A Systematic Review of Lipid-Focused Cardiovascular Disease Research: Trends and Opportunities
Abstract
:1. Introduction
2. Methods
2.1. Selection of PubMed Records and Data Visualization
2.2. Association between Terms
2.3. Construction of a Lipid–Protein Network
3. Results and Discussion
3.1. Temporal Trends in Lipid-Focused CVD Research
3.2. Qualifiers of Lipid Research in CVD
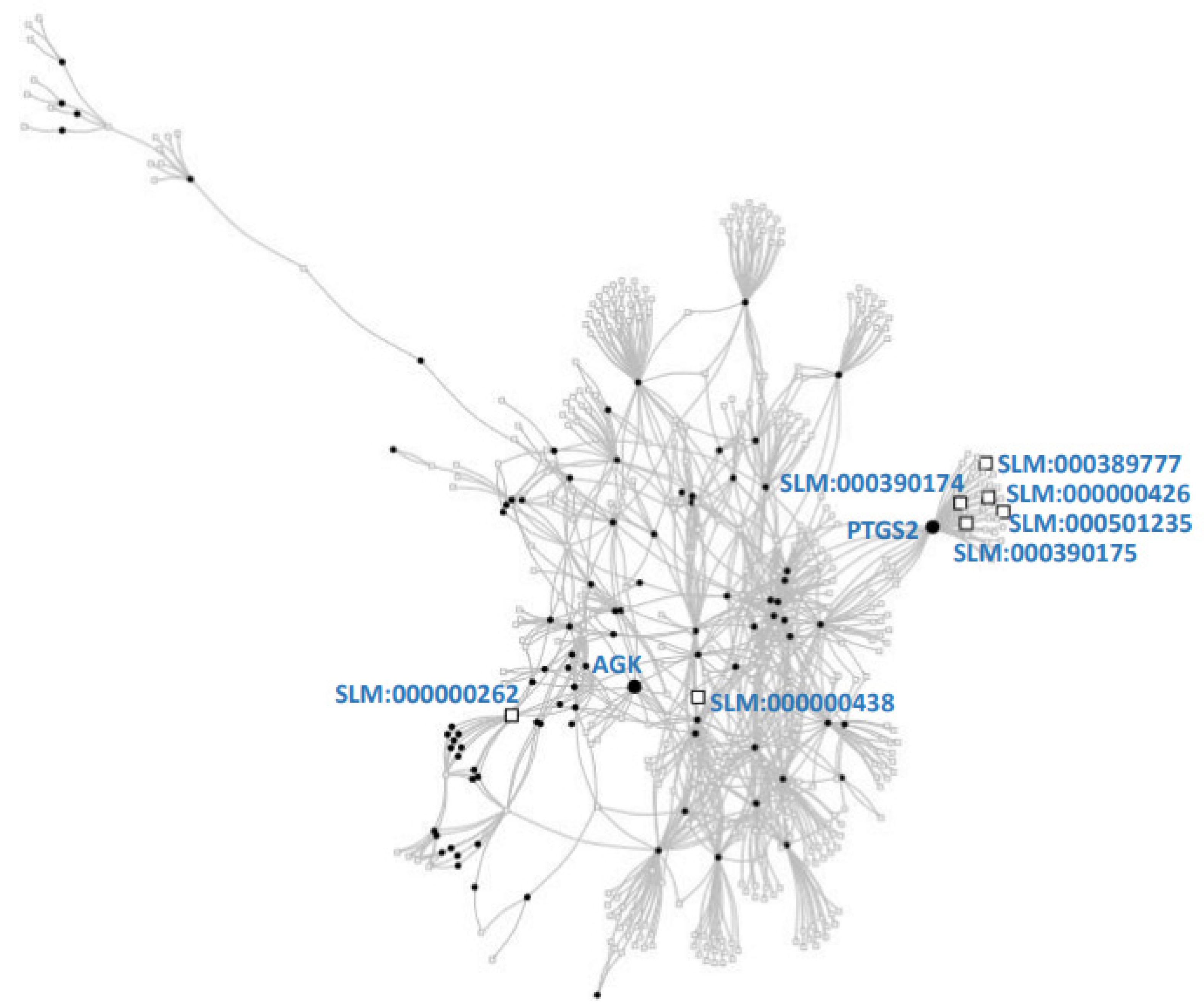
3.3. Research of Lipids in CVD and the Lipid-Protein Network
| UniProt | Score | Protein Name | Gene ID | CVD References |
|---|---|---|---|---|
| P35354 | 5.8 | Prostaglandin G/H synthase 2 | PTGS2 | [22,23,24,25] |
| Q53H12 | 4.0 | Acylglycerol kinase, mitochondrial | AGK | [26,27,28,29] |
| Q9NUN7 | 3.5 | Alkaline ceramidase 3 | ACER3 | |
| Q9NWW9 | 3.5 | Phospholipase A and acyltransferase 2 | PLAAT2 | |
| P53816 | 3.5 | Phospholipase A and acyltransferase 3 | PLAAT3 | [32,33] |
| Q9HDD0 | 3.5 | Phospholipase A and acyltransferase 1 | PLAAT1 | |
| Q9UL19 | 3.5 | Phospholipase A and acyltransferase 4 | PLAAT4 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Wijk, D.F.; Stroes, E.S.G.; Kastelein, J.J.P. Lipid measures and cardiovascular disease prediction. Dis. Markers 2009, 26, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.-T.; Gudnason, V.; A, V.; et al. Triglycerides and the Risk of Coronary Heart Disease: 10,158 Incident Cases among 262,525 Participants in 29 Western Prospective Studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The ApoB/ApoA-I Ratio: A Strong, New Risk Factor for Cardiovascular Disease and a Target for Lipid-Lowering Therapy—A Review of the Evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.J.; Lichtenstein, A.H.; Pittas, A.G.; Roberts, S.B.; Fuss, P.J.; Greenberg, A.S.; McCrory, M.A.; Bouchard, T.J., Jr.; Saltzman, E.; Neale, M.C. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid. Res. 2009, 50, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.V. Diet-Heart: End of an era. N. Engl. J. Med. 1977, 297, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Kandutsch, A.A.; Chen, H.W. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J. Biol. Chem. 1973, 248, 8408–8417. [Google Scholar] [CrossRef]
- Ravnskov, U. The fallacies of the lipid hypothesis. Scand. Cardiovasc. J. 2008, 42, 236–239. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Sørensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental risk factors and cardiovascular diseases: A comprehensive expert review. Cardiovasc. Res. 2022, 118, 2880–2902. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid. Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.B. Medical subject headings. Bull. Med. Libr. Assoc. 1963, 51, 114–116. [Google Scholar] [PubMed]
- Perez-Iratxeta, C.; Andrade-Navarro, M.A.; Wren, J.D. Evolving research trends in bioinformatics. Brief. Bioinform. 2007, 8, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ilgisonis, E.V.; Pyatnitskiy, M.A.; Tarbeeva, S.N.; Aldushin, A.A.; Ponomarenko, E.A. How to catch trends using MeSH terms analysis? Scientometrics 2022, 127, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- More, P.; Bindila, L.; Wild, P.; Andrade-Navarro, M.; Fontaine, J.-F. LipiDisease: Associate lipids to diseases using literature mining. Bioinformatics 2021, 37, 3981–3982. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid. Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Lerner, R.; Baker, D.; Schwitter, C.; Neuhaus, S.; Hauptmann, T.; Post, J.M.; Kramer, S.; Bindila, L. Four-dimensional trapped ion mobility spectrometry lipidomics for high throughput clinical profiling of human blood samples. Nat. Commun. 2023, 14, 937. [Google Scholar] [CrossRef]
- Aimo, L.; Liechti, R.; Hyka-Nouspikel, N.; Niknejad, A.; Gleizes, A.; Götz, L.; Kuznetsov, D.; David, F.P.; van der Goot, F.G.; Riezman, H.; et al. The SwissLipids knowledgebase for lipid biology. Bioinformatics 2015, 31, 2860–2866. [Google Scholar] [CrossRef]
- Michael, L.H.; Entman, M.L.; Hartley, C.J.; Youker, K.A.; Zhu, J.; Hall, S.R.; Hawkins, H.K.; Berens, K.; Ballantyne, C.M. Myocardial ischemia and reperfusion: A murine model. Am. J. Physiol. 1995, 269 Pt 2, H2147–H2154. [Google Scholar] [CrossRef]
- Tanaka, N.; Dalton, N.; Mao, L.; Rockman, H.A.; Peterson, K.L.; Gottshall, K.R.; Hunter, J.J.; Chien, K.R.; Ross, J. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 1996, 94, 1109–1117. [Google Scholar] [CrossRef]
- McNeill, E.; Channon, K.M.; Greaves, D.R. Inflammatory cell recruitment in cardiovascular disease: Murine models and potential clinical applications. Clin. Sci. 2010, 118, 641–655. [Google Scholar] [CrossRef]
