Expressions of Type I and III Interferons, Endogenous Retroviruses, TRIM28, and SETDB1 in Children with Respiratory Syncytial Virus Bronchiolitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Total RNA Extraction
2.3. Reverse Transcription
2.4. Transcription Levels of IFNs, ISGs, TRIM28, SETDB1, Pol Genes of HERV-H, -K, and -W, and Env Genes of SYN1 and SYN2 via Real-Time PCR Assays
2.5. Statistical Analysis
3. Results
3.1. Study Populations
3.2. Influence of Age on IFN Signature Scores and on Expression Levels of HERVs, TRIM28, and SETDB1
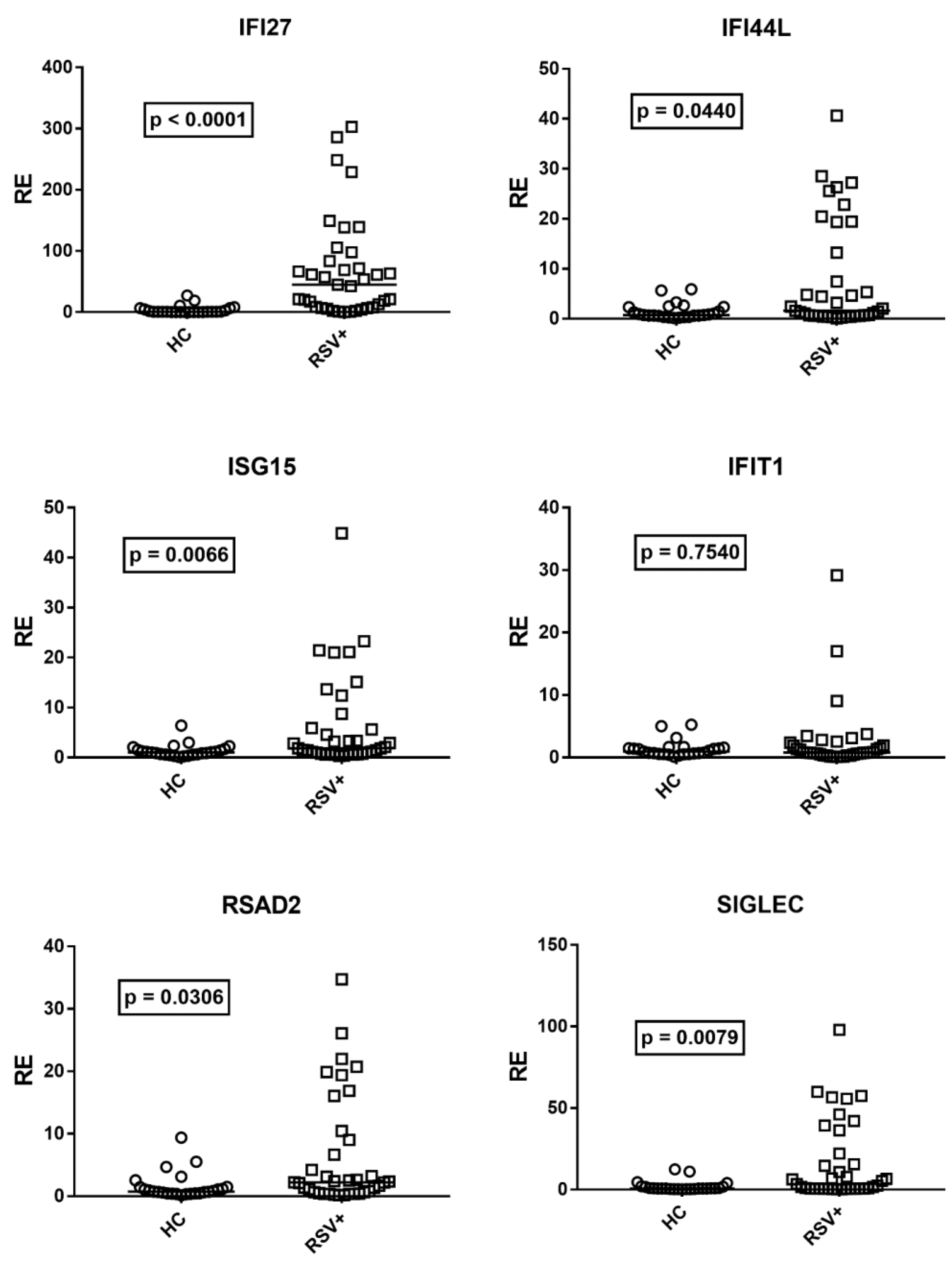
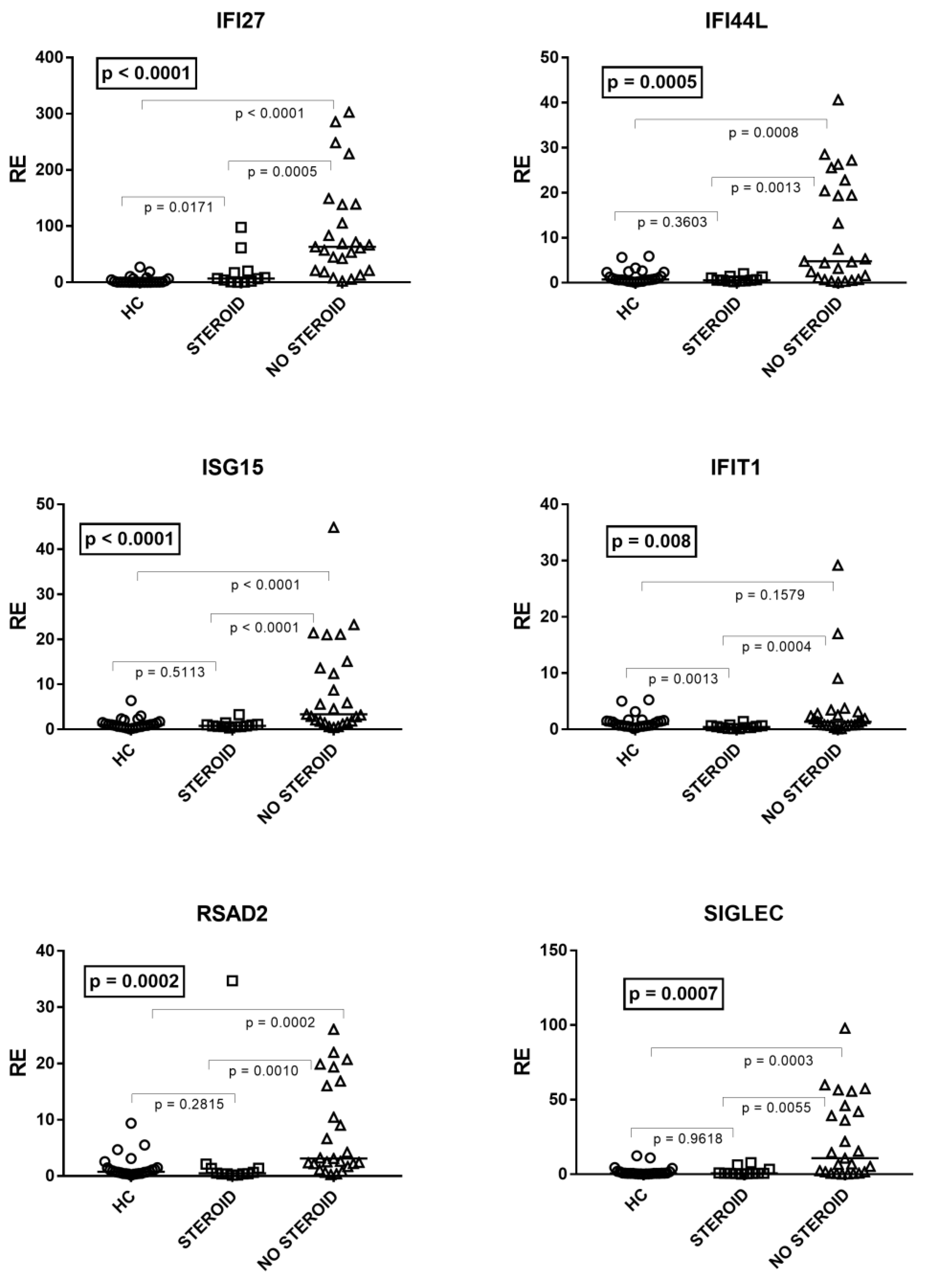
3.3. Type I IFN Signature
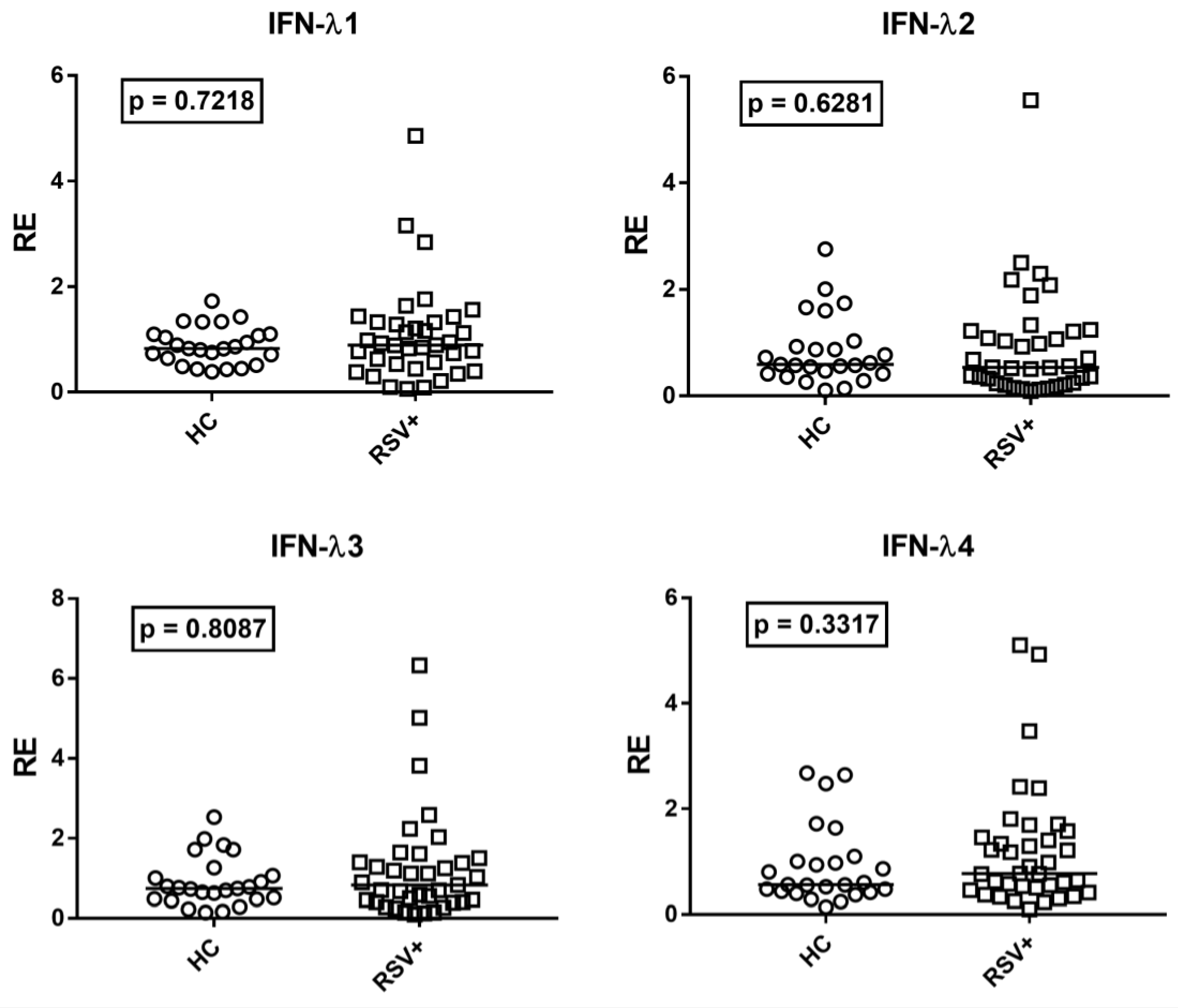
3.4. Type III IFNs
3.5. Expressions of HERV-H-Pol, HERV-K-Pol, HERV-W-Pol, SYN1-Env, and SYN2-Env
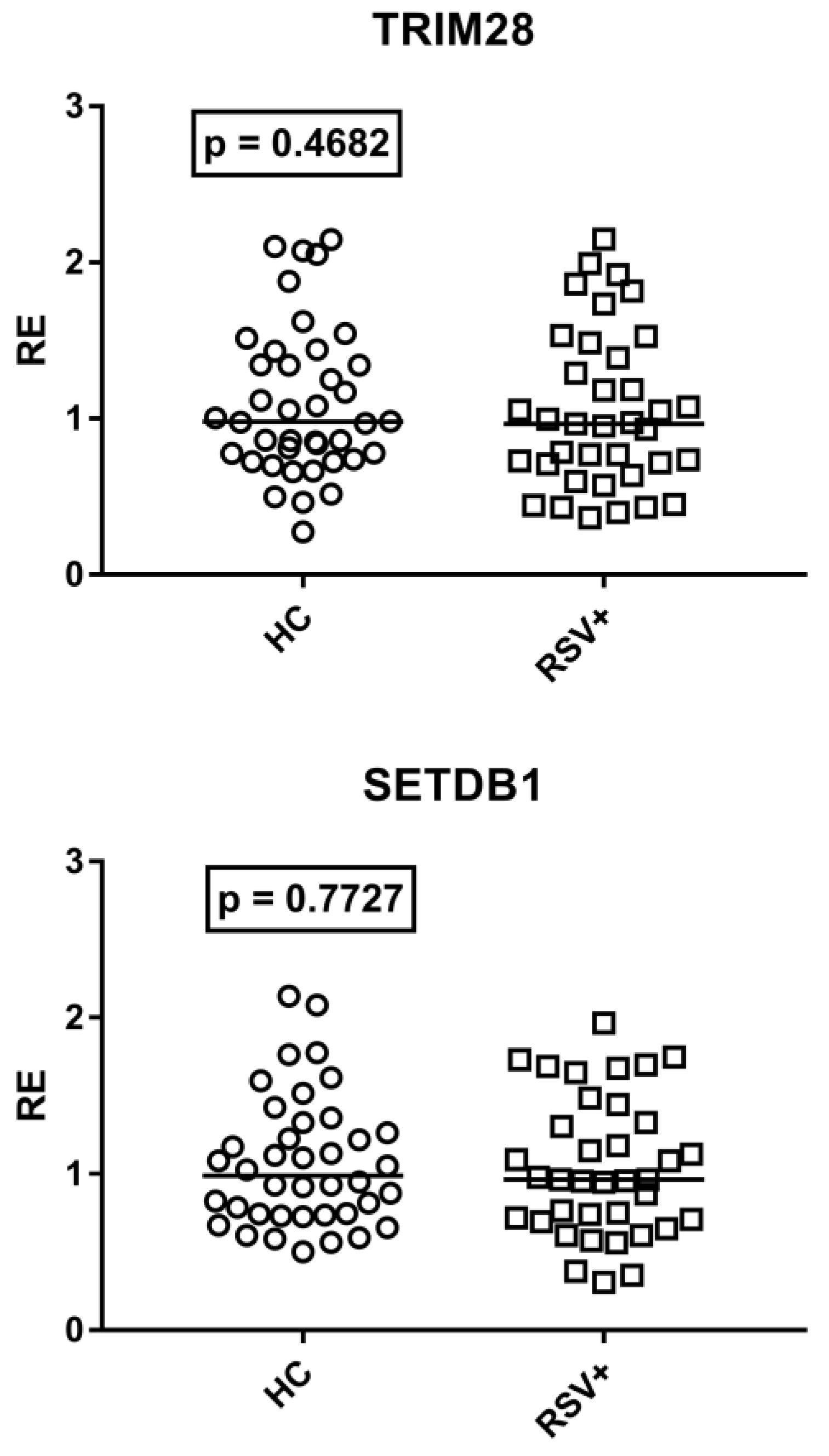
3.6. Expressions of TRIM28 and SETDB1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HERVs | human endogenous retroviruses |
| IFN | interferon |
| ISGs | interferon-stimulated genes |
| KRAB-ZFPs | Kruppel-associated box domain zinc finger proteins |
| NS1 | RSV-nonstructural protein 1 |
| NS2 | RSV-nonstructural protein 2 |
| PICU | pediatric intensive care unit |
| PBMCs | peripheral blood mononuclear cells |
| PRR | pattern recognition receptor |
| RSV | respiratory syncytial virus |
| SETDB1 | domain bifurcated histone lysine methyltrasferase 1 |
