Scholar] [CrossRef]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef]
- Kajal, A.; Singh, R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE 2019, 14, e0213147. [Google Scholar] [CrossRef]
- Pandiri, I.; Moni, A. Ocimum herb species: A potential treatment strategy for diabetic kidney disease. J. Adv. Biotechnol. Exp. Ther. 2018, 1, 88–91. [Google Scholar] [CrossRef]
- Halim, M.E.; Misra, A. The effects of the aqueous extract of Pterocarpus santalinus heartwood and vitamin E supplementation in streptozotocin-induced diabetic rats. J. Med. Plants Res. 2011, 5, 398–409. [Google Scholar]
- Al-Tahtawy, R.H.M.; El-Bastawesy, A.M.; Monem, M.G.A.; Zekry, Z.K.; Al-Mehdar, H.A.; El-Merzabani, M.M. Antioxidant activity of the volatile oils of Zingiber officinale (ginger). Spatula DD 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Dissanayake, K.G.; Waliwita, W.A.; Liyanage, R.P. A review on medicinal uses of Zingiber officinale (ginger). Int. J. Health Sci. 2020, 10, 142–148. [Google Scholar]
- Mishra, S. Bhavaprakasha Nighantu Vidyodhinihindi Commentary, 10th ed.; Chaukhamba Orientalia: Varanasi, India, 2002; p. 15. [Google Scholar]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS ONE 2013, 8, e67078. [Google Scholar]
- Kato, A.; Higuchi, Y.; Goto, H.; Kizu, H.; Okamoto, T.; Asano, N.; Hollinshead, J.; Nash, R.J.; Adachi, I. Inhibitory effects of Zingiber officinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J. Agric. Food Chem. 2006, 54, 6640–6644. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Asad, M. The Potential Benefits of Using Garlic Oil and Its Active Constituent, Dially Disulphide, in Combination with Carvedilol in Ameliorating Isoprenaline-Induced Cardiac Damage in Rats. Front. Pharmacol. 2021, 12, 739758. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Cardioprotective Potential of Garlic Oil and Its Active Constituent, Diallyl Disulphide, in Presence of Carvedilol during Chronic Isoprenaline Injection-Mediated Myocardial Necrosis in Rats. Molecules 2021, 26, 5137. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Historical perspective on garlic and cardiovascular disease. J. Nutr. 2001, 131, 977S–979S. [Google Scholar] [CrossRef] [Green Version]
- Asdaq, S.M.B.; Lokaraja, S.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Nagaraja, S.; Imran, M. Potential Interaction of Fresh Garlic with Metformin during Ischemia-Reperfusion Induced Cardiac Injury in Diabetic Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 9739089. [Google Scholar] [CrossRef]
- Davis, S.R. An overview of the antifungal properties of allicin and its breakdown products-the possibility of a safe and effective antifungal prophylactic. Mycoses 2005, 48, 95–100. [Google Scholar] [CrossRef]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory effects of the nutraceutical garlic derivative allicin in the progression of diabetic nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef] [Green Version]
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Alshammari, M.S. Cytoprotective Potential of Aged Garlic Extract (AGE) and Its Active Constituent, S-allyl-l-cysteine, in Presence of Carvedilol during Isoproterenol-Induced Myocardial Disturbance and Metabolic Derangements in Rats. Molecules 2021, 26, 3203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.Y.; Son, D.J.; Lee, H.; Yoo, H.S.; Song, S.; Oh, K.W.; Han, D.C.; Kwon, B.M.; Hong, J.T. Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem. Pharmacol. 2005, 69, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Hussain, S.; Rahman, R.; Mushtaq, A.; Zerey-Belaskri, A.E. Clove: A review of a precious species with multiple uses. Int. J. Chem. Biochem. 2017, 11, 129–133. [Google Scholar]
- Jonah, A.O.; Enoh, E. In vitro Anti-trypanosomal activity of curcumin isolated from Curcuma longa (Turmeric) rhizomes. J. Entomol. Zool. Stud. 2020, 8, 729–731. [Google Scholar]
- Sahebkar, A.; Serbanc, M.C.; Ursoniuc, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Lysiuk, R.; Mansuetusmboya, J. Herbal drugs for the treatment of diabetic nephropathy: Current status and prospects for the application. J. Kidney Treat. Diagn. 2020, 3, 3–4. [Google Scholar]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Srinivasan, K. Amelioration of renal lesions associated with diabetes by dietary curcumin in strepozotocine diabetic rats. Mol. Cell. Biochem. 1998, 181, 87–96. [Google Scholar] [CrossRef]
