1,2-13C2-Glucose Tracing Approach to Assess Metabolic Alterations of Human Monocytes under Neuroinflammatory Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Human Monocyte Isolation and Incubation
2.2. Metabolite Extraction and Sample Preparation
2.2.1. Culture Medium
2.2.2. Cell Extract
2.3. HPLC-MS/MS Setup and Analysis
2.4. Data Analysis
3. Results
3.1. Increased Glucose Conversion in Monocytes after Exposure to CSF
3.2. Differential Concentration of Secreted Glucose-Derived Metabolites
3.3. Endogenous Glucose-Derived Metabolites in CSF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B. Neuroinflammation: No rose by any other name. Brain Pathol. 2014, 24, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.C.; Boutin, H.; Allan, S.M. Interleukin-1 in the brain: Mechanisms of action in acute neurodegeneration. Ann. N. Y. Acad. Sci. 2003, 992, 39–47. [Google Scholar] [CrossRef]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Cribbs, D.H.; Anderson, A.J.; Cummings, B.J.; Su, J.H.; Wasserman, A.J.; Cotman, C.W. The induction of the TNFalpha death domain signaling pathway in Alzheimer’s disease brain. Neurochem. Res. 2003, 28, 307–318. [Google Scholar] [CrossRef]
- Kouwenhoven, M.; Teleshova, N.; Ozenci, V.; Press, R.; Link, H. Monocytes in multiple sclerosis: Phenotype and cytokine profile. J. Neuroimmunol. 2001, 112, 197–205. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Stolp, H.B.; Dziegielewska, K.M. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2009, 35, 132–146. [Google Scholar] [CrossRef]
- De Vries, H.E.; Kooij, G.; Frenkel, D.; Georgopoulos, S.; Monsonego, A.; Janigro, D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: Implications for clinical disease. Epilepsia 2012, 53 (Suppl. 6), 45–52. [Google Scholar] [CrossRef] [Green Version]
- Paterka, M.; Siffrin, V.; Voss, J.O.; Werr, J.; Hoppmann, N.; Gollan, R.; Belikan, P.; Bruttger, J.; Birkenstock, J.; Jung, S.; et al. Gatekeeper role of brain antigen-presenting CD11c+ cells in neuroinflammation. EMBO J. 2016, 35, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Siffrin, V.; Brandt, A.U.; Herz, J.; Zipp, F. New Insights into Adaptive Immunity in Chronic Neuroinflammation. Adv. Immunol. 2007, 96, 1–40. [Google Scholar]
- Jolivel, V.; Luessi, F.; Masri, J.; Kraus, S.H.P.; Hubo, M.; Poisa-Beiro, L.; Klebow, S.; Paterka, M.; Yogev, N.; Tumani, H.; et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 2013, 136, 1048–1066. [Google Scholar] [CrossRef] [Green Version]