| SOCS | suppressor of cytokine signaling |
| SYN1 | syncytin 1 |
| SYN2 | syncytin 2 |
| TRIM28 | tripartite motif containing 28 |
| TLR | Toll-like receptor |
References
- Hasegawa, K.; Tsugawa, Y.; Brown, D.F.; Mansbach, J.M.; Camargo, C.A., Jr. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics 2013, 132, 28–36. [Google Scholar] [CrossRef]
- Ghazaly, M.; Nadel, S. Characteristics of children admitted to intensive care with acute bronchiolitis. Eur. J. Pediatr. 2018, 177, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Checchia, P.A.; Paes, B.; Bont, L.; Manzoni, P.; Simoes, E.A.; Fauroux, B.; Figueras-Aloy, J.; Carbonell-Estrany, X. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with congenital heart disease. Infect. Dis. Ther. 2017, 6, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.T.; Bont, L.J.; Zar, H.; Polack, F.P.; Park, C.; Claxton, A.; Borok, G.; Butylkova, Y.; Wegzyn, C. Respiratory syncytial virus hospitalization and mortality: Systematic review and meta-analysis. Pediatr. Pulmonol. 2017, 52, 556–569. [Google Scholar] [CrossRef]
- Lu, S.; Hartert, T.V.; Everard, M.L.; Giezek, H.; Nelsen, L.; Mehta, A.; Patel, H.; Knorr, B.; Reiss, T.F. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr. Pulmonol. 2016, 51, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Dupont, W.; Wu, P.; Gebretsadik, T.; Hartert, T. RSV prevention in infancy and asthma in later life. Lancet Respir. Med. 2018, 6, e32. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Carvajal, J.J.; Avellaneda, A.M.; Salazar-Ardiles, C.; Maya, J.E.; Kalergis, A.M.; Lay, M.K. Host components contributing to respiratory syncytial virus pathogenesis. Front. Immunol. 2019, 10, 2152. [Google Scholar] [CrossRef]
- Hardy, M.P.; Owczarek, C.M.; Jermiin, L.S.; Ejdebäck, M.; Hertzog, P.J. Characterization of the type I interferon locus and identification of novel genes. Genomics 2004, 84, 331–345. [Google Scholar] [CrossRef]
- Schreiber, G. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 2017, 292, 7285–7294. [Google Scholar] [CrossRef] [Green Version]
- Lamot, L.; Niemietz, I.; Brown, K.L. Comparable type I interferon score determination from PAXgene and Tempus whole blood RNA collection and isolation systems. BMC Res. Notes. 2019, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.A.; Garazzino, S.; Daprà, V.; Pruccoli, G.; Calvi, C.; Mignone, F.; Alliaudi, C.; Denina, M.; Scolfaro, C.; Zoppo, M.; et al. COVID-19 in children: Expressions of type I/II/III interferons, TRIM28, SETDB1, and endogenous retroviruses in mild and severe cases. Int. J. Mol. Sci. 2021, 22, 7481. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Zanoni, I.; Galani, I.E. Lambda interferons come to light: Dual function cytokines mediating antiviral immunity and damage control. Curr. Opin. Immunol. 2019, 56, 67–75. [Google Scholar] [CrossRef]