- Sun, L.N.; Yang, Z.Y.; Lv, S.S.; Liu, X.C.; Guan, G.J.; Liu, G. Curcumin prevents diabetic nephropathy against inflammatory response via reversing caveolin-1 Tyr14 phosphorylation influenced TLR4 activation. Int. Immunopharmacol. 2014, 23, 236–246. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Zhang, W.; Zhang, J.; Yang, J.; Li, K.; He, Y. ATP-P2X4 signalling mediates NLRP3 inflammasome activation: A novel pathway of diabetic nephropathy. Int. J. Biochem. Cell Biol. 2013, 45, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Geng, H.; Liu, Z.; Li, H.; Zu, Z. Effect of curcumin on rats/mice with diabetic nephropathy: A systematic review and meta-analysis of randomized controlled trials. J. Tradit. Chin. Med. 2014, 34, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.P.; Feng, L.; Zhu, M.M.; Wang, R.S.; Zhang, M.H.; Hu, S.Y.; Jia, X.B.; Wu, J.J. The in vitro protective effects of curcumin and dimethoxy curcumin in Curcuma longa extract on advanced glycation end products-induced mesangial cell apoptosis and oxidative stress. Planta Med. 2012, 78, 1757–1760. [Google Scholar]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Zhu, H.M.; Cai, J.Q.; Huang, Y.Z.; Xu, J.; Zhou, Y.; Chen, X.H.; Li, X.Q.; Yang, Z.M.; Deng, L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1407–1415. [Google Scholar] [CrossRef]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [Green Version]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roccaro, S.A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartippour, M.R.; Shao, Z.M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.M.; Ruzindana-Umunyana, A.; Imbeault, L.; Sircar, S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir. Res. 2003, 58, 167–173. [Google Scholar] [CrossRef]
- Ahn, T.G.; Kim, H.K.; Park, S.W.; Kim, S.A.; Lee, B.R.; Han, S.J. Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats. Obstet. Gynecol. Sci. 2014, 57, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Barzegar, K. Green tea and diabetes effect of green tea and sour tea on blood pressure of patients with type 2 diabetes: A randomized clinical trial. J. Diet. Suppl. 2013, 10, 105–115. [Google Scholar] [CrossRef]
- Renno, W.M.; Abdeen, S.; Alkhalaf, M.; Asfar, S. Effect of green tea on kidney tubules of diabetic rats. Br. J. Nutr. 2008, 100, 652–659. [Google Scholar] [CrossRef]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Shahi, M. Green Tea (Camellia sinensis) Supplementation to Diabetic Rats Improves Serum and Hepatic Oxidative Stress Markers. Iran J. Pharm. Res. 2013, 12, 109–114. [Google Scholar]
- Nasri, H.; Ahmadi, A.; Baradaran, A.; Nasri, P.; Hajian, S.; Pour-Arian, A.; Kohi, G.; Rafieian-Kopaei, M. A biochemical study on ameliorative effect of green tea (Camellia sinensis) extract against contrast media induced acute kidney injury. J. Ren. Inj. Prev. 2013, 3, 47–49. [Google Scholar]
- Deguchi, Y.; Miyazaki, K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr. Metab. 2010, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Antiglycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar]
- Jain, N.; Dhawan, K.; Malhotra, S.; Singh, R. Biochemistry of fruit ripening of guava (Psidium guajava L.): Compositional and enzymatic changes. Plant Foods Hum. Nutr. 2003, 58, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dixit, Y.; Kar, A. Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Hum. Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Morcos, S.R.; Elhawary, Z.; Gabrial, G.N. Protein rich food mixtures for feeding the young in Egypt. 1. Formulation. Z. Ernährungswissenschaft 1981, 20, 275–282. [Google Scholar] [CrossRef]
- Bdel-Bary, J.A.; Abdel Hassan, I.A.; Al-Hakiem, M.H.H. Hypoglycemic and antihyperglycemic effect of Trigonellafoenumgraecum leaf in normal and alloxan induced diabetic rats. J. Ethnopharmacol. 1997, 58, 149–155. [Google Scholar] [CrossRef]
- Sayed, A.A.; Khalifa, M.; Abd el-Latif, F.F. Fenugreek attenuation of diabetic nephropathy in alloxan-diabetic rats. J. Physiol. Biochem. 2012, 68, 263–269. [Google Scholar] [CrossRef]
