Genome-Wide Identification and Characterization of the AlkB Gene Family in Sweet Orange (Citrus sinensis)
Abstract
:1. Introduction
2. Results
2.1. Identification of ALKBH Gene Family Members in C. sinensis
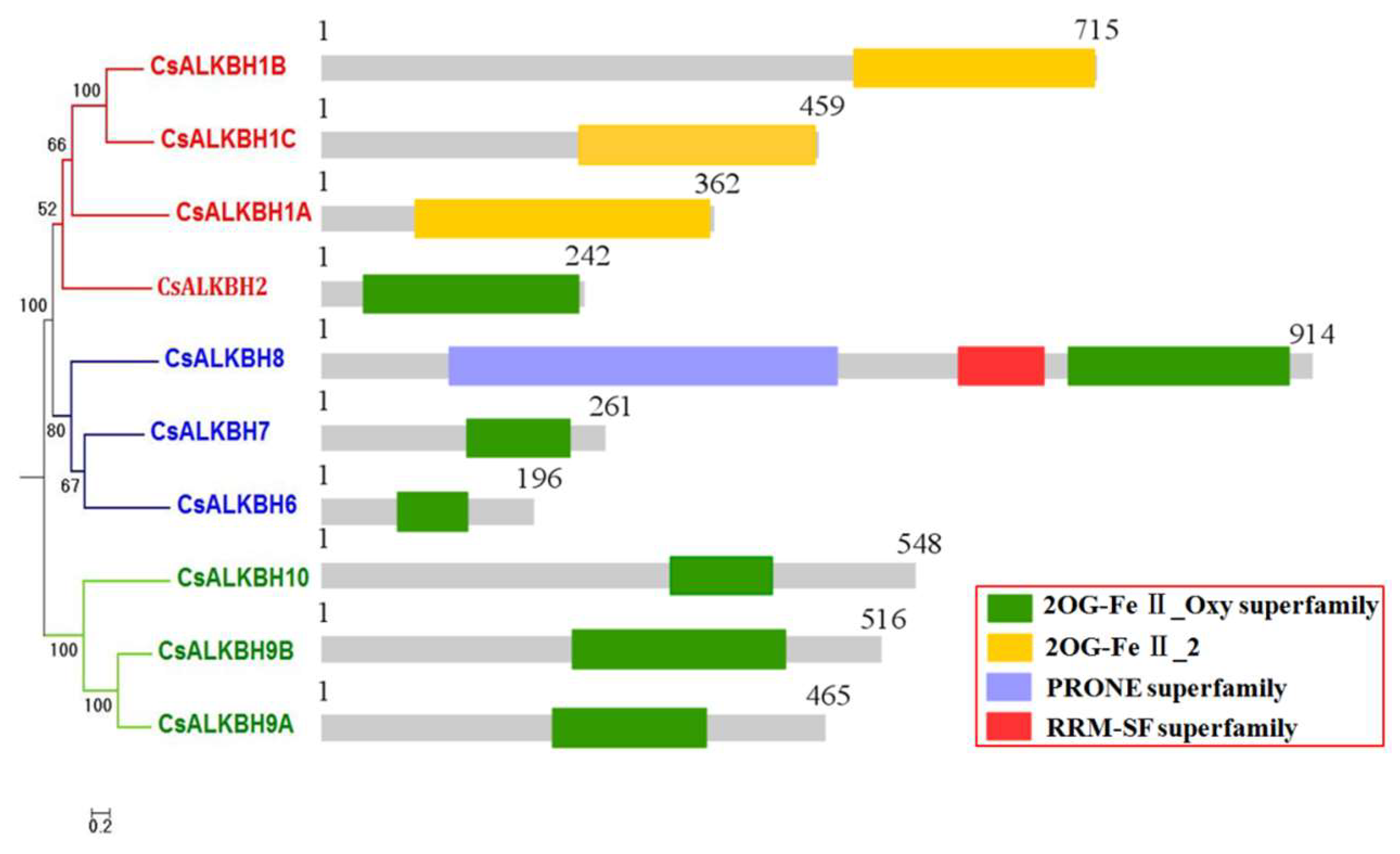
2.2. Phylogenetic Analysis and Classification of CsALKBHs
2.3. The Chromosomal Location of CsALKBHs
2.4. Gene Structural and Conserved Motif Analysis of CsALKBHs
2.5. Cis-Element Analysis of the CsALKBH Promoter in Citrus
2.6. Synteny Analysis of CsALKBHs
2.7. Tissue-Specific Expression Profiles of CsALKBH Genes
2.8. Change in Relative Expression of the CsALKBH Gene under Biotic Stress
3. Discussion
4. Materials and Methods
4.1. Identification of ALKBH Gene Family in C. sinensis
4.2. Alignment and Phylogenetic Analysis
4.3. Gene Structure, Conserved Motif, and Cis-Element Analysis
4.4. Chromosomal Location and Syntenic Analysis
4.5. Expression Patterns Analysis of ALKBH Genes by RNA-Seq Data
4.6. Plant Materials and Quantitative Real-Time PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcinkowski, M.; Pilzys, T.; Garbicz, D.; Steciuk, J.; Zugaj, D.; Mielecki, D.; Sarnowski, T.J.; Grzesiuk, E. Human and arabidopsis alpha-ketoglutarate-dependent dioxygenase homolog proteins—New players in important regulatory processes. IUBMB Life 2020, 72, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Manduzio, S.; Kang, H. Epitranscriptomic RNA methylation in plant development and abiotic stress responses. Front. Plant Sci. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arribas-Hernández, L.; Brodersen, P. Occurrence and functions of m6A and other covalent modifications in plant mRNA. Plant Physiol. 2020, 182, 79–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Riaz, A.; Chachar, S.; Ding, Y.; Du, H.; Gu, X. Epigenetic modifications of mRNA and DNA in plants. Mol. Plant 2020, 13, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wei, L.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.; Chen, P.R.; He, C.; Jia, G.F. ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Pérez, M.; Aparicio, F.; López-Gresa, M.P.; Bellés, J.M.; Sánchez-Navarro, J.A.; Pallás, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef] [Green Version]
- Kawarada, L.; Suzuki, T.; Ohira, T.; Hirata, S.; Miyauchi, K.; Suzuki, T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017, 45, 7401–7415. [Google Scholar] [CrossRef] [Green Version]