- Syedbasha, M.; Egli, A. Interferon lambda: Modulating immunity in infectious diseases. Front. Immunol. 2017, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Rivera, A.; Parker, D.; Durbin, J.E. Type III IFNs: Beyond antiviral protection. Semin. Immunol. 2019, 43, 101303. [Google Scholar] [CrossRef]
- Ye, L.; Schnepf, D.; Becker, J.; Ebert, K.; Tanriver, Y.; Bernasconi, V.; Gad, H.H.; Hartmann, R.; Lycke, N.; Staeheli, P. Interferon-λ enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat. Immunol. 2019, 20, 593–601. [Google Scholar] [CrossRef]
- Xia, C.; Vijayan, M.; Pritzl, C.J.; Fuchs, S.Y.; McDermott, A.B.; Hahm, B. Hemagglutinin of influenza A virus antagonizes type I interferon (IFN) responses by inducing degradation of type I IFN receptor 1. J. Virol. 2015, 90, 2403–2417. [Google Scholar] [CrossRef]
- Konno, Y.; Kimura, I.; Uriu, K.; Fukushi, M.; Irie, T.; Koyanagi, Y.; Sauter, D.; Gifford, R.J.; Nakagawa, S.; Sato, K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020, 32, 108185. [Google Scholar] [CrossRef]
- Graham, B.S.; Anderson, L.J. Challenges and opportunities for respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 2013, 372, 391–404. [Google Scholar]
- Hijano, D.R.; Vu, L.D.; Kauvar, L.M.; Tripp, R.A.; Polack, F.P.; Cormier, S.A. Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front. Immunol. 2019, 10, 566. [Google Scholar] [CrossRef]