- Hu, N.; Han, X.; Lane, E.K.; Gao, F.; Zhang, Y.; Ren, J. Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage. PLoS ONE 2013, 8, e57151. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Babu, S.S.; Palaniyandi, S.S.; Watanabe, K.; Cooke, J.P.; Thandavarayan, R.A. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res. Rev. 2017, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.-F.; Priller, F.; Barbosa-Silva, A.; Andrade-Navarro, M.A. Genie: Literature-based gene prioritization at multi genomic scale. Nucleic Acids Res. 2011, 39, W455–W461. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.F.; Andrade-Navarro, M.A. Gene set to Diseases (GS2D): Disease enrichment analysis on human gene sets with literature data. Genom. Comput. Biol. 2016, 2, 33. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, H.; Hua, L.; Hou, C.; Jia, Q.; Chen, J.; Zhang, S.; Wang, Y.; He, S.; Jia, E. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic. Biol. Med. 2021, 171, 55–68. [Google Scholar] [CrossRef]
- Wan, Q.; Kong, D.; Liu, Q.; Guo, S.; Wang, C.; Zhao, Y.; Ke, Z.-J.; Yu, Y. Congestive heart failure in COX2 deficient rats. Sci. China Life Sci. 2021, 64, 1068–1076. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, Y.; Feng, N.; Guo, Z.; Wang, S.; Xing, D. New horizons in the roles and associations of COX-2 and novel natural inhibitors in cardiovascular diseases. Mol. Med. 2021, 27, 123. [Google Scholar] [CrossRef]
- Haghighi, A.; Haack, T.B.; Atiq, M.; Mottaghi, H.; Haghighi-Kakhki, H.; Bashir, R.A.; Ahting, U.; Feichtinger, R.G.; Mayr, J.A.; Rötig, A.; et al. Sengers syndrome: Six novel AGK mutations in seven new families and review of the phenotypic and mutational spectrum of 29 patients. Orphanet. J. Rare Dis. 