(–)-Xanthatin as a Killer of Human Breast Cancer MCF-7 Mammosphere Cells: A Comparative Study with Salinomycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Cell Viability Analysis
2.4. Mammosphere Formation Assay and Chemical Treatment
2.5. Preparation of Total RNA and Real-Time Reverse Transcription-Polymerase Chain Reaction (Real-Time RT-PCR) Analysis
2.6. Statistical Analysis
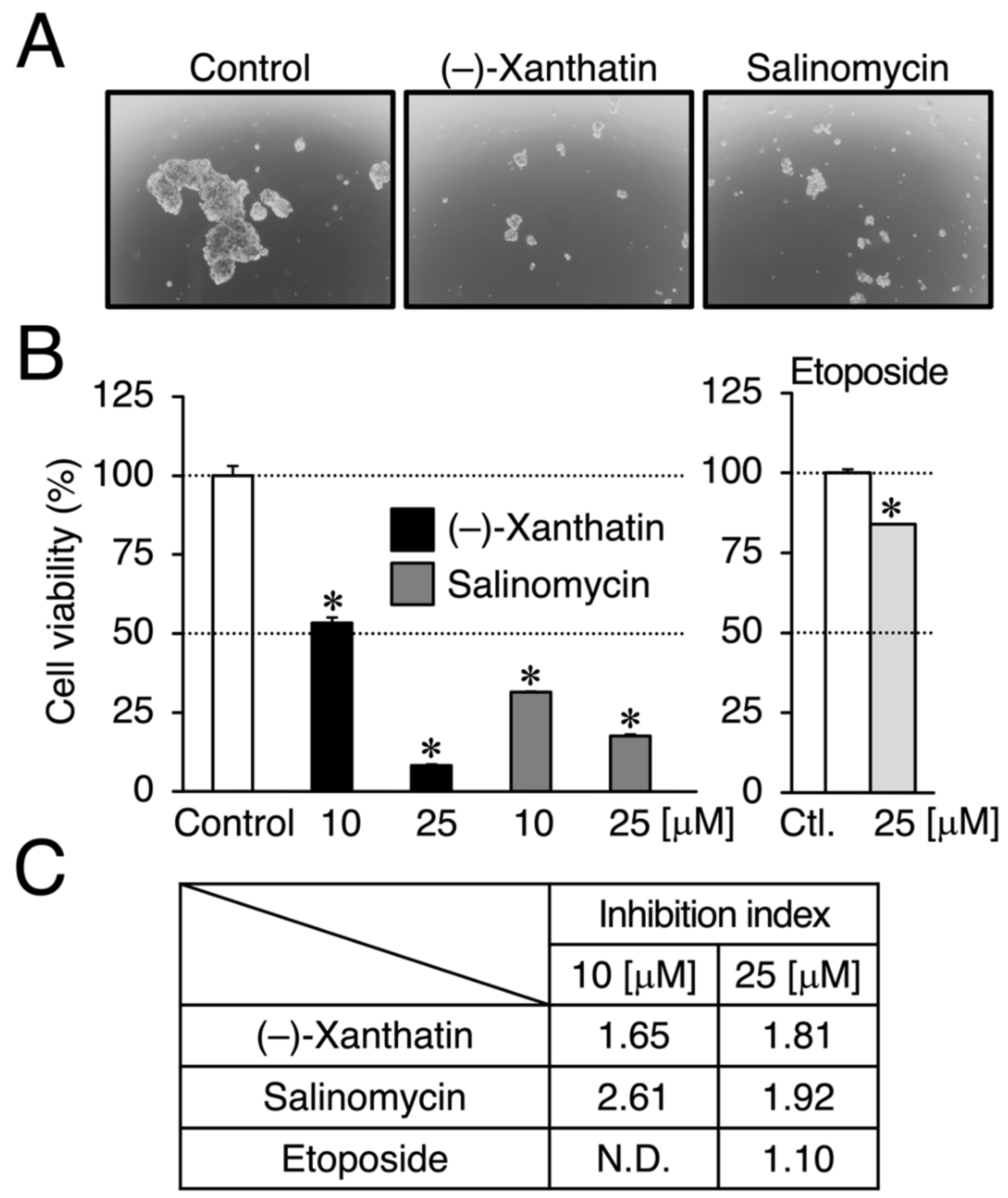
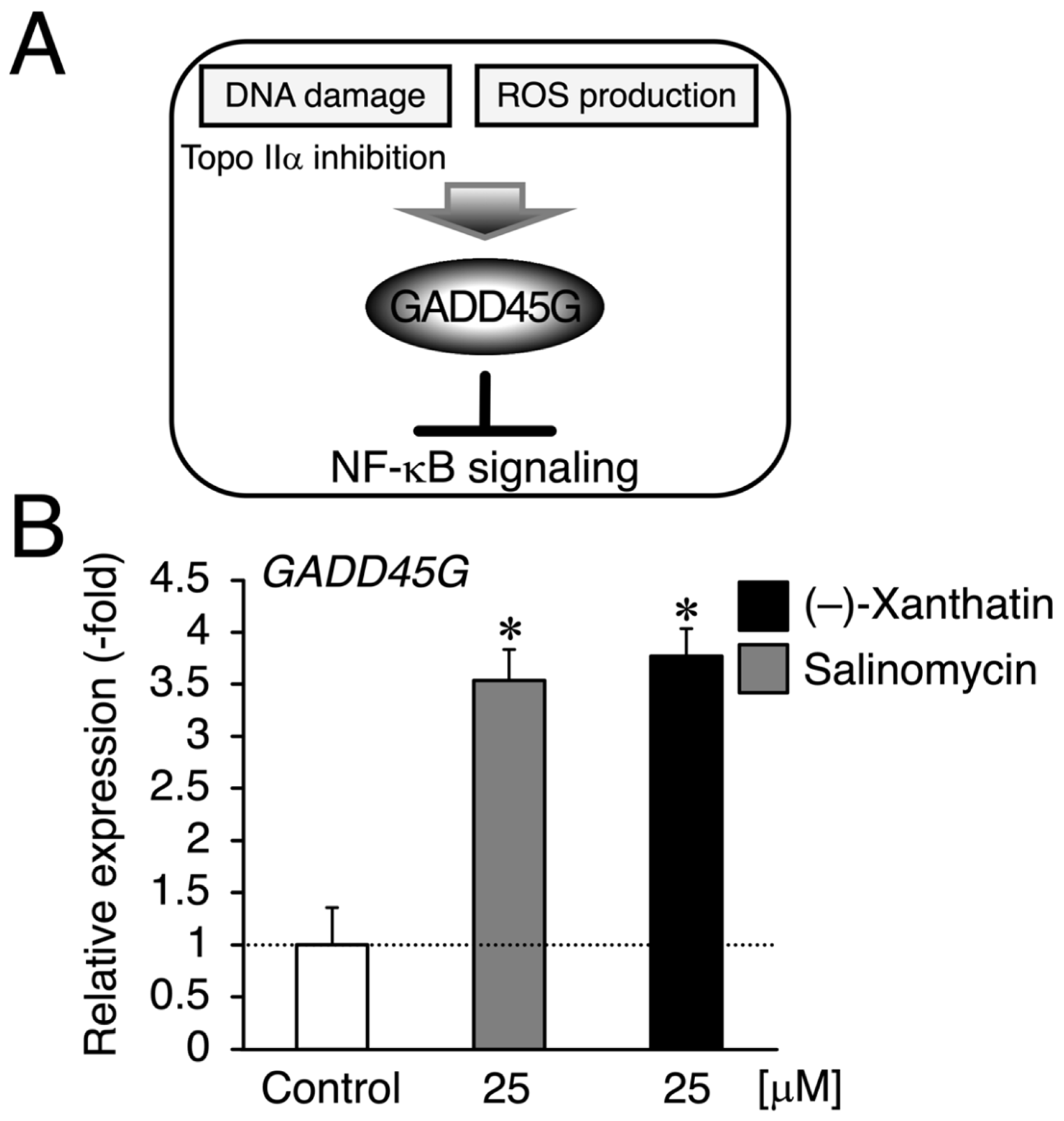
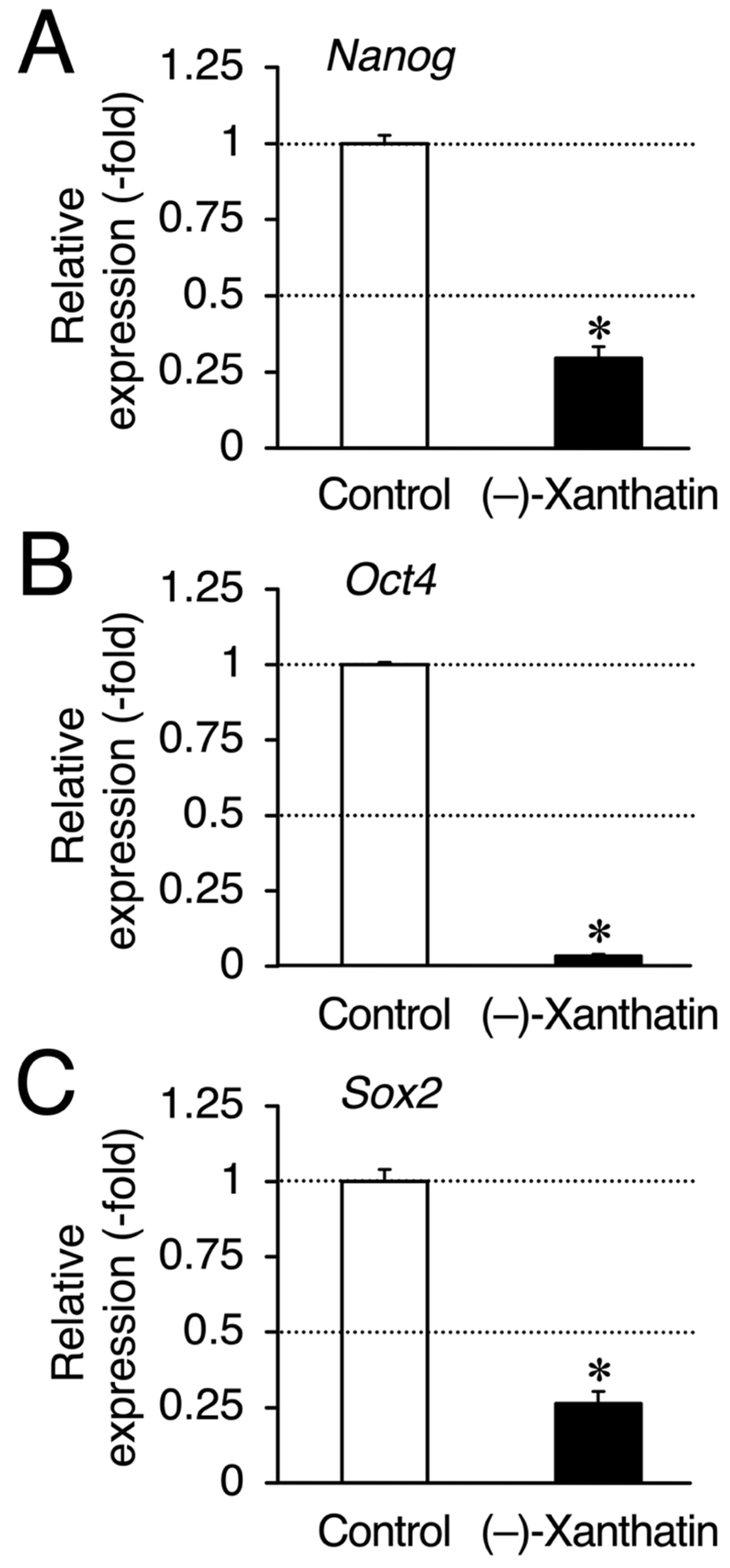
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamboj, A.; Saluja, A. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. 2010, 4, 129–139. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, C.; Chinou, I.; Vayas, C.; Harvala, C.; Verbist, J.F. Cytotoxic activity of xanthatin and the crude extracts of Xanthium strumarium. Planta Med. 1994, 60, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Erosa, I.; Huang, Y.; Hickie, R.A.; Sutherland, R.G.; Barl, B. Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can. J. Physiol. Pharmacol. 2007, 85, 1160–1172. [Google Scholar] [CrossRef]
- Takeda, S.; Matsuo, K.; Yaji, K.; Okajima-Miyazaki, S.; Harada, M.; Miyoshi, H.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Omiecinski, C.J.; et al. (–)-Xanthatin selectively induces GADD45γ and stimulates caspase-independent cell death in human breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011, 24, 855–865. [Google Scholar] [CrossRef]
- Takeda, S.; Noguchi, M.; Matsuo, K.; Yamaguchi, Y.; Kudo, T.; Nishimura, H.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Omiecinski, C.J.; et al. (–)-Xanthatin up-regulation of the GADD45γ tumor suppressor gene in MDA-MB-231 breast cancer cells: Role of topoisomerase IIα inhibition and reactive oxygen species. Toxicology 2013, 305, 1–9. [Google Scholar] [CrossRef]