- Huong, T.T.; Ngoc, L.N.T.; Hunseung, K. Functional characterization of a putative RNA demethylase ALKBH6 in Arabidopsis growth and abiotic stress response. Int. J. Mol. Sci. 2020, 21, 6707. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Cao, S.; Tian, Y.; Han, K.; Sun, Y.; Li, J.; Yang, Q.; Ji, Q.; Sederoff, R.; et al. Genome-wide identification of the AlkB homologs gene family, PagALKBH9B and PagALKBH10B regulated salt stress response in Populus. Front. Plant Sci. 2022, 3491. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, S.; Qin, G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SIDML2 in tomato fruit repening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Li, J.; Cheng, D.; Dai, C. Genome-wide sequence identification and expression analysis of N6-methyladenosine demethylase in sugar beet (Beta vularis L.) under salt stress. PeerJ 2022, 10, e12719. [Google Scholar] [CrossRef] [PubMed]
- Mielecki, D.; Zugaj, D.; Muszewska, A.; Piwowarski, J.; Chojnacka, A.; Mielecki, M.; Nieminuszczy, J.; Grynberg, M.; Grzesiuk, E. Novel AlkB dioxygenases alternative models for in silico and in vivo studies. PLoS ONE 2012, 7, e30588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Luo, B.; Wang, Y.; Li, J.; Hu, Z.; Xie, Q.; Wu, T.; Chen, G. Genome-wide identification, classification and expression analysis of m6A gene family in Solanum lycopersicum. Int. J. Mol. Sci. 2022, 23, 4522. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Waadt, R.; Schroeder, J.I. Release of GTP exchage factor mediated down-regulation of abscisic acid signal transduction through ABA-induced rapid degradation of PopGEFs. PLoS Biol. 2016, 14, e1002461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierzek, E. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyadenosines. Nucleic Acids Res. 2003, 31, 4472–4480. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, Y.; Jia, G. The detection and functions of RNA modification m6A based on m6A writers and erasers. J. Biol. Chem. 2021, 297, 100973. [Google Scholar] [CrossRef]
- Yue, J.; Wei, Y.; Sun, Z.; Chen, Y.; Wei, X.; Wang, H.; Pasin, F.; Zhao, M. AlkB RNA demethylase homologues and N6-methyladenosine are involved in Potyvirus infection. Mol. Plant Pathol. 2022, 23, 1555–1564. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liu, S.; Huang, Y.; Guo, Y.; Xie, W.; Liu, H.; Muhammad, T.Q.; Xu, Q.; Chen, L. Citrus Pan-genome to breeding database (CPBD): A comprehensive genome database for citrus breeding. Mol. Plant 2022, 15, 1503–1505. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.D.C.E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, 597–603. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA 7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Lescon, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]








| Genome ID | Gene Name | Protein Length (aa) | Molecular Weight (KD) | Isoelectric Point (PI) | Instability Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| Cs7g10770 | CsALKBH1A | 361 | 40.83 | 6.12 | 55.08 | −0.433 | Nucleus |
| Cs1g16870 | CsALKBH1B | 714 | 78.26 | 6.54 | 44.41 | −0.604 | Nucleus |
| Cs6g09650 | CsALKBH1C | 458 | 51.33 | 9.11 | 53.01 | −0.625 | Chloroplast |
| Cs2g01630 | CsALKBH2 | 241 | 28.22 | 9.53 | 31.97 | −0.754 | Nucleus |
| Cs6g01500 | CsALKBH6 | 195 | 22.27 | 6.05 | 54.00 | −0.172 | Nucleus |
| Cs5g32950 | CsALKBH7 | 260 | 29.61 | 4.46 | 50.05 | −0.510 | Nucleus |
| orange1.1t03596 | CsALKBH8 | 913 | 102.33 | 6.25 | 55.40 | −0.399 | Nucleus |
| Cs3g04140 | CsALKBH9A | 464 | 51.92 | 8.52 | 52.44 | −0.542 | Nucleus |
| Cs1g25260 | CsALKBH9B | 515 | 57.87 | 6.46 | 47.36 | −0.618 | Nucleus |
| Cs5g31840 | CsALKBH10 | 547 | 59.93 | 5.44 | 54.43 | −0.340 | Nucleus |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| CsALKBH1A | TTCATACAATCAGAACGGTCA | CTTCCATACTTAACGCACCT |
| CsALKBH1B | AATATGAAACACCCCGAGT | TAAAACAATCATGGGCGAGA |
| CsALKBH1C | GCCGTCTGTTATTCCTTGTGA | AAGTATGTCTTCCACGTTGCT |
| CsALKBH2 | TTAATTTACAGTGGCTACAGG | TTAAGCCAAATAGAAGAACTGA |
| CsALKBH6 | CTTACAATGATTACGCGAAG | CATTATGCCTTGGTTAGGTT |
| CsALKBH7 | ATAGATAACCCACATGCGGTA | CTGTTTGCGATTTATCTCGT |
| CsALKBH8 | ATGGCTTCCGAATTCTACACCAG | TGCGAAATGTGAAAGATACCCT |
| CsALKBH9A | TTCCTTCTGATGATACCGAA | ATCCTGCTCTAGTGAACCTG |
| CsALKBH9B | TTTTCAAATCTGATGGCCTA | CACCCTCGACTTATCCGTA |
| CsALKBH10 | TTCAGTGGCAACTAATACCAGA | TCACAAACAAGACTACGTCCA |
| F-box | TTGGAAACTCTTTCGCCACT | CAGCAACAAAATACCCGTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.; Wang, Y.; Gu, P.; Yang, Z.; Han, J.; Yi, L. Genome-Wide Identification and Characterization of the AlkB Gene Family in Sweet Orange (Citrus sinensis). Curr. Issues Mol. Biol. 2023, 45, 122-133. https://doi.org/10.3390/cimb45010009
Huang A, Wang Y, Gu P, Yang Z, Han J, Yi L. Genome-Wide Identification and Characterization of the AlkB Gene Family in Sweet Orange (Citrus sinensis). Current Issues in Molecular Biology. 2023; 45(1):122-133. https://doi.org/10.3390/cimb45010009
Chicago/Turabian StyleHuang, Aijun, Ying Wang, Peipei Gu, Zhixun Yang, Junna Han, and Long Yi. 2023. "Genome-Wide Identification and Characterization of the AlkB Gene Family in Sweet Orange (Citrus sinensis)" Current Issues in Molecular Biology 45, no. 1: 122-133. https://doi.org/10.3390/cimb45010009