2014, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Gouveia, S.; Vázquez-Mosquera, M.E.; Gonzalez-Vioque, E.; Hermida-Ameijeiras, Á.; Valverde, L.L.; Armstrong-Moron, J.; Fons-Estupiña, M.d.C.; Wintjes, L.T.; Kappen, A.; Rodenburg, R.J.; et al. Characterization of a Novel Splicing Variant in Acylglycerol Kinase (AGK) Associated with Fatal Sengers Syndrome. Int. J. Mol. Sci. 2021, 22, 13484. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Yang, K.-Q.; Han, B.; Kong, D.; Yin, W.-H.; Li, J.-H.; Yang, Z.-X.; Niu, L.-L.; Fu, C.-S.; Rong, C.-Z.; et al. A novel AGK splicing mutation in a patient with Sengers syndrome and left ventricular non-compaction cardiomyopathy. Pediatr. Res. 2023, 94, 683–690. [Google Scholar] [CrossRef]
- Siriwardena, K.; MacKay, N.; Levandovskiy, V.; Blaser, S.; Raiman, J.; Kantor, P.F.; Ackerley, C.; Robinson, B.H.; Schulze, A.; Cameron, J.M. Mitochondrial citrate synthase crystals: Novel finding in Sengers syndrome caused by acylglycerol kinase (AGK) mutations. Mol. Genet. Metab. 2013, 108, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Yang, M.; Guo, J.; Li, D.; Li, Y. Lipidomics Analysis Reveals a Protective Effect of Myriocin on Cerebral Ischemia/Reperfusion Model Rats. J. Mol. Neurosci. 2022, 72, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Bender, D.; Law, S.-H.; Mishra, V.K.; Chen, C.-C.; Ke, L.-Y. Role of Ceramidases in Sphingolipid Metabolism and Human Diseases. Cells 2019, 8, 1573. [Google Scholar] [CrossRef] [PubMed]