- Morcos, M.; Schlotterer, A.; Sayed, A.A.R.; Kukudov, G.; Oikomonou, D.; Ibrahim, Y.; Pfisterer, F.; Schneider, J.; Bozorgmehr, F.; Rudofsky, G., Jr.; et al. Rosiglitazone reduces angiotensin ii and advanced glycation end product-dependent sustained nuclear factor-κB activation in cultured human proximal tubular epithelial cells. Horm. Metab. Res. 2008, 40, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Unander, D.W.; Webster, G.L.; Blumberg, B.S. Records of usage or assays in Phyllanthus (Euphorbiaceae) I. Subgenera Isocladus, Kirganelia, Cicca and Emblica. J. Ethnopharmacol. 1990, 30, 233–264. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; Marques, M.; Witcher, M. Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxidative Med. Cell Longev. 2015, 2015, 950890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Malki, A.L. Oat protects against diabetic nephropathy in rats via attenuating advanced glycation end products and nuclear factor kappa B. Evid. Based Complement. Altern. Med. 2013, 2013, 609745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcaro, C.A.; Gutierres, V.O.; Assis, R.P.; Moreira, T.F.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PLoS ONE 2014, 9, e113993. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food. sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Lee, E.B.; Shin, K.H.; Woo, W.S. Pharmacological study on piperine. Arch. Pharm. Res. 1984, 7, 127–132. [Google Scholar] [CrossRef]
- Önder, A. Coriander and its Phytoconstituents for the Beneficial Effects. Potential of Essential Oils; IntechOpen: London, UK, 2018; Volume 26, pp. 165–185. [Google Scholar]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Uitterhaegen, E.; Sampaio, K.A.; Delbeke, E.I.P.; Greyt, W.D.; Cerny, M.; Evon, P.; OthmaneMerah, O.; Talou, T.; Stevens, C.V. Characterization of French coriander oil as a source of petroselinic acid. Molecules 2016, 21, 1202. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Hahbazi, F.; Dashti-Khavidaki, S.; Khalili, H.; Lessan-Pezeshki, M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J. Pharm. Sci. 2012, 15, 112–123. [Google Scholar]
- Loguerico, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Baboota, S.; Kohli, K.; Ahmad, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007, 39, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Roozbeh, J.; Shahriyari, B.; Akmali, M.; Vessal, G.; Pakfetrat, M.; Jalali, G.A.R.; Afshariani, R.; Hasheminasab, M.; Ghahramani, N. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren. Fail. 2011, 33, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and milk thistle may prevent the progression of dibatic nephropathy in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Rafieian-Kopaie, M.; Nasri, H. Silymarin and diabetic nephropathy. J. Ren. Inj. Prev. 2012, 1, 3–5. [Google Scholar]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M. Tulsi—Ocimum sanctum: An herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Shafi, S.; Tabassum, N.; Ahmad, F. Diabetic nephropathy and herbal medicines. Int. J. Phytopharm. 2012, 3, 10–17. [Google Scholar]
- Alanazi, A.Z.; Mohany, M.; Alasmari, F.; Mothana, R.A.; Alshehri, A.O.; Alhazzani, K.; Ahmed, M.M.; Al-Rejaie, S.S. Amelioration of Diabetes-Induced Nephropathy by Loranthusregularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia. Appl. Sci. 2021, 11, 4548. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Soleimani, A.; Bahmani, F.; Moravveji, A.; Asadi, A.; Amirani, E.; Farzin, N.; Sharifi, N.; Naseri, A.; Dastorani, M.; et al. Metabolic response to mulberry extract supplementation in patients with diabetic nephropathy: A randomized controlled trial. Iran J. Kidney Dis. 2017, 11, 438–446. [Google Scholar] [PubMed]
- Vanaie, A.; Shahidi, S.; Iraj, B.; Siadat, Z.D.; Kabirzade, M.; Shakiba, F.; Mohammadi, M.; Parvizian, H. Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J. Res. Med. Sci. 2019, 28, 77. [Google Scholar]
- Asdaq, S.M.; Inamdar, M.N. The potential for interaction of hydrochlorothiazide with garlic in rats. Chem. Biol. Interact. 2009, 181, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.; Inamdar, M.N. The potential benefits of a garlic and hydrochlorothiazide combination as antihypertensive and cardioprotective in rats. J. Nat. Med. 2011, 65, 81–88. [Google Scholar] [CrossRef]
- Singh, R.; Rao, H.K.; Singh, T.G. Advanced glycated end products (ages) in diabetes and its complications: An insight. Plant Arch. 2020, 20, 3838–3841. [Google Scholar]
- Rodríguez-Pérez, M.D.; López-Villodres, J.A.; Arrebola, M.M.; Martín-Aurioles, E.; Fernández-Prior, Á.; Bermúdez-Oria, A.; Ríos, M.C.; De La Cruz, J.P.; González-Correa, J.A. Nephroprotective Effect of the Virgin Olive Oil Polyphenol Hydroxytyrosol in Type 1-like Experimental Diabetes Mellitus: Relationships with Its Antioxidant Effect. Antioxidants 2021, 10, 1783. [Google Scholar] [CrossRef]
| Botanical Name | Family | Common Name | Parts Used | Mechanism of Action | References |
|---|---|---|---|---|---|
| Zingiber officinale | Zingiberacae | Ginger | Rhizomes | Lowers lipid peroxidation and enhances plasma antioxidant capacity | [8] |
| Allium sativum | Amaryllidaceae | Garlic | Bulb | The anti-glycation and hypolipidemic properties of aged garlic extract may be responsible for its protection against DN. | [9] |
| Cinnamon cassia | Lauraceae | Cinnamon | Bark | Prevents AGE accumulation in vivo and reduced alteration in the renal function of diabetic rats. | [10] |
| Syzygiumaromaticum | Myrtaceae | Clove | Roots | Decreases lipid peroxidation and cytokine release. | [11] |
| Curcuma longa | Zingiberaceae | Turmeric | Seeds | Curcumin’s protective impact in DN may be due to the suppression of NLRP3 inflammasome activation. | [12] |
| Camellia sinensis | Theaceae, | Green tea | Leaves | Green tea protects the kidneys from DN by preventing glomerular hyperfiltration, hypertrophic alterations, and protein loss in the urine. | [13] |
| Psidium guajava | Myrtaceae | Guava | Fruits | Anti-inflammatory, antioxidant and anti-gylcative properties. | [14] |
| Trigonella foenumgraecum | Myrtaceae | Fenugreek | Seeds | By reducing renal oxidative stress and inhibiting the TGF-β1/CTGF signaling pathway. | [15] |
| Phyllanthus emblica | Grossulariaceae | Gooseberry | Fruits | Gallotanin, an important ingredient of gooseberry, was found to be efficient in lowering plasma creatinine levels and reducing apoptosis by blocking poly ADP-ribose polymerase cleavage. | [16] |
| Piper nigrum | Piperaceae | Pepper | Fruit | Inhibited NF-κB and NLRP3 activation. | [17] |
| Coriandrum sativum | Apiaceae | Coriander | Seed | Delayed progression of DN, by inhibiting AGE. | [18] |
| Ocimum sanctum | Labiatae | Tulasi | Leaves | Antioxidant and anti-inflammatory activity. | [19] |
| Pterocarpus santalinus | Fabaceae | Red sandalwood | Bark | Antioxidant activity. | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahin D. H., H.; Sultana, R.; Farooq, J.; Taj, T.; Khaiser, U.F.; Alanazi, N.S.A.; Alshammari, M.K.; Alshammari, M.N.; Alsubaie, F.H.; Asdaq, S.M.B.; et al. Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review. Curr. Issues Mol. Biol. 2022, 44, 2887-2902. https://doi.org/10.3390/cimb44070199
Shahin D. H. H, Sultana R, Farooq J, Taj T, Khaiser UF, Alanazi NSA, Alshammari MK, Alshammari MN, Alsubaie FH, Asdaq SMB, et al. Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review. Current Issues in Molecular Biology. 2022; 44(7):2887-2902. https://doi.org/10.3390/cimb44070199
Chicago/Turabian StyleShahin D. H., Haleema, Rokeya Sultana, Juveriya Farooq, Tahreen Taj, Umaima Farheen Khaiser, Nader Sulaiman Ayyt Alanazi, Mohammed Kanan Alshammari, Mohammad Nazal Alshammari, Firas Hamdan Alsubaie, Syed Mohammed Basheeruddin Asdaq, and et al. 2022. "Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review" Current Issues in Molecular Biology 44, no. 7: 2887-2902. https://doi.org/10.3390/cimb44070199
APA StyleShahin D. H., H., Sultana, R., Farooq, J., Taj, T., Khaiser, U. F., Alanazi, N. S. A., Alshammari, M. K., Alshammari, M. N., Alsubaie, F. H., Asdaq, S. M. B., Alotaibi, A. A., Alamir, A. a., Imran, M., & Jomah, S. (2022). Insights into the Uses of Traditional Plants for Diabetes Nephropathy: A Review. Current Issues in Molecular Biology, 44(7), 2887-2902. https://doi.org/10.3390/cimb44070199








