Myrrh Essential Oil Mitigates Renal Ischemia/Reperfusion-Induced Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Acquisition and Myrrh Essential Oil Isolation and Analysis
2.2. Animals’ Acquisition and Ethical Approval
2.3. Experimental Design
2.4. Renal Ischemia/Reperfusion (I/R) Surgery Procedures
2.5. Histopathological and Immunohistochemistry Investigations
2.6. Assessment of the Kidney Function
2.7. Assessment of the Kidney Injury Biomarkers
2.8. Assessment of the Kidney Oxidative Stress
2.9. Gene Expression by Real-Time PCR (qPCR)
2.10. Assessment of the Kidney Inflammatory Mediators
2.11. Statistical Analysis
3. Results
3.1. Myrrh Essential Oil Constituents
3.2. Myrrh Essential Oil Improved Renal Function Subsequent to Renal I/R
3.3. Myrrh Essential Oil Deterred Renal Injury Biomarkers
3.4. Myrrh Essential Oil Amended Renal Histological Alterations Subsequent to Renal I/R
3.5. Myrrh Essential Oil Deterred Renal Oxidative Stress Subsequent to Renal I/R
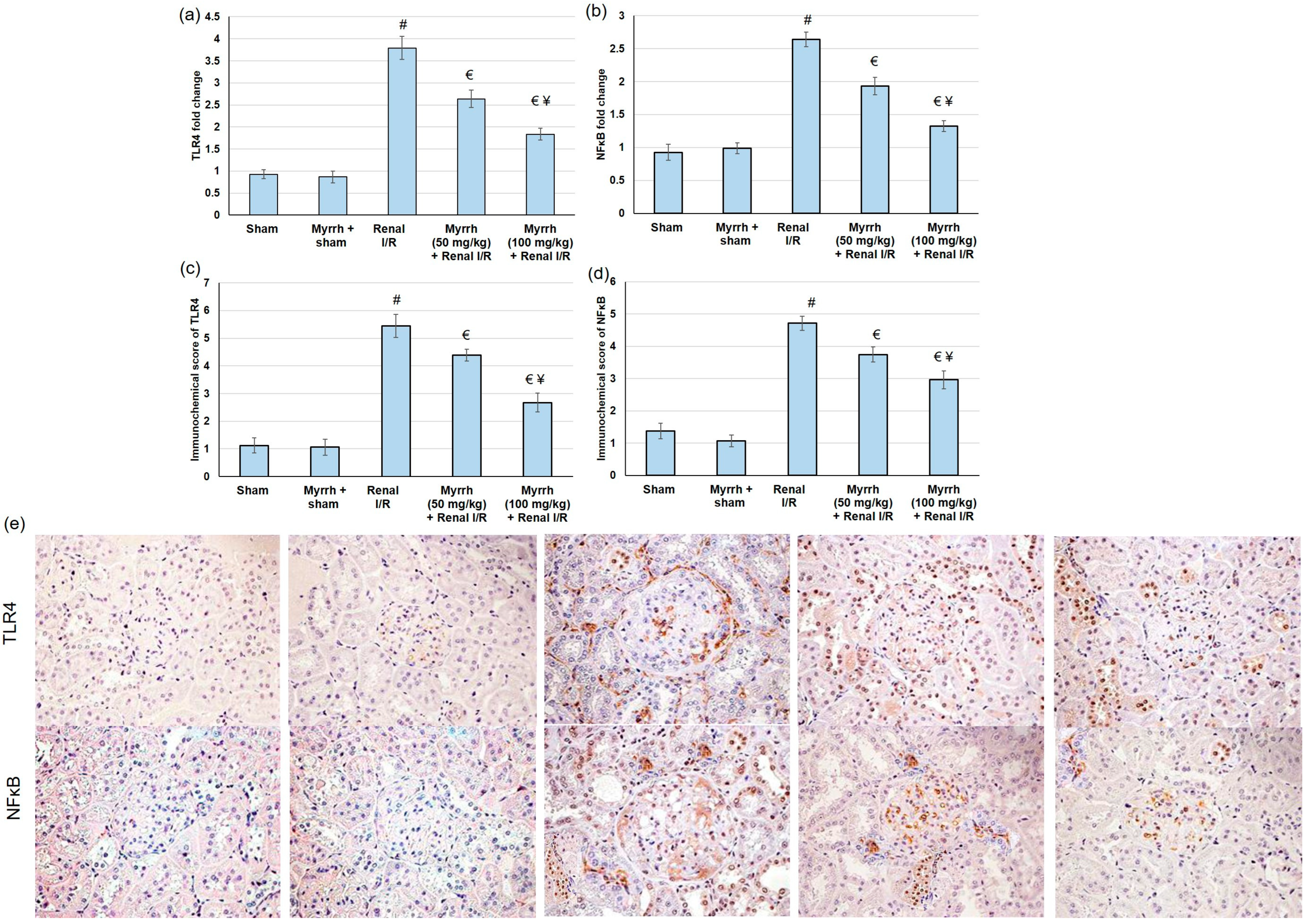
3.6. Myrrh Essential Oil Deterred TLR4/NFκB Pathway Activation Subsequent to Renal I/R
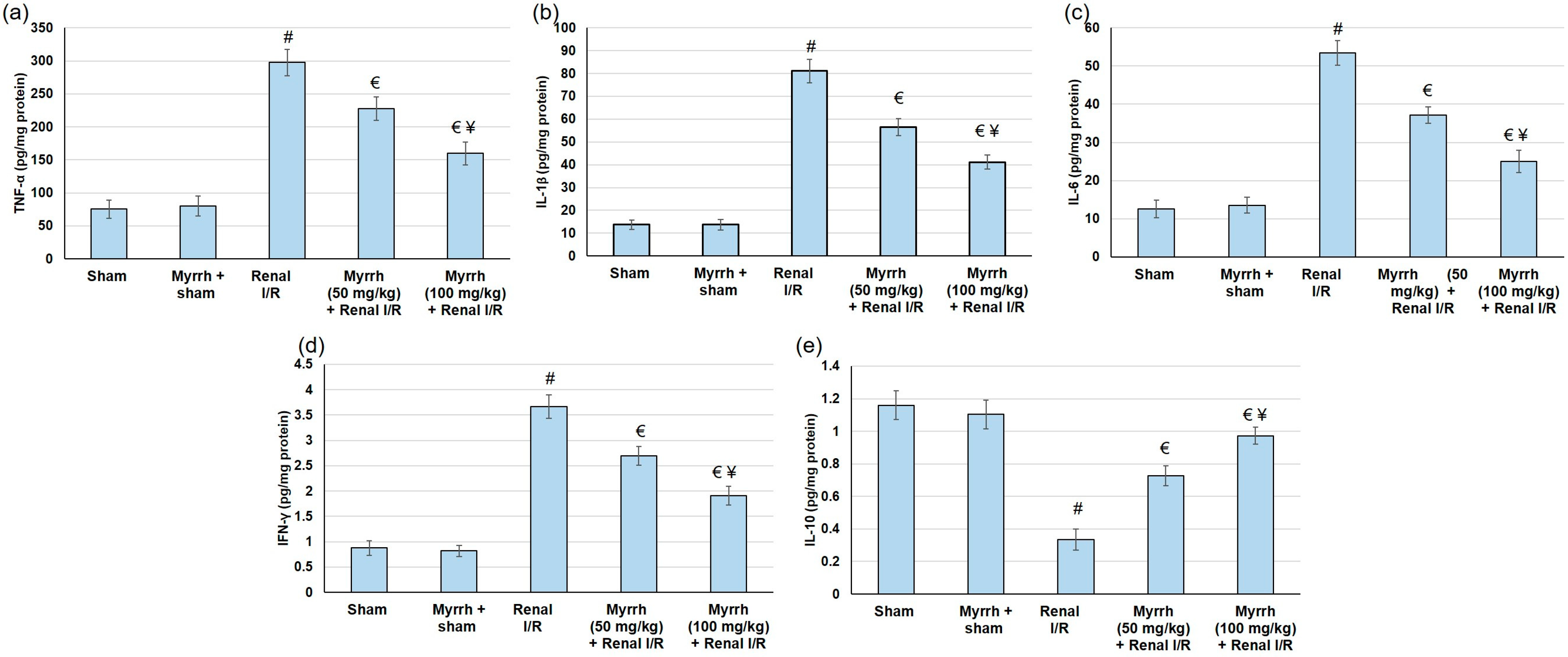
3.7. Myrrh Essential Oil Deterred Renal Inflammation Subsequent to Renal I/R
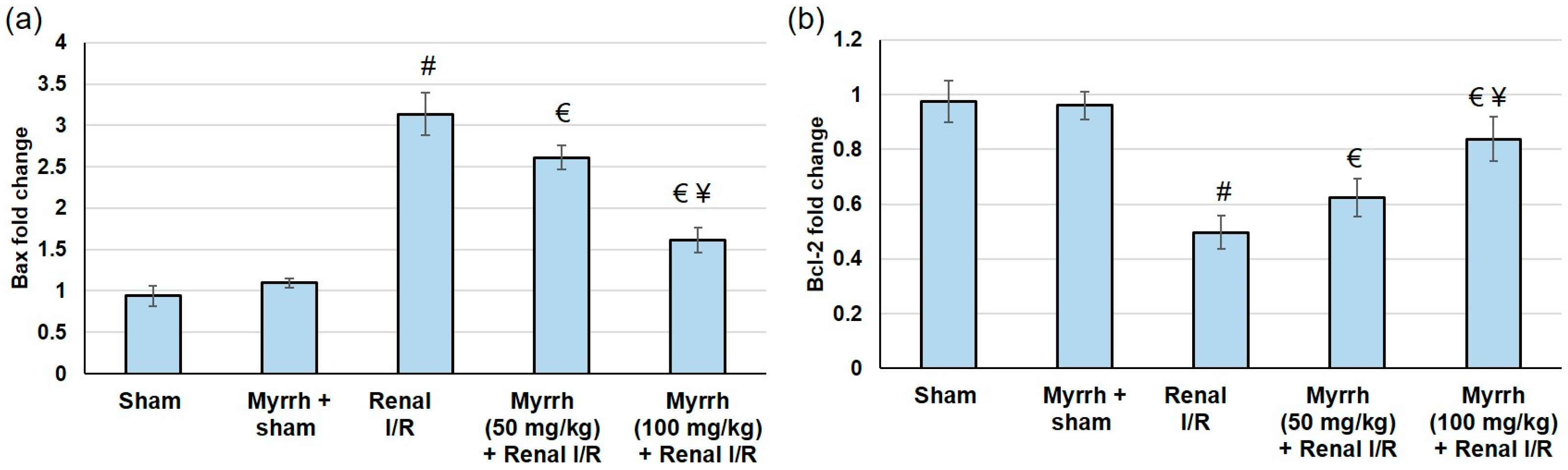
3.8. Myrrh Essential Oil Deterred Renal Apoptosis Subsequent to Renal I/R
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, M.E.; Tawfeek, N.; Elbaramawi, S.S.; Elbatreek, M.H.; Fikry, E. Agathis robusta Bark Extract Protects from Renal Ischemia-Reperfusion Injury: Phytochemical, In Silico and In Vivo Studies. Pharmaceuticals 2022, 15, 1270. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Chen, D.; Hu, B.; Xu, G.; Li, W.; Jin, Y.; Jin, X.; Jin, X.; Jin, L. Allicin ameliorates renal ischemia/reperfusion injury via inhibition of oxidative stress and inflammation in rats. Biomed. Pharmacother. 2021, 142, 112077. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, X.; Gui, T.; Wang, T.; Wang, Z.; Kullak-Ublick, G.A.; Gai, Z. Effects of Farnesiferol B on Ischemia-Reperfusion-Induced Renal Damage, Inflammation, and NF-κB Signaling. Int. J. Mol. Sci. 2019, 20, 6280. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Lindner, J.R.; Coggins, M.P.; Kaul, S.; Klibanov, A.L.; Brandenburger, G.H.; Ley, K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin-and complement-mediated adherence to activated leukocytes. Circulation 2000, 101, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Prophylactic Treatment with Hydrogen Sulphide Can Prevent Renal Ischemia-Reperfusion Injury in L-NAME Induced Hypertensive Rats with Cisplatin-Induced Acute Renal Failure. Life 2022, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Ahmadi, G.; Ahmadi, H. A review on antifungal and antibacterial activities of some medicinal plants. Micro Nano Bio Asp. 2022, 1, 10–17. [Google Scholar]
- Amraei, S.; Ahmadi, S. Recent studies on antimicrobial and anticancer activities of saponins: A mini-review. Nano Micro. Biosyst. 2022, 1, 22–26. [Google Scholar]
- Haffor, A.-S.A. Effect of myrrh (Commiphora molmol) on leukocyte levels before and during healing from gastric ulcer or skin injury. J. Immunotoxicol. 2010, 7, 68–75. [Google Scholar] [CrossRef]
- Feng, J.; Shi, W.; Miklossy, J.; Tauxe, G.M.; McMeniman, C.J.; Zhang, Y. Identification of Essential Oils with Strong Activity against Stationary Phase Borrelia burgdorferi. Antibiotics 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Lee, A.; Kim, T.; Jang, S.A.; Ha, H. Anti-Osteoporotic Effects of Commiphora Myrrha and Its Poly-Saccharide via Osteoclastogenesis Inhibition. Plants 2021, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Hammouda, A.A.-E. Analgesic, anti-inflammatory and anti-hyperlipidemic activities of Commiphora molmol extract (Myrrh). J. Intercult. Ethnopharmacol. 2014, 3, 56. [Google Scholar] [CrossRef]
- Fatani, A.J.; Alrojayee, F.S.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Exp. Ther. Med. 2016, 12, 730–738. [Google Scholar] [CrossRef]
- Orabi, S.H.; Al-Sabbagh, E.S.; Khalifa, H.K.; Mohamed, M.A.E.-G.; Elhamouly, M.; Gad-Allah, S.M.; Abdel-Daim, M.M.; Eldaim, M.A.A. Commiphora myrrha Resin Alcoholic Extract Ameliorates High Fat Diet Induced Obesity via Regulation of UCP1 and Adiponectin Proteins Expression in Rats. Nutrients 2020, 12, 803. [Google Scholar] [CrossRef]
- Ramesh, B.; Saralakumari, D. Antihyperglycemic, hypolipidemic and antioxidant activities of ethanolic extract of Commiphora mukul gum resin in fructose-fed male Wistar rats. J. Physiol. Biochem. 2012, 68, 573–582. [Google Scholar] [CrossRef]
- Ahmad, A.; Raish, M.; Ganaie, M.A.; Ahmad, S.R.; Mohsin, K.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Alkharfy, K.M. Hepatoprotective effect of Commiphora myrrha against d-GalN/LPS-induced hepatic injury in a rat model through attenuation of pro inflammatory cytokines and related genes. Pharm. Biol. 2015, 53, 1759–1767. [Google Scholar] [CrossRef]