- Casares-Marfil, D.; Kerick, M.; Andrés-León, E.; Bosch-Nicolau, P.; Molina, I.; Martin, J.; Acosta-Herrera, M.; Network, C.G.C. GWAS loci associated with Chagas cardiomyopathy influences DNA methylation levels. PLoS Negl. Trop. Dis. 2021, 15, e0009874. [Google Scholar] [CrossRef]
- Parmer, C.; De Sousa-Coelho, A.L.; Cheng, H.S.; Daher, G.; Burkart, A.; Dreyfuss, J.M.; Pan, H.; Prenner, J.C.; Keilson, J.M.; Pande, R.; et al. Skeletal muscle expression of adipose-specific phospholipase in peripheral artery disease. Vasc. Med. 2020, 25, 401–410. [Google Scholar] [CrossRef]
- Ledmyr, H.; McMahon, A.D.; Ehrenborg, E.; Nielsen, L.B.; Neville, M.; Lithell, H.; MacFarlane, P.W.; Packard, C.J.; Karpe, F. The microsomal triglyceride transfer protein gene-493T variant lowers cholesterol but increases the risk of coronary heart disease. Circulation 2004, 109, 2279–2284. [Google Scholar] [CrossRef]
- Mirzaei, H.; Di Biase, S.; Longo, V.D. Dietary Interventions, Cardiovascular Aging, and Disease: Animal Models and Human Studies. Circ. Res. 2016, 118, 1612–1625. [Google Scholar] [CrossRef]
- Wishart, D.S.; Bartok, B.; Oler, E.; Liang, K.Y.H.; Budinski, Z.; Berjanskii, M.; Guo, A.; Cao, X.; Wilson, M. MarkerDB: An online database of molecular biomarkers. Nucleic Acids Res. 2021, 49, D1259–D1267. [Google Scholar] [CrossRef]
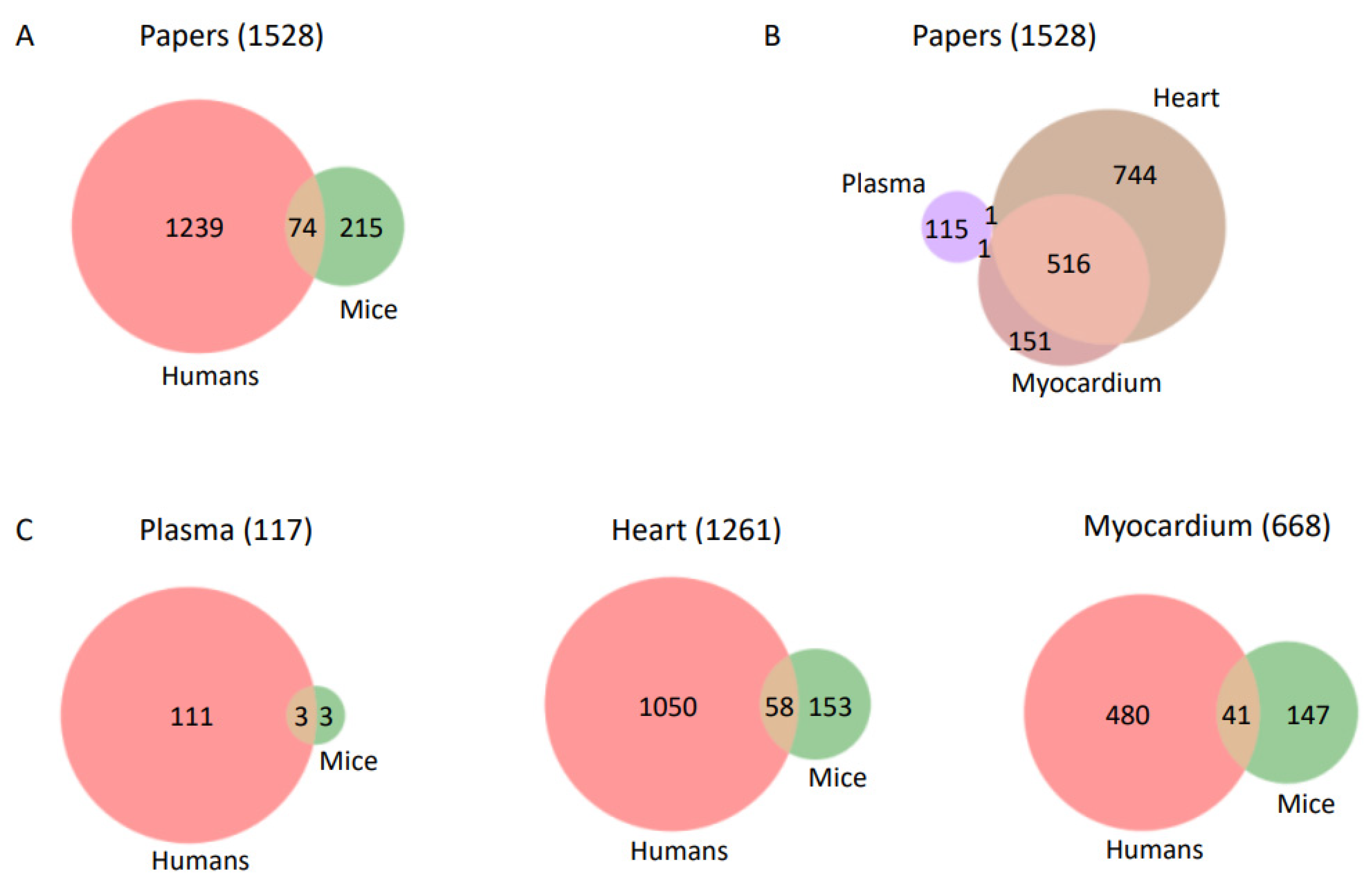
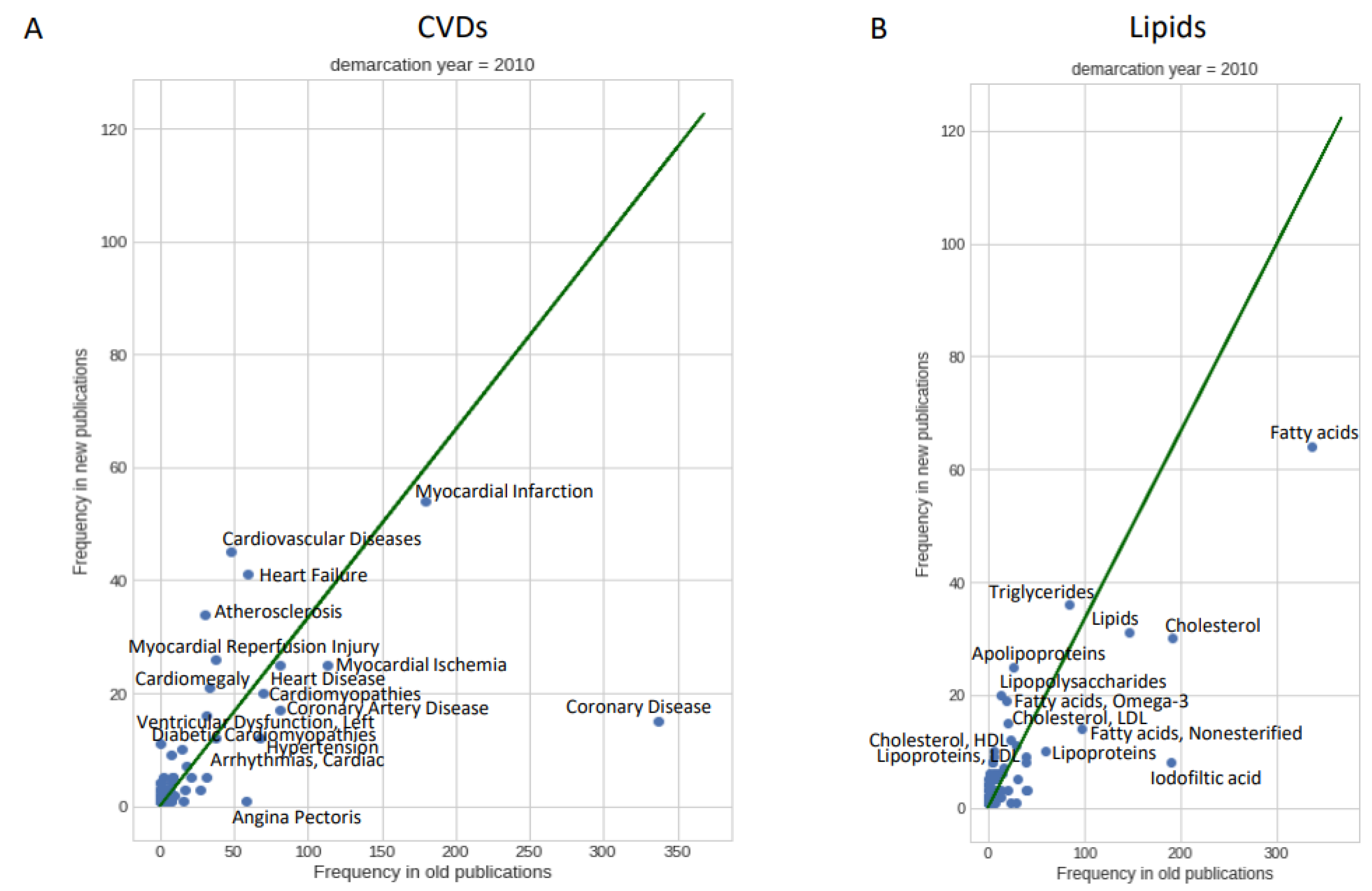
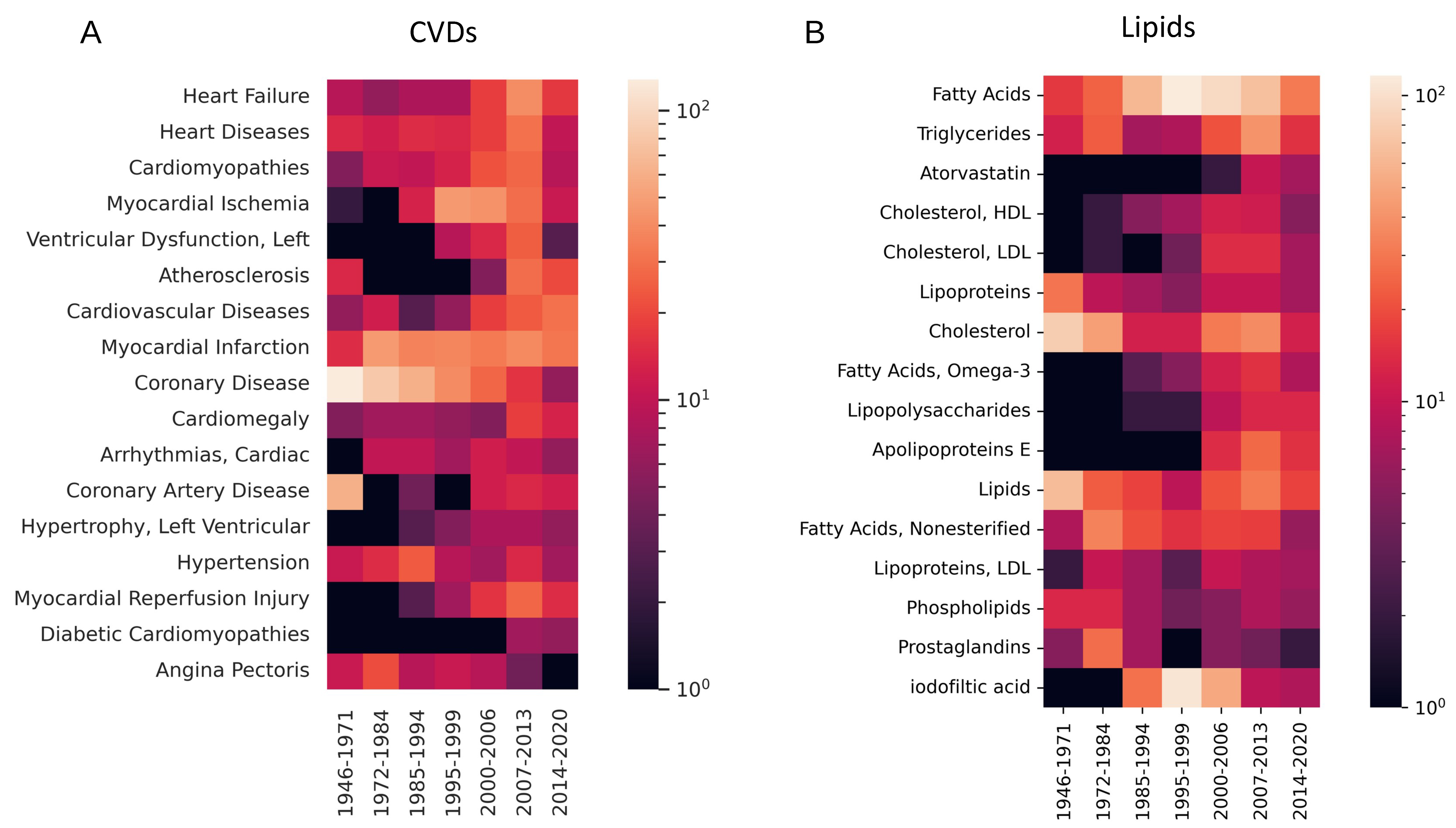
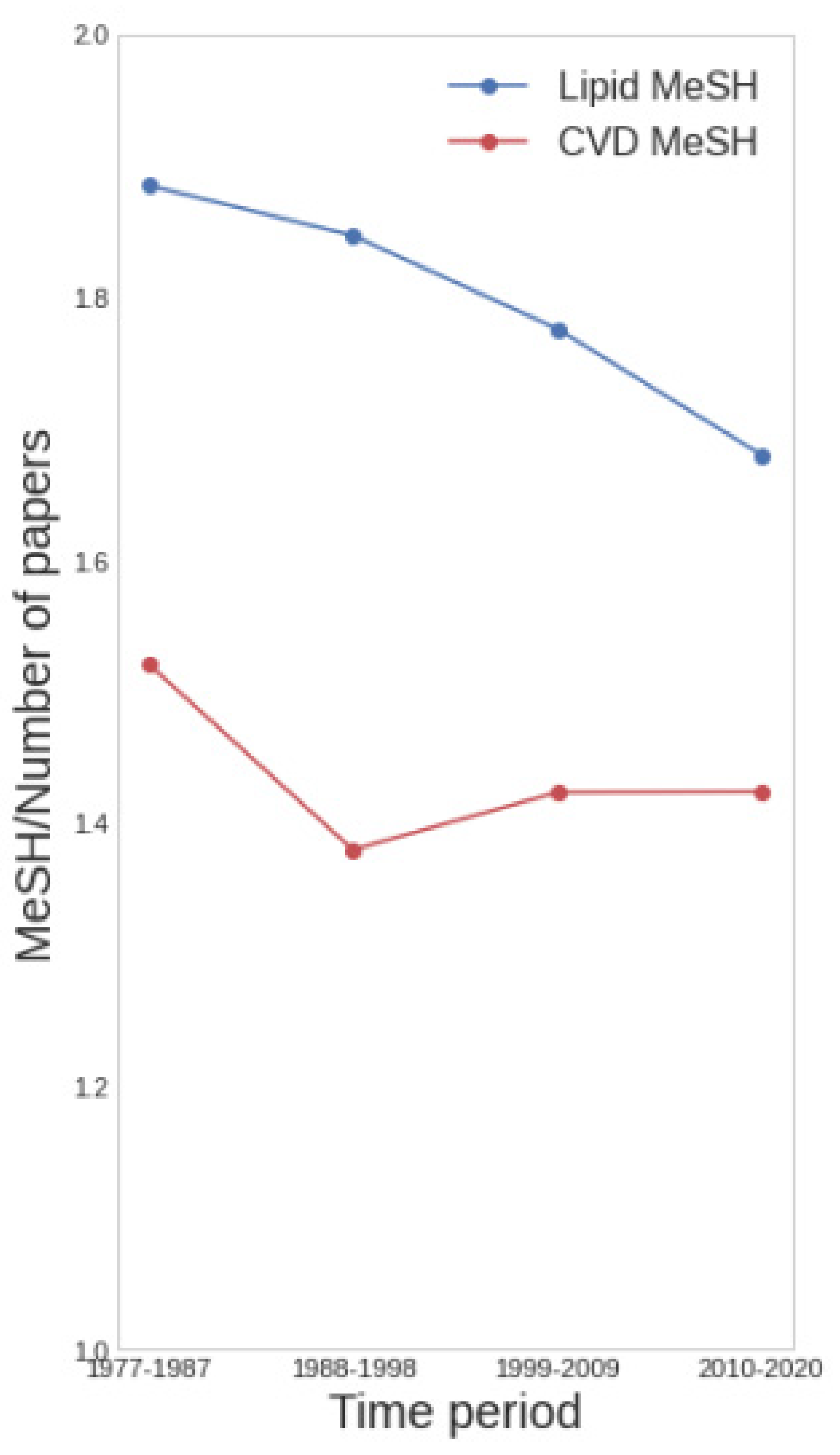
| Lipid | CVD | #Papers Lipid | #Papers CVD | #Papers Lipid & CVD | p-Value |
|---|---|---|---|---|---|
| Apolipoproteins E | Atherosclerosis | 52 | 64 | 24 | 4.06 × 10−14 |
| Trihexosylceramides | Fabry Disease | 7 | 11 | 7 | 2.45 × 10−11 |
| Globotriaosylceramide | Fabry Disease | 6 | 11 | 6 | 5.76 × 10−10 |
| Fatty Acids, Omega-3 | Death, Sudden, Cardiac | 38 | 25 | 11 | 2.32 × 10−9 |
| Cholesterol | Arteriosclerosis | 222 | 84 | 37 | 6.26 × 10−7 |
| Fatty Acids, Omega-3 | Arrhythmias, Cardiac | 38 | 49 | 10 | 4.1 × 10−6 |
| Fish Oils | Death, Sudden, Cardiac | 21 | 25 | 6 | 7.63 × 10−6 |
| Fats | Coronary Artery Disease | 31 | 98 | 12 | 1 × 10−5 |
| Glycolipids | Fabry Disease | 3 | 11 | 3 | 1.17 × 10−5 |
| Lipoproteins | Arteriosclerosis | 70 | 84 | 16 | 2.01 × 10−5 |
| Lipid | MeSH Qualifier | #Papers Lipid | #Papers MeSH q | #Papers Lipid & MeSH q | p-Value |
| Iodofiltic acid | diagnostic imaging | 617 | 819 | 195 | 4.59 × 10−27 |
| Fatty Acids | diagnostic imaging | 1552 | 819 | 273 | 5.5 × 10−20 |
| Apolipoproteins E | deficiency | 352 | 142 | 29 | 1.45 × 10−17 |
| Fatty Acids, Omega-3 | prevention & control | 230 | 301 | 30 | 5.18 × 10−13 |
| Cholesterol | blood | 1027 | 988 | 181 | 9.54 × 10−13 |
| Apolipoproteins E | genetics | 352 | 405 | 38 | 1.47 × 10−11 |
| Iodofiltic acid | pharmacokinetics | 617 | 134 | 38 | 5.94 × 10−10 |
| Lipids | blood | 785 | 988 | 137 | 2.36 × 10−9 |
| Fish Oils | prevention & control | 143 | 301 | 17 | 2.84 × 10−8 |
| Lipopolysaccharides | toxicity | 272 | 84 | 12 | 7.93 × 10−8 |
| CVD | MeSH Qualifier | #Papers CVD | #Papers MeSH q | #Papers Lipid & MeSH q | p-Value |
| Chagas Cardiomyopathy | parasitology | 6 | 6 | 6 | 4.46 × 10−11 |
| Death, Sudden, Cardiac | prevention & control | 25 | 159 | 16 | 6.74 × 10−7 |
| Myocarditis | immunology | 13 | 45 | 7 | 1.47 × 10−6 |