- Takeda, S.; Nishimura, H.; Koyachi, K.; Matsumoto, K.; Yoshida, K.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Aramaki, H. (–)-Xanthatin induces the prolonged expression of c-Fos through an N-acetyl-L-cysteine (NAC)-sensitive mechanism in human breast cancer MDA-MB-231 cells. J. Toxicol. Sci. 2013, 38, 547–557. [Google Scholar] [CrossRef]
- Li, W.D.; Wu, Y.; Zhang, L.; Yan, L.G.; Yin, F.Z.; Ruan, J.S.; Chen, Z.P.; Yang, G.M.; Yan, C.P.; Zhao, D.; et al. Characterization of xanthatin: Anticancer properties and mechanisms of inhibited murine melanoma in vitro and in vivo. Phytomedicine 2013, 20, 865–873. [Google Scholar] [CrossRef]
- Takeda, S.; Okajima, S.; Miyoshi, H.; Koyachi, K.; Matsumoto, K.; Shindo, M.; Aramaki, H. (–)-Xanthatin-mediated marked up-regulation of RhoB, a sensor for damaged DNA. Fundam. Toxicol. Sci. 2015, 2, 233–238. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Pei, C.G.; Li, Y.Y.; Tu, P.; Gao, G.P.; Shao, Y. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 10355–10364. [Google Scholar]
- Takeda, S.; Okajima, S.; Noguchi, M.; Miyoshi, H.; Koyachi, K.; Matsumoto, K.; Shindo, M.; Aramaki, H. Possible involvement of FosB in (–)-xanthatin-mediated anti-proliferative effects in human cancer MDA-MB-231 cells. Fundam. Toxicol. Sci. 2016, 3, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Sheng, X.; Zhang, L.; Li, W.; Wei, Z.; Zhu, P.; Zhang, F.; Wang, A.; Woodgett, J.R.; Lu, Y. Xanthatin anti-tumor cytotoxicity is mediated via glycogen synthase kinase-3β and β-catenin. Biochem. Pharmacol. 2016, 115, 18–27. [Google Scholar] [CrossRef]
- Matsuo, K.; Ohtsuki, K.; Yoshikawa, T.; Shisho, K.; Yokotani-Tomita, K.; Shindo, M. Total synthesis of xanthanolides. Tetrahedron 2010, 66, 8407–8419. [Google Scholar] [CrossRef]
- Matsumoto, K.; Koyachi, K.; Shindo, M. Asymmetric total syntheses of xanthatin and 11,13-dihydroxanthatin using a stereocontrolled conjugate allylation to γ-butenolide. Tetrahedron 2013, 69, 1043–1049. [Google Scholar] [CrossRef]
- Humayun, A.; Fornace, A.J. GADD45 in stress signaling, cell cycle control, and apoptosis. Adv. Exp. Med. Biol. 2022, 1360, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ji, J.; Wang, X.; Zhang, M.; Li, X.; Zhang, Y.; Zhu, Z.; Ye, S.-D.; Wang, X. Gadd45g initiates embryonic stem cell differentiation and inhibits breast cell carcinogenesis. Cell death Discov. 2021, 7, 271. [Google Scholar] [CrossRef]
- Wesolowski, R.; Ramaswamy, B. Gene expression profiling: Changing face of breast cancer classification and management. Gene Expr. 2011, 15, 105–115. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Kennedy, D. Multidrug-resistant cancer cells and cancer stem cells hijack cellular systems to circumvent systemic therapies, can natural products reverse this? Cell. Mol. Life Sci. 2017, 74, 777–801. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer 2015, 15, 261–275. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988, Erratum in Proc. Natl. Acad. Sci. USA 2003, 100, 6890. [Google Scholar] [CrossRef]
- Manuel Iglesias, J.; Beloqui, I.; Garcia-Garcia, F.; Leis, O.; Vazquez-Martin, A.; Eguiara, A.; Cufi, S.; Pavon, A.; Menendez, J.A.; Dopazo, J.; et al. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS ONE 2013, 8, e77281. [Google Scholar] [CrossRef]
- Morrison, B.J.; Schmidt, C.W.; Lakhani, S.R.; Reynolds, B.A.; Lopez, J.A. Breast cancer stem cells: Implications for therapy of breast cancer. Breast Cancer Res. 2008, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Hirao-Suzuki, M.; Koga, T.; Sakai, G.; Kobayashi, T.; Ishii, Y.; Miyazawa, H.; Takiguchi, M.; Sugihara, N.; Toda, A.; Ohara, M.; et al. Fatty acid 2-hydroxylase (FA2H) as a stimulatory molecule responsible for breast cancer cell migration. Biochem. Biophys. Res. Commun. 2020, 531, 215–222. [Google Scholar] [CrossRef]
- Zhang, X.; Yalcin, S.; Lee, D.F.; Yeh, T.Y.; Lee, S.M.; Su, J.; Mungamuri, S.K.; Rimmelé, P.; Kennedy, M.; Sellers, R.; et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat. Cell. Biol. 2011, 13, 1092–1099. [Google Scholar] [CrossRef]
- Kurosu, T.; Fukuda, T.; Miki, T.; Miura, O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in B-cell lymphoma cells. Oncogene 2003, 22, 4459–4468. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, M.; Kim, W.K.; Kim, Y.J.; Kang, H.S.; Kim, H.S.; Yoon, S. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br. J. Pharmacol. 2011, 162, 773–784. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, K.I.; Kim, S.H.; Yu, S.N.; Lee, D.; Kim, Y.W.; Noh, K.T.; Ma, J.Y.; Seo, Y.K.; Ahn, S.C. Salinomycin induces reactive oxygen species and apoptosis in aggressive breast cancer cells as mediated with regulation of autophagy. Anticancer Res. 2017, 37, 1747–1758. [Google Scholar] [CrossRef]
- Zerbini, L.F.; Wang, Y.; Czibere, A.; Correa, R.G.; Cho, J.Y.; Ijiri, K.; Wei, W.; Joseph, M.; Gu, X.; Grall, F.; et al. NF-κB-mediated repression of growth arrest- and DNA-damage-inducible proteins 45α and γ is essential for cancer cell survival. Proc. Natl. Acad. Sci. USA 2004, 101, 13618–13623, Erratum in Proc. Natl. Acad. Sci. USA 2004, 101, 15271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tao, L.; Ruan, J.; Li, W.; Wu, Y.; Yan, L.; Zhang, F.; Fan, F.; Zheng, S.; Wang, A.; et al. Xanthatin induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma MKN-45 cells. Planta Med. 2012, 78, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, L.F.; Libermann, T.A. Life and death in cancer. GADD45 and are critical regulators of NF-κB mediated escape from programmed cell death. Cell Cycle 2005, 4, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Luo, J.L.; Karin, M. IκB kinase α kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15852–15857. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sakamaki, T.; Casimiro, M.C.; Willmarth, N.E.; Quong, A.A.; Ju, X.; Ojeifo, J.; Jiao, X.; Yeow, W.S.; Katiyar, S.; et al. The canonical NF-κB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res. 2010, 70, 10464–10473. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yaji, K.; Matsumoto, K.; Amamoto, T.; Shindo, M.; Aramaki, H. Xanthocidin derivatives as topoisomerase IIα enzymatic inhibitors. Biol. Pharm. Bull. 2014, 37, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, S.; Hirao-Suzuki, M.; Shindo, M.; Aramaki, H. (–)-Xanthatin as a Killer of Human Breast Cancer MCF-7 Mammosphere Cells: A Comparative Study with Salinomycin. Curr. Issues Mol. Biol. 2022, 44, 3849-3858. https://doi.org/10.3390/cimb44090264
Takeda S, Hirao-Suzuki M, Shindo M, Aramaki H. (–)-Xanthatin as a Killer of Human Breast Cancer MCF-7 Mammosphere Cells: A Comparative Study with Salinomycin. Current Issues in Molecular Biology. 2022; 44(9):3849-3858. https://doi.org/10.3390/cimb44090264
Chicago/Turabian StyleTakeda, Shuso, Masayo Hirao-Suzuki, Mitsuru Shindo, and Hironori Aramaki. 2022. "(–)-Xanthatin as a Killer of Human Breast Cancer MCF-7 Mammosphere Cells: A Comparative Study with Salinomycin" Current Issues in Molecular Biology 44, no. 9: 3849-3858. https://doi.org/10.3390/cimb44090264
APA StyleTakeda, S., Hirao-Suzuki, M., Shindo, M., & Aramaki, H. (2022). (–)-Xanthatin as a Killer of Human Breast Cancer MCF-7 Mammosphere Cells: A Comparative Study with Salinomycin. Current Issues in Molecular Biology, 44(9), 3849-3858. https://doi.org/10.3390/cimb44090264