- Al-Harbi, M.; Qureshi, S.; Raza, M.; Ahmed, M.; Afzal, M.; Shah, A. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J. Ethnopharmacol. 1997, 55, 141–150. [Google Scholar] [CrossRef]
- AbouLaila, M.; El-Sayed, S.A.E.-S.; Omar, M.A.; Al-Aboody, M.S.; Abdel Aziz, A.R.; Abdel-Daim, M.M.; Rizk, M.A.; Igarashi, I. Myrrh oil in vitro inhibitory growth on bovine and equine piroplasm parasites and Babesia microti of mice. Pathogens 2020, 9, 173. [Google Scholar] [CrossRef]
- Madia, V.N.; De Angelis, M.; De Vita, D.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Chiara, G.; Garzoli, S.; et al. Investigation of Commiphora myrrha (Nees) Engl. Oil and Its Main Components for Antiviral Activity. Pharmaceuticals 2021, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.; Aljammaz, N.A.; Huwaizi, S.; Suliman, R.; Kahtani, K.M.; Albadrani, G.M.; Barhoumi, T.; et al. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals 2022, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; Mostafa, R.E.; Taha, N.M.; Abd El-Maksoud, E.M.; Tohamy, H.G.; Al Jaouni, S.K.; El-Far, A.H.; Elfeky, M.S. Commiphora myrrh Supplementation Protects and Cures Ethanol-Induced Oxidative Alterations of Gastric Ulceration in Rats. Antioxidants 2021, 10, 1836. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Cho, D.H.; Pariyar, R.; Yoon, C.S.; Chang, B.Y.; Kim, D.S.; Cho, H.K.; Kim, S.Y.; Oh, H.; Kim, Y.C.; et al. The Ameliorating Effect of Myrrh on Scopolamine-Induced Memory Impairments in Mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 925432. [Google Scholar] [CrossRef]
- Lee, D.; Ju, M.K.; Kim, H. Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Nutrients 2020, 12, 1477. [Google Scholar] [CrossRef]
- ElMosbah, D.E.; Khattab, M.S.; Emam, S.R.; Miniawy, H. The anti-inflammatory effect of myrrh ethanolic extract in comparison with prednisolone on an autoimmune disease rat model induced by silicate. Inflammopharmacology 2022, 30, 2537–2546. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. Protective effects of myrrh essential oil on isoproterenol-induced myocardial infarction in rats through antioxidant, anti-inflammatory, Nrf2/HO-1 and apoptotic pathways. J. Ethnopharmacol. 2021, 270, 113793. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef]