- Johnson, W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019, 17, 355–370. [Google Scholar] [CrossRef]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An envelope glycoprotein of the human endogenous retrovirus herv-w is expressed in the human placenta and fuses cells expressing the type d mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Benit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses 2014, 6, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Human endogenous retroviruses are ancient acquired elements still shaping innate immune responses. Front. Immunol. 2018, 9, 2039. [Google Scholar]
- Isbel, L.; Whitelaw, E. Endogenous retroviruses in mammals: An emerging picture of how ERVs modify expression of adjacent genes. BioEssays 2012, 34, 734–738. [Google Scholar] [CrossRef]
- Rolland, A.; Jouvin-Marche, E.; Viret, C.; Faure, M.; Perron, H.; Marche, P.N. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 2006, 76, 7636–7644. [Google Scholar] [CrossRef]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef]
- Mu, X.; Ahmad, S.; Hur, S. Endogenous retroelements and the host innate immune sensors. Adv. Immunol. 2016, 132, 47–69. [Google Scholar]
- Madeira, A.; Burgelin, I.; Perron, H.; Curtin, F.; Lang, A.B.; Faucard, R. MSRV envelope protein is a potent, endogenous and pathogenic agonist of human toll-like receptor 4: Relevance of GNbAC1 in multiple sclerosis treatment. J. Neuroimmunol. 2016, 291, 29–38. [Google Scholar] [CrossRef]
