Genetically Engineered Hepatitis C Virus-like Particles (HCV-LPs) Tagged with SP94 Peptide to Acquire Selectivity to Liver Cancer Cells via Grp78
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of VLPs Tagged with RGD and SP94 Cancer-Specific Peptides
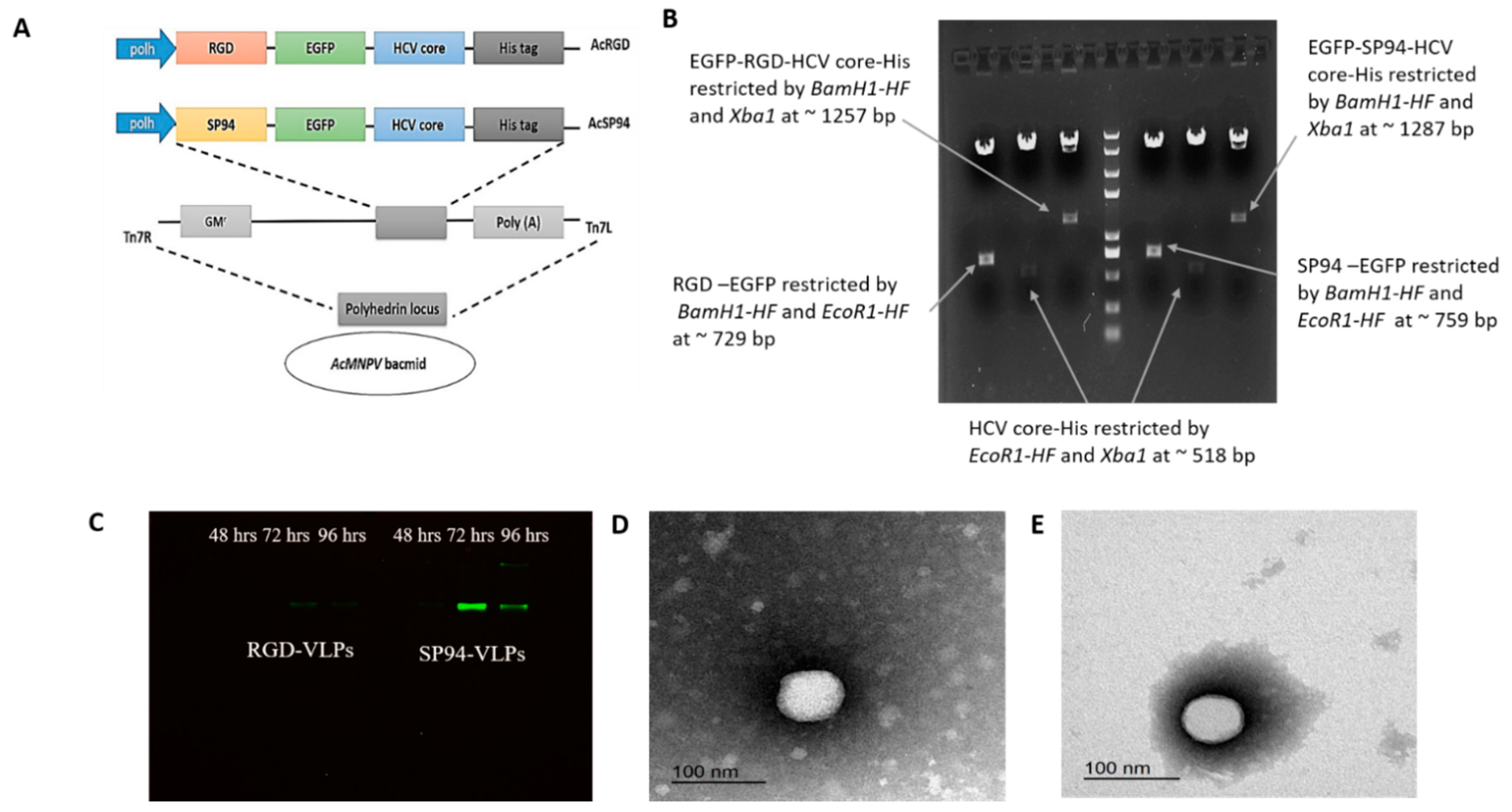
- (A)
- SP94-EGFP was constructed after three sequential PCR reactions using Phusion High Fidelity DNA Taq polymerase (ThermoFisher Scientific, Waltham, MA, USA), using specific primers listed in (Table 1). The first PCR amplicon was amplified by primers 1 and 7; then it was purified by a gel purification kit (Qiagen, Hilden, Germany). The same process was followed with the sequential PCR reactions using primers 2 and 7 and finally by primers 3 and 7 to produce the final amplicon. The same procedures for RGD-EGFP were conducted, but by using primers 4 and 7, then primers 5 and 7, and finally primers 6 and 7. The purified PCR products of SP94-EGFP and RGD-EGFP were digested with restriction endonucleases BamH1- and EcoR1 (New England Biolabs-NEB, Ipswich, MA, USA); the same enzymes were used to cut the plasmid pFastBac Dual (ThermoFisher Scientific, Waltham, MA, USA). Digested products were purified and ligated with T4 DNA ligase (New England Biolabs-NEB, Ipswich, MA, USA) to obtain pFast-SP94-EGFP and pFast-RGD-EGFP constructs. The ligated products were transformed into competent E. coli DH5a, as in our previous work [13].
- (B)
- For the second cloning fragment, HCV core-His was constructed by two sequential PCR reactions using Phusion High Fidelity DNA Taq polymerase (ThermoFisher Scientific, Waltham, MA, USA); (a) First PCR amplicon was amplified by primers 8 and 9, then primers 8 and 10. The purified amplicon was digested with restriction endonucleases EcoR1 and Xbal (New England Biolabs-NEB, Ipswich, MA, USA); the same enzymes were also applied to plasmids pFast-SP94-EGFP and pFast-RGD-EGFP. Digested products were purified and then ligated with T4 DNA ligase (New England Biolabs-NEB, Ipswich, MA, USA) to form pFast-SP94-EGFP-core-His (pSP94-core) and pFast-RGD-EGFP-core-His (pRGD-core). The ligated product transformed into competent E. coli DH5a as indicated above.
2.2. Construction of Recombinant Baculoviruses
2.3. Sf9 Transfection
2.4. Purification and Identification of HCV-LP
2.5. Binding Assay
2.6. Overexpression of Grp78 in HeLa and HepG2 Cells
3. Results
3.1. Expression and Validation of SP94-Core and RGD-Core Proteins
3.2. RGD-Core Protein Demonstrated Specificity to Cancer Cells
3.3. SP94-Core Protein Demonstrated Specificity to Liver Cancer Cells
3.4. Overexpression of Grp78 Increased Binding Efficiency of RGD-Core Protein to Cancer Cells
3.5. Overexpression of Grp78 Increases Binding Efficiency of SP94-Core Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, W.; Mofed, D.; Zekri, A.; El-Sayed, N.; Rahouma, M.; Sabet, S. Antioxidant activity and apoptotic induction as mechanisms of action of Withania somnifera (Ashwagandha) against a hepatocellular carcinoma cell line. Int. J. Med. Res. 2018, 46, 1358–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.; Cai, H.; Steinmetz, N. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, J.C.; Perrine, M.; Pericle, F.; Cavallo, F. Virus-Like Particles as an Immunogenic Platform for Cancer Vaccines. Viruses 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xing, L.; Stark, M.; Ou, T.; Holla, P.; Xiao, K.; Kamita, S.; Hammock, B.; Lam, K.; Cheng, R. Chemically activatable viral capsid functionalized for cancer targeting. Nanomedicine 2016, 11, 377–390. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, J.H.; Hur, W.; Choi, J.E.; Kim, S.M.; Park, D.J.; Kang, B.Y.; Lee, G.W.; Yoon, S.K. Liver-Specific Gene Delivery Using Engineered Virus-like Particles of Hepatitis E Virus. Sci. Rep. 2019, 9, 1616. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Jin, A.; Jia, Q.; Zhou, H.; Kang, S.; Lou, Y.; Gao, J.; Lu, J. Self-Assembled HCV Core Virus-like Particles Targeted And Inhibited Tumor Cell Migration And Invasion. Nanoscale Res. Lett. 2013, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Guzzetti, I.; Civera, M.; Vasile, F.; Araldi, E.; Belvisi, L.; Gennari, C.; Potenza, D.; Fanelli, R.; Piarulli, U. Determination of the binding epitope of RGD-peptidomimetics to αvβ3 and αIIbβ3 integrin-rich intact cells by NMR and computational studies. Org. Biomol. Chem. 2013, 11, 3886. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Kim, C.; Ginsberg, M. Molecular mechanism of inside-out integrin regulation. J. Thromb. Haemost. 2011, 9, 20–25. [Google Scholar] [CrossRef]
- Toita, R.; Murata, M.; Abe, K.; Narahara, S.; Piao, J.S.; Kang, J.H.; Hashizume, M. A Nanocarrier Based on a Genetically Engineered Protein Cage to Deliver Doxorubicin to Human Hepatocellular Carcinoma Cells. Chem. Commun. 2013, 49, 7442. [Google Scholar] [CrossRef]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef] [Green Version]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A.; Banerjee, S. ER Stress Sensor, Glucose Regulatory Protein 78 (GRP78) Regulates Redox Status In Pancreatic Cancer Thereby Maintaining “Stemness”. Cell Death Dis. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.; Salem, T.Z.; Wahba, M.A.; Mofeed, D.; Morsy, O.E.; Abdelbaset, R. A Capacitive Sensor For Differentiation Between Virus-Infected And Uninfected Cells. Sens. Bio-Sens. Res. 2022, 36, 100497. [Google Scholar] [CrossRef]
- O’Reilly, D.R.; Miller, L.K.; Luckow, V.A. Baculovirus Expression Vectors: A laboratory Manual; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Salem, T.Z.; Zhang, F.; Thiem, S. Reduced expression of Autographa californica nucleopolyhedrovirus ORF34, an essential gene, enhances heterologous gene expression. Virology 2013, 435, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, T.Z.; Zhang, F.; Sahly, N.; Thiem, S. Effect of Temporal Expression of Integral Membrane Proteins by Baculovirus Expression Vector System. Mol. Biotechnol. 2018, 60, 576–584. [Google Scholar] [CrossRef]
- Hundt, J. Post-translational modifications of hepatitis C viral proteins and their biological significance. World J. Gastroenterol. 2013, 19, 8929–8939. [Google Scholar] [CrossRef]
- Acosta-Rivero, N.; Alvarez-Obregón, J.C.; Musacchio, A.; Falcón, V.; Dueñas-Carrera, S.; Marante, J.; Menéndez, I.; Morales, J. In Vitro Self-Assembled HCV Core Virus-like Particles Induce A Strong Antibody Immune Response in Sheep. Biochem. Biophys. Res. Commun. 2002, 290, 300–304. [Google Scholar] [CrossRef]
- Acosta-Rivero, N.; Rodriguez, A.; Musacchio, A.; Falcón, V.; Suarez, V.M.; Martinez, G.; Guerra, I.; Paz-Lago, D.; Morera, Y.; María, C.; et al. In Vitro Assembly Into Virus-like Particles Is an Intrinsic Quality of Pichia Pastoris Derived HCV Core Protein. Biochem. Biophys. Res. Commun. 2004, 325, 68–74. [Google Scholar] [CrossRef]
- Yvon, S.; Rolland, D.; Charrier, J.P.; Jolivet, M. An Alternative For Purification of Low Soluble Recombinant Hepatitis C Virus Core Protein: Preparative Two-Dimensional Electrophoresis. Electrophoresis 1998, 19, 1300–1305. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD Peptides For Directing Cell Association With Biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [Green Version]
- Manes, T.; Zheng, D.; Tognin, S.; Woodard, A.; Marchisio, P.; Languino, L. αvβ3 integrin expression up-regulates cdc2, which modulates cell migration. J. Cell Biol. 2003, 161, 817–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayoral, R.; Fernández-Martínez, A.; Boscá, L.; Martín-Sanz, P. Prostaglandin E 2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 2005, 26, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshiba, T.; Nemoto, E.; Sato, K.; Orui, T.; Otaki, T.; Yoshihiro, A.; Tanaka, M. Regulation of the Contribution of Integrin to Cell Attachment on Poly(2-Methoxyethyl Acrylate) (PMEA) Analogous Polymers for Attachment-Based Cell Enrichment. PLoS ONE 2015, 10, e0136066. [Google Scholar]
- Hou, J.; Yan, D.; Liu, Y.; Huang, P.; Cui, H. The Roles of Integrin α5β1 in Human Cancer. Onco Targets Ther. 2020, 13, 13329–13344. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential Transformation of Human Neuronal Cells By Human Adenoviruses And The Origin of HEK 293 Cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.; Li, X.; Liu, Y.; A Haas, T. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer 2011, 11, 293. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, Y.; Xiao, J.; Liu, G.; Li, X.; Zhao, Y.; Tan, H.; Shi, H.; Cheng, D. Investigation of SP94 Peptide as a Specific Probe for Hepatocellular Carcinoma Imaging and Therapy. Sci. Rep. 2016, 6, 33511. [Google Scholar] [CrossRef]
- Chopra, M.; Sgro, A.; Norret, M.; Blancafort, P.; Iyer, K.; Evans, C. SP94-Targeted Nanoparticles Enhance the Efficacy of So-rafenib and Improve Liver Cancer Cell Discrimination. ACS Appl. Bio Mater. 2020, 4, 1023–1029. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Cheng, L.; Yuan, J.; Zhong, Z. SP94 peptide mediating highly specific and efficacious delivery of polymersomal doxorubicin hydrochloride to hepatocellular carcinoma in vivo. Colloids Surf. B Biointerfaces Colloids 2021, 197, 111399. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Casas, C. GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Front. Neurosci. 2017, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Suzuki, S.; Harkin, D.G.; Chirila, T.V. Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes. J. Funct. Biomater. 2013, 4, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, L.; Li, Z.; Fan, Y.; Li, H.; Li, Z.; Li, Y. Overexpressed GRP78 affects EMT and cell-matrix adhesion via autocrine TGF-β/Smad2/3 signaling. Int. J. Biochem. Cell Biol. 2015, 64, 202–211. [Google Scholar]




| Item | Primer | Sequence of Primer |
|---|---|---|
| 1 | SP94_linker_F | 5′ CTTTAGCATTATTCATACCCCGATTCTGCCGCTGGGAGGTGGAGGA 3′ |
| 2 | SP94_linker_EGFP_F | 5′ CGCTGGGAGGTGGAGGAGTGAGCAAGGGCGAGGAG 3′ |
| 3 | BamH1_SP94_F | 5′ GCGGATCCGCCACCATGGTGAGCTTTAGCATTATTCATACCCCGA |
| 4 | RGD_linker_F | 5′ ACCATGGTG CGTGGCGATGGAGGTGGAGGA 3′ |
| 5 | RGD_linker_EGFP_F | 5′ GCGAT GGAGGTGGAGGAGTGAGCAAGGGCGAGGAG 3′ |
| 6 | BamH1_RGD_F | 5′ GCGGATCCGCCACCATGGTGCGTGGCGAT |
| 7 | EcoR1_EGFP_R | 5′ GCGAATTCCTTGTACAGCTCGTCCATG 3′ |
| 8 | EcoR1_Core_F_ | 5′ GCGAATTC AGCACGAATCCTAAACCT 3′ |
| 9 | Core_linker_R | 5′ ATGATGTCCTCCACCTCCTCCGGAGCAACCGGGGAGATT 3′ |
| 10 | linker_xba1_R | 5′ GCTCTAGATTAATGGTGATGGTG ATGATGTCCTCCACCTCCTCC 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofed, D.; Wahba, M.A.; Salem, T.Z. Genetically Engineered Hepatitis C Virus-like Particles (HCV-LPs) Tagged with SP94 Peptide to Acquire Selectivity to Liver Cancer Cells via Grp78. Curr. Issues Mol. Biol. 2022, 44, 3746-3756. https://doi.org/10.3390/cimb44080256
Mofed D, Wahba MA, Salem TZ. Genetically Engineered Hepatitis C Virus-like Particles (HCV-LPs) Tagged with SP94 Peptide to Acquire Selectivity to Liver Cancer Cells via Grp78. Current Issues in Molecular Biology. 2022; 44(8):3746-3756. https://doi.org/10.3390/cimb44080256
Chicago/Turabian StyleMofed, Dina, Mohamed A. Wahba, and Tamer Z. Salem. 2022. "Genetically Engineered Hepatitis C Virus-like Particles (HCV-LPs) Tagged with SP94 Peptide to Acquire Selectivity to Liver Cancer Cells via Grp78" Current Issues in Molecular Biology 44, no. 8: 3746-3756. https://doi.org/10.3390/cimb44080256
APA StyleMofed, D., Wahba, M. A., & Salem, T. Z. (2022). Genetically Engineered Hepatitis C Virus-like Particles (HCV-LPs) Tagged with SP94 Peptide to Acquire Selectivity to Liver Cancer Cells via Grp78. Current Issues in Molecular Biology, 44(8), 3746-3756. https://doi.org/10.3390/cimb44080256