- Pasten, C.; Lozano, M.; Rocco, J.; Carrión, F.; Alvarado, C.; Liberona, J.; Michea, L.; Irarrázabal, C.E. Aminoguanidine Prevents the Oxidative Stress, Inhibiting Elements of Inflammation, Endothelial Activation, Mesenchymal Markers, and Confers a Renoprotective Effect in Renal Ischemia and Reperfusion Injury. Antioxidants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Yan, Y.; Liu, W.; Li, G.; Li, R. Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats. Ren. Fail. 2015, 37, 727–733. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abdelnaby, R.M.; Younis, N.S. β-caryophyllene ameliorates hepatic ischemia reperfusion-induced injury: The involvement of Keap1/Nrf2/HO 1/NQO 1 and TLR4/NF-κB/NLRP3 signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8551–8566. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Dieterle, F.; Perentes, E.; Cordier, A.; Roth, D.R.; Verdes, P.; Grenet, O.; Pantano, S.; Moulin, P.; Wahl, D.; Mahl, A.; et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 2010, 28, 463–469. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomości. Lekarskie. 2004, 57, 453–455. [Google Scholar]
- Park, J.; Lee, E.G.; Yi, H.J.; Kim, N.H.; Rhee, S.G.; Woo, H.A. Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice. Antioxidants 2020, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhu, H.; Tao, Q.; Guo, L.; Jiang, T.; Xu, L.; Yang, R.; Wei, X.; Wu, J.; Li, X.; et al. FGF10 Protects Against Renal Ischemia/Reperfusion Injury by Regulating Autophagy and Inflammatory Signaling. Front. Genet. 2018, 9, 556. [Google Scholar] [CrossRef]
- Sotoudeh, R.; Gholamnezhad, Z.; Aghaei, A. The anti-diabetic and antioxidant effects of a combination of Commiphora mukul, Commiphora myrrha and Terminalia chebula in diabetic rats. Avicenna J. Phytomed. 2019, 9, 454. [Google Scholar]
- Bondi, C.D.; Rush, B.M.; Hartman, H.L.; Wang, J.; Al-Bataineh, M.M.; Hughey, R.P.; Tan, R.J. Suppression of NRF2 Activity by HIF-1α Promotes Fibrosis after Ischemic Acute Kidney Injury. Antioxidants 2022, 11, 1810. [Google Scholar]
- Xiao, J.J.; Liu, Q.; Li, Y.; Peng, F.F.; Wang, S.; Zhang, Z.; Liu, H.; Yu, H.; Tao, S.; Zhang, B.F. Regulator of calcineurin 1 deletion attenuates mitochondrial dysfunction and apoptosis in acute kidney injury through JNK/Mff signaling pathway. Cell Death Dis. 2022, 13, 774. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand. J. Clin. Lab. Investig. 2008, 68, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.B.; Bengaard, A.K.; Iversen, E.; Nguyen, C.N.; Kallemose, T.; Juul-Larsen, H.G.; Jawad, B.N.; Hornum, M.; Andersen, O.; Eugen-Olsen, J.; et al. Utility of suPAR and NGAL for AKI Risk Stratification and Early Optimization of Renal Risk Medications among Older Patients in the Emergency Department. Pharmaceuticals 2021, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Nevo, A.; Armaly, Z.; Abd El Kadir, A.; Douvdevani, A.; Tovbin, D. Elevated neutrophil gelatinase lipocalin levels are associated with increased oxidative stress in hemodialysis patients. J. Clin. Med. Res. 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.-J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Knoppert, S.N.; Pissas, G.; Rodrigues-Diez, R.R.; Marquez-Exposito, L.; Broekhuizen, R.; Mokry, M.; Kester, L.A.; Falke, L.L.; Goldschmeding, R.; et al. CCN2 Aggravates the Immediate Oxidative Stress-DNA Damage Response Following Renal Ischemia-Reperfusion Injury. Antioxidants 2021, 10, 2020. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Ghazy, E.W.; Fayez, M. Synergistic protective role of mirazid (Commiphora molmol) and ascorbic acid against tilmicosin-induced cardiotoxicity in mice. Can. J. Physiol. Pharmacol. 2015, 93, 45–51. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Alqahtani, S.; Othman, S.I.; Germoush, M.O.; Hussein, O.E.; Al-Basher, G.; Khim, J.S.; Al-Qaraawi, M.A.; Al-Harbi, H.M.; Fadel, A.; et al. Commiphora molmol Modulates Glutamate-Nitric Oxide-cGMP and Nrf2/ARE/HO-1 Pathways and Attenuates Oxidative Stress and Hematological Alterations in Hyperammonemic Rats. Oxid. Med. Cell Longev. 2017, 2017, 7369671. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Zaki, A.R.; Hassan, M.E.; Mostafa-Hedeab, G. Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and Nrf2/ARE/HO-1 signaling. Chem. Biol. Interact. 2017, 270, 41–50. [Google Scholar] [CrossRef]
- Kar, F.; Hacioglu, C.; Senturk, H.; Donmez, D.B.; Kanbak, G. The Role of Oxidative Stress, Renal Inflammation, and Apoptosis in Post Ischemic Reperfusion Injury of Kidney Tissue: The Protective Effect of Dose-Dependent Boric Acid Administration. Biol. Trace Elem. Res. 2020, 195, 150–158. [Google Scholar] [CrossRef]
- Liu, M.; Zen, K. Toll-Like Receptors Regulate the Development and Progression of Renal Diseases. Kidney Dis. 2021, 7, 14–23. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, J.; Ji, K.; Liu, H.; Degen, A.; Zhai, M.; Jiao, D.; Guo, J.; Zhao, Z.; Yang, G. Protective Effect of Resveratrol Improves Systemic Inflammation Responses in LPS-Injected Lambs. Animals 2019, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.H.; Wu, C.T.; Deng, J.S.; Jiang, W.P.; Huang, W.C.; Huang, G.J. Salvianolic Acid C Protects against Cisplatin-Induced Acute Kidney Injury through Attenuation of Inflammation, Oxidative Stress and Apoptotic Effects and Activation of the CaMKK-AMPK-Sirt1-Associated Signaling Pathway in Mouse Models. Antioxidants 2021, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nozaki, Y.; Murao, Y.; Yano, T.; Ri, J.