- Luessi, F.; Siffrin, V.; Zipp, F. Neurodegeneration in multiple sclerosis: Novel treatment strategies. Expert Rev. Neurother. 2012, 12, 1061–1077. [Google Scholar] [CrossRef] [Green Version]
- Siffrin, V.; Vogt, J.; Radbruch, H.; Nitsch, R.; Zipp, F. Multiple sclerosis-candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010, 33, 202–210. [Google Scholar] [CrossRef]
- Palmer, C.S.; Cherry, C.L.; Sada-Ovalle, I.; Singh, A.; Crowe, S.M. Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis. EBioMedicine 2016, 6, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Torretta, S.; Scagliola, A.; Ricci, L.; Mainini, F.; Di Marco, S.; Cuccovillo, I.; Kajaste-Rudnitski, A.; Sumpton, D.; Ryan, K.M.; Cardaci, S. D-mannose suppresses macrophage IL-1beta production. Nat. Commun. 2020, 11, 6343. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [Green Version]
- Fernandez Zapata, C.; Giacomello, G.; Spruth, E.J.; Middeldorp, J.; Gallaccio, G.; Dehlinger, A.; Dames, C.; Leman, J.K.H.; van Dijk, R.E.; Meisel, A.; et al. Differential compartmentalization of myeloid cell phenotypes and responses towards the CNS in Alzheimer’s disease. Nat. Commun. 2022, 13, 7210. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Baruch, K. The resolution of neuroinflammation in neurodegeneration: Leukocyte recruitment via the choroid plexus. EMBO J. 2014, 33, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Varvel, N.H.; Neher, J.J.; Bosch, A.; Wang, W.; Ransohoff, R.M.; Miller, R.J.; Dingledine, R. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc. Natl. Acad. Sci. USA 2016, 113, E5665–E5674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Hisamatsu, T.; Chiba, S.; Mori, K.; Kitazume, M.T.; Shimamura, K.; Nakamoto, N.; Matsuoka, K.; Ebinuma, H.; Naganuma, M.; et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol. Lett. 2016, 176, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Bottcher, C.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Paza, E.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; Kahn, R.S.; et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef]
- Zia, S.; Rawji, K.S.; Michaels, N.J.; Burr, M.; Kerr, B.J.; Healy, L.M.; Plemel, J.R. Microglia Diversity in Health and Multiple Sclerosis. Front. Immunol. 2020, 11, 588021. [Google Scholar] [CrossRef]
- Mathias, A.; Perriard, G.; Canales, M.; Soneson, C.; Delorenzi, M.; Schluep, M.; Du Pasquier, R.A. Increased ex vivo antigen presentation profile of B cells in multiple sclerosis. Mult. Scler. 2017, 23, 802–809. [Google Scholar] [CrossRef]
- Schulz, D.; Severin, Y.; Zanotelli, V.R.T.; Bodenmiller, B. In-Depth Characterization of Monocyte-Derived Macrophages using a Mass Cytometry-Based Phagocytosis Assay. Sci. Rep. 2019, 9, 1925. [Google Scholar] [CrossRef] [Green Version]
- Dreschers, S.; Ohl, K.; Lehrke, M.; Mollmann, J.; Denecke, B.; Costa, I.; Vogl, T.; Viemann, D.; Roth, J.; Orlikowsky, T.; et al. Impaired cellular energy metabolism in cord blood macrophages contributes to abortive response toward inflammatory threats. Nat. Commun. 2019, 10, 1685. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, Y.; Sonntag, G.V.H.; Braun, L.C.; Lagache, S.M.M.; Liebmann, M.; Klotz, L.; Godfrey, R.; Kahles, F.; Waltenberger, J.; Findeisen, H.M. LXR Activation Induces a Proinflammatory Trained Innate Immunity-Phenotype in Human Monocytes. Front. Immunol. 2020, 11, 353. [Google Scholar] [CrossRef]
- Pence, B.D.; Yarbro, J.R. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp. Gerontol. 2018, 108, 112–117. [Google Scholar] [CrossRef]
- McGarry, T.; Hanlon, M.M.; Marzaioli, V.; Cunningham, C.C.; Krishna, V.; Murray, K.; Hurson, C.; Gallagher, P.; Nagpal, S.; Veale, D.J.; et al. Rheumatoid arthritis CD14(+) monocytes display metabolic and inflammatory dysfunction, a phenotype that precedes clinical manifestation of disease. Clin. Transl. Immunol. 2021, 10, e1237. [Google Scholar] [CrossRef]
- Ong, S.M.; Hadadi, E.; Dang, T.M.; Yeap, W.H.; Tan, C.T.; Ng, T.P.; Larbi, A.; Wong, S.C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Michlmayr, D.; Kim, E.Y.; Rahman, A.H.; Raghunathan, R.; Kim-Schulze, S.; Che, Y.; Kalayci, S.; Gumus, Z.H.; Kuan, G.; Balmaseda, A.; et al. Comprehensive Immunoprofiling of Pediatric Zika Reveals Key Role for Monocytes in the Acute Phase and No Effect of Prior Dengue Virus Infection. Cell Rep. 2020, 31, 107569. [Google Scholar] [CrossRef]
- Mason, G.F.; Gruetter, R.; Rothman, D.L.; Behar, K.L.; Shulman, R.G.; Novotny, E.J. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cereb. Blood Flow Metab. 1995, 15, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Mason, G.F.; Falk Petersen, K.; de Graaf, R.A.; Kanamatsu, T.; Otsuki, T.; Shulman, G.I.; Rothman, D.L. A comparison of (13)C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-(13)C]glucose. Brain Res. Brain Res. Protoc. 2003, 10, 181–190. [Google Scholar] [CrossRef]
- Fuchs, A.L.; Schiller, S.M.; Keegan, W.J.; Ammons, M.C.B.; Eilers, B.; Tripet, B.; Copie, V. Quantitative (1)H NMR Metabolomics Reveal Distinct Metabolic Adaptations in Human Macrophages Following Differential Activation. Metabolites 2019, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Noga, M.J.; Dane, A.; Shi, S.; Attali, A.; van Aken, H.; Suidgeest, E.; Tuinstra, T.; Muilwijk, B.; Coulier, L.; Luider, T.; et al. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics 2012, 8, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Ahn, W.S.; Antoniewicz, M.R. Parallel labeling experiments with [1,2-(13)C]glucose and [U-(13)C]glutamine provide new insights into CHO cell metabolism. Metab. Eng. 2013, 15, 34–47. [Google Scholar] [CrossRef]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS Method for the Simultaneous Detection of Tricarboxylic Acid Cycle Intermediates in a Range of Biological Matrices. J. Anal. Methods Chem. 2017, 2017, 5391832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Meyers, A.; Long, D.; Ingram, B.; Liu, T.; Yoza, B.K.; Vachharajani, V.; McCall, C.E. Frontline Science: Monocytes sequentially rewire metabolism and bioenergetics during an acute inflammatory response. J. Leukoc. Biol. 2019, 105, 215–228. [Google Scholar] [CrossRef]
- Agilent Technologies. How Agilent Seahorse XF Analyzers Work. Available online: https://www.agilent.com/en/products/cell-analysis/how-seahorse-xf-analyzers-work (accessed on 8 January 2022).
- Giacomello, G.; Boettcher, C.; Parr, M.K. Isotopic tracing of glucose-metabolites in human monocytes to assess changes in inflammatory conditions. STAR Protoc. 2022, 3, 101715. [Google Scholar] [CrossRef] [PubMed]
- Spalding, J.L.; Naser, F.J.; Mahieu, N.G.; Johnson, S.L.; Patti, G.J. Trace Phosphate Improves ZIC-pHILIC Peak Shape, Sensitivity, and Coverage for Untargeted Metabolomics. J. Proteome Res. 2018, 17, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sadilek, M.; Lidstrom, M.E. Streamlined pentafluorophenylpropyl column liquid chromatography–tandem quadrupole mass spectrometry and global 13C-labeled internal standards improve performance for quantitative metabolomics in bacteria. J. Chromatogr. A 2010, 1217, 7401–7410. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, J.J.; Van de Bittner, G.C.; Kennedy, A.P.; Wei, T.C. The Use of HILIC Zwitterionic Phase Superficially Porous Particles for Metabolomics Analysis. Lc Gc N. Am. 2018, 36, 30–35. [Google Scholar]
- Regenold, W.T.; Phatak, P.; Makley, M.J.; Stone, R.D.; Kling, M.A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 2008, 275, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Omerhoca, S.; Akkas, S.Y.; Icen, N.K. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Noro Psikiyatr Ars 2018, 55, S1–S9. [Google Scholar] [CrossRef]
- Dumurgier, J.; Paquet, C.; Peoc’h, K.; Lapalus, P.; Mouton-Liger, F.; Benisty, S.; Chasseigneaux, S.; Chabriat, H.; Hugon, J. CSF Abeta(1)(-)(4)(2) levels and glucose metabolism in Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 845–851. [Google Scholar] [CrossRef]
- Redjems-Bennani, N.; Jeandel, C.; Lefebvre, E.; Blain, H.; Vidailhet, M.; Gueant, J.L. Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology 1998, 44, 300–304. [Google Scholar] [CrossRef]
- Diskin, C.; Palsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.; Cuevas, V.D.; Fernandez-Arroyo, S.; Riera-Borrull, M.; Orta-Zavalza, E.; Joven, J.; Rial, E.; Corbi, A.L.; Escribese, M.M. Reshaping of Human Macrophage Polarization through Modulation of Glucose Catabolic Pathways. J. Immunol. 2015, 195, 2442–2451. [Google Scholar] [CrossRef]
- Raulien, N.; Friedrich, K.; Strobel, S.; Rubner, S.; Baumann, S.; von Bergen, M.; Korner, A.; Krueger, M.; Rossol, M.; Wagner, U. Fatty Acid Oxidation Compensates for Lipopolysaccharide-Induced Warburg Effect in Glucose-Deprived Monocytes. Front. Immunol. 2017, 8, 609. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.S.; Al-Sharea, A.; Shihata, W.A.; Bertuzzo Veiga, C.; Cooney, O.D.; Fleetwood, A.J.; Flynn, M.C.; Claeson, E.; Palmer, C.S.; Lancaster, G.I.; et al. Glycolysis Is Required for LPS-Induced Activation and Adhesion of Human CD14(+)CD16(-) Monocytes. Front. Immunol. 2019, 10, 2054. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Andres, J.; Arts, R.J.W.; Ter Horst, R.; Gresnigt, M.S.; Smeekens, S.P.; Ratter, J.M.; Lachmandas, E.; Boutens, L.; van de Veerdonk, F.L.; Joosten, L.A.B.; et al. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog. 2017, 13, e1006632. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Spiljar, M.; Kuchroo, V.K. Metabolic regulation and function of T helper cells in neuroinflammation. Semin. Immunopathol. 2022, 44, 581–598. [Google Scholar] [CrossRef]
- Stienstra, R.; Netea-Maier, R.T.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017, 26, 142–156. [Google Scholar] [CrossRef]
- Nishizawa, T.; Kanter, J.E.; Kramer, F.; Barnhart, S.; Shen, X.; Vivekanandan-Giri, A.; Wall, V.Z.; Kowitz, J.; Devaraj, S.; O’Brien, K.D.; et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014, 7, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Westergaard, N.; Waagepetersen, H.S.; Belhage, B.; Schousboe, A. Citrate, a Ubiquitous Key Metabolite with Regulatory Function in the CNS. Neurochem. Res. 2017, 42, 1583–1588. [Google Scholar] [CrossRef]
- Mellerup, E.T.; Rafaelsen, O.J. Depression and cerebrospinal fluid citrate. Acta Psychiatr. Scand. 1981, 63, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Convertini, P.; Cucci, L.; Panaro, M.A.; Di Noia, M.A.; Calvello, R.; Palmieri, F.; Iacobazzi, V. The mitochondrial citrate carrier: A new player in inflammation. Biochem. J. 2011, 438, 433–436. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. A critical role for citrate metabolism in LPS signalling. Biochem. J. 2011, 438, e5–e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Glutamate, T cells and multiple sclerosis. J. Neural Transm. 2017, 124, 775–798. [Google Scholar] [CrossRef]
- Kuzmina, U.S.; Zainullina, L.F.; Vakhitov, V.A.; Bakhtiyarova, K.Z.; Vakhitova, Y.V. The Role of Glutamate in the Pathogenesis of Multiple Sclerosis. Neurosci. Behav. Physiol. 2020, 50, 669–675. [Google Scholar] [CrossRef]
- Muhlert, N.; Atzori, M.; De Vita, E.; Thomas, D.L.; Samson, R.S.; Wheeler-Kingshott, C.A.; Geurts, J.J.; Miller, D.H.; Thompson, A.J.; Ciccarelli, O. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry 2014, 85, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Lima, M.M.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Spittler, A.; Reissner, C.M.; Oehler, R.; Gornikiewicz, A.; Gruenberger, T.; Manhart, N.; Brodowicz, T.; Mittlboeck, M.; Boltz-Nitulescu, G.; Roth, E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999, 13, 563–571. [Google Scholar] [CrossRef]
- Zhang, J.; Ahn, W.S.; Gameiro, P.A.; Keibler, M.A.; Zhang, Z.; Stephanopoulos, G. 13C isotope-assisted methods for quantifying glutamine metabolism in cancer cells. Methods Enzymol. 2014, 542, 369–389. [Google Scholar] [CrossRef] [Green Version]
- Antoniewicz, M.R. A guide to (13)C metabolic flux analysis for the cancer biologist. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef]
- Wolfe, H.; Mela, V.; Minogue, A.M.; Miller, A.M.; McGuigan, C.; Williams, L.; Lohan, D.; Lawlor, B.A.; Lynch, M.A. Monocytes exposed to plasma from patients with Alzheimer’s disease undergo metabolic reprogramming. Neurosci. Res. 2019, 148, 54–60. [Google Scholar] [CrossRef]


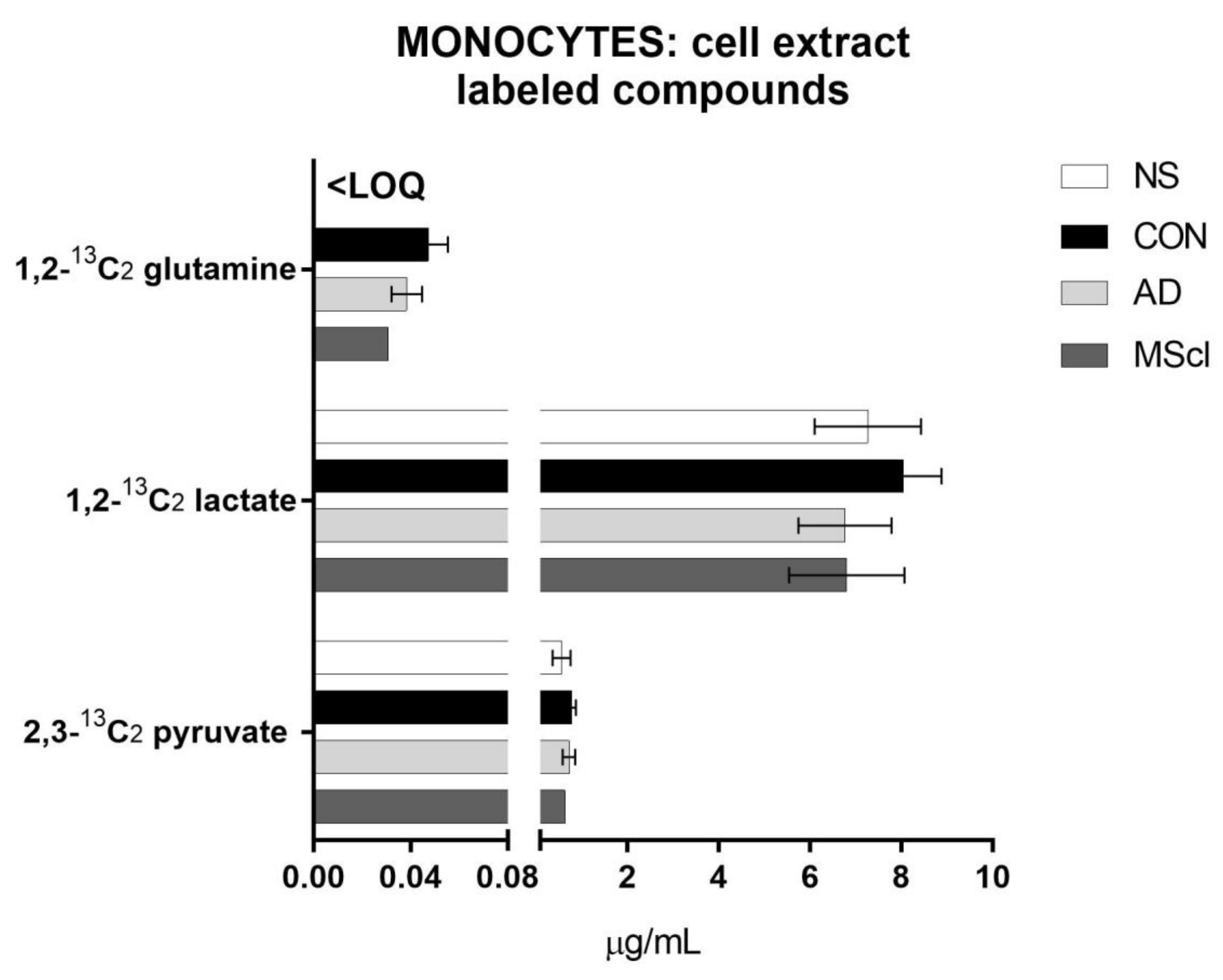

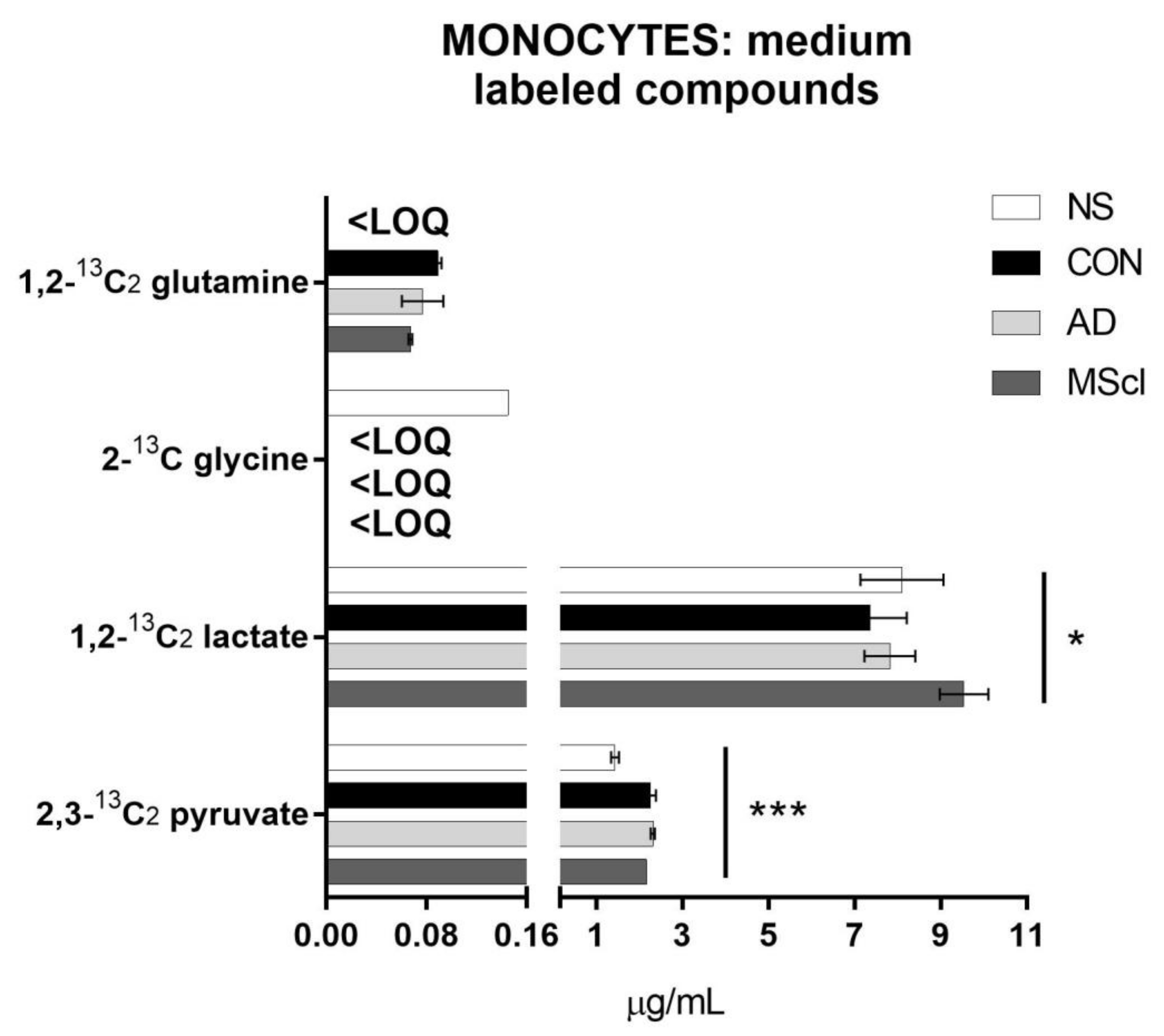


| Analyte | Cell Lysate | Incubation Medium | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | NS vs. CON | NS vs. AD | NS vs. MScl | CON vs. AD | CON vs. MScl | AD vs. MScl | p-value | NS vs. CON | NS vs. AD | NS vs. MScl | CON vs. AD | CON vs. MScl | AD vs. MScl | |
| Pyruvate | 0.0387 | # | 6.9 × 10−5 | # | # | # | ||||||||
| 2,3-13C2 pyruvate | 0.3611 | 7.9 × 10−5 | # | # | # | |||||||||
| Lactate | 0.4955 | 0.0125 | # | # | ||||||||||
| 1,2-13C2 lactate | 0.3963 | 0.0318 | # | |||||||||||
| Glycine | 0.0166 | * | * | 1.3 × 10−6 | * | * | * | |||||||
| 1,2-13C2 glutamine | 0.2199 | 0.0665 | ||||||||||||
| Glutamine | 0.0004 | # | # | # | * | 9.0 × 10−7 | # | # | # | * | * | |||
| Serine | 0.0356 | * | * | 1.5 × 10−10 | * | * | * | * | # | |||||
| Glutamic acid | 0.7333 | 4.6 × 10−5 | * | * | * | * | ||||||||
| Citric acid | 0.0073 | # | # | 3.2 × 10−6 | # | # | # | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomello, G.; Otto, C.; Priller, J.; Ruprecht, K.; Böttcher, C.; Parr, M.K. 1,2-13C2-Glucose Tracing Approach to Assess Metabolic Alterations of Human Monocytes under Neuroinflammatory Conditions. Curr. Issues Mol. Biol. 2023, 45, 765-781. https://doi.org/10.3390/cimb45010051
Giacomello G, Otto C, Priller J, Ruprecht K, Böttcher C, Parr MK. 1,2-13C2-Glucose Tracing Approach to Assess Metabolic Alterations of Human Monocytes under Neuroinflammatory Conditions. Current Issues in Molecular Biology. 2023; 45(1):765-781. https://doi.org/10.3390/cimb45010051
Chicago/Turabian StyleGiacomello, Ginevra, Carolin Otto, Josef Priller, Klemens Ruprecht, Chotima Böttcher, and Maria Kristina Parr. 2023. "1,2-13C2-Glucose Tracing Approach to Assess Metabolic Alterations of Human Monocytes under Neuroinflammatory Conditions" Current Issues in Molecular Biology 45, no. 1: 765-781. https://doi.org/10.3390/cimb45010051
APA StyleGiacomello, G., Otto, C., Priller, J., Ruprecht, K., Böttcher, C., & Parr, M. K. (2023). 1,2-13C2-Glucose Tracing Approach to Assess Metabolic Alterations of Human Monocytes under Neuroinflammatory Conditions. Current Issues in Molecular Biology, 45(1), 765-781. https://doi.org/10.3390/cimb45010051