| Atherosclerosis | deficiency | 64 | 68 | 15 | 3.15 × 10−6 |
| Cardiomegaly | cytology | 54 | 61 | 12 | 1.60 × 10−5 |
| Myocardial Reperfusion Injury | prevention & control | 63 | 159 | 22 | 1.86 × 10−5 |
| Myocardial Reperfusion Injury | pharmacology | 63 | 368 | 38 | 2.87 × 10−5 |
| Heart Neoplasms | secondary | 3 | 2 | 2 | 5.08 × 10−5 |
| Cardiomyopathy, Hypertrophic | diagnostic imaging | 30 | 432 | 26 | 6.13 × 10−5 |
| Arrhythmias, Cardiac | chemically induced | 49 | 92 | 13 | 6.58 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyaegbunam, U.A.; More, P.; Fontaine, J.-F.; Cate, V.t.; Bauer, K.; Distler, U.; Araldi, E.; Bindila, L.; Wild, P.; Andrade-Navarro, M.A. A Systematic Review of Lipid-Focused Cardiovascular Disease Research: Trends and Opportunities. Curr. Issues Mol. Biol. 2023, 45, 9904-9916. https://doi.org/10.3390/cimb45120618
Anyaegbunam UA, More P, Fontaine J-F, Cate Vt, Bauer K, Distler U, Araldi E, Bindila L, Wild P, Andrade-Navarro MA. A Systematic Review of Lipid-Focused Cardiovascular Disease Research: Trends and Opportunities. Current Issues in Molecular Biology. 2023; 45(12):9904-9916. https://doi.org/10.3390/cimb45120618
Chicago/Turabian StyleAnyaegbunam, Uchenna Alex, Piyush More, Jean-Fred Fontaine, Vincent ten Cate, Katrin Bauer, Ute Distler, Elisa Araldi, Laura Bindila, Philipp Wild, and Miguel A. Andrade-Navarro. 2023. "A Systematic Review of Lipid-Focused Cardiovascular Disease Research: Trends and Opportunities" Current Issues in Molecular Biology 45, no. 12: 9904-9916. https://doi.org/10.3390/cimb45120618