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Protective effect and mechanism of IL-10 on renal ischemia-reperfusion injury. Lab. Investig. 2019, 99, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhang, Y.; Zhang, L.; Li, X.; Yu, J.B.; Zhang, H.T.; Tan, B.B.; Jiang, L.H.; Wang, Y.X.; Liang, Y.; et al. Protective effect of tea polyphenols on renal ischemia/reperfusion injury via suppressing the activation of TLR4/NF-κB p65 signal pathway. Gene 2014, 542, 46–51. [Google Scholar] [CrossRef]
- Meng, X.; Wei, M.; Wang, D.; Qu, X.; Zhang, K.; Zhang, N.; Li, X. The protective effect of hesperidin against renal ischemia-reperfusion injury involves the TLR-4/NF-κB/iNOS pathway in rats. Physiol. Int. 2020, 107, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhao, J.; Cui, D.; Wang, F.; Song, Y.; Cheng, L.; Gao, K.; Wang, J.; Li, L.; Li, S.; et al. Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-κB signaling pathway. Sci. Rep. 2018, 8, 9173. [Google Scholar] [CrossRef]
- Haffor, A.S. Effect of Commiphora molmol on leukocytes proliferation in relation to histological alterations before and during healing from injury. Saudi J. Biol. Sci. 2010, 17, 139–146. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Pazoki Toroudi, H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Huang, J.; Chen, X.Y.; Xie, J.; Yang, Q.; Wang, J.F.; Deng, X.M. Senkyunolide I alleviates renal Ischemia-Reperfusion injury by inhibiting oxidative stress, endoplasmic reticulum stress and apoptosis. Int. Immunopharmacol. 2022, 102, 108393. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younis, N.S. Myrrh Essential Oil Mitigates Renal Ischemia/Reperfusion-Induced Injury. Curr. Issues Mol. Biol. 2023, 45, 1183-1196. https://doi.org/10.3390/cimb45020078
Younis NS. Myrrh Essential Oil Mitigates Renal Ischemia/Reperfusion-Induced Injury. Current Issues in Molecular Biology. 2023; 45(2):1183-1196. https://doi.org/10.3390/cimb45020078
Chicago/Turabian StyleYounis, Nancy S. 2023. "Myrrh Essential Oil Mitigates Renal Ischemia/Reperfusion-Induced Injury" Current Issues in Molecular Biology 45, no. 2: 1183-1196. https://doi.org/10.3390/cimb45020078
APA StyleYounis, N. S. (2023). Myrrh Essential Oil Mitigates Renal Ischemia/Reperfusion-Induced Injury. Current Issues in Molecular Biology, 45(2), 1183-1196. https://doi.org/10.3390/cimb45020078