- Yu, P. The potential role of retroviruses in autoimmunity. Immunol. Rev. 2016, 269, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Holder, B.S.; Tower, C.L.; Forbes, K.; Mulla, M.J.; Aplin, J.D.; Abrahams, V.M. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology 2012, 136, 184–191. [Google Scholar] [PubMed]
- Hummel, J.; Kämmerer, U.; Müller, N.; Avota, E.; Schneider-Schaulies, S. Human endogenous retrovirus envelope proteins target dendritic cells to suppress T-cell activation. Eur. J. Immunol. 2015, 45, 1748–1759. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, É.; Lafond, J.; LeDuc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Rodriguez-Martin, E.; Ramos-Mozo, P.; Ortega-Madueño, I.; Dominguez-Mozo, M.I.; Arias-Leal, A.; García-Martínez, M.Á.; Casanova, I.; Galan, V.; Arroyo, R.; et al. Syncytin-1/HERV-W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur. J. Immunol. 2020, 50, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.A.; Rabbone, I.; Tinti, D.; Galliano, I.; Trada, M.; Daprà, V.; Cerutti, F.; Bergallo, M. Enhanced expression of human endogenous retroviruses in new-onset type 1 diabetes: Pathogenetic and therapeutic implications. Autoimmunity 2020, 53, 283–288. [Google Scholar]
- Tovo, P.A.; Monti, G.; Daprà, V.; Montanari, P.; Calvi, C.; Alliaudi, C.; Sardo, A.; Galliano, I.; Bergallo, M. Enhanced expression of endogenous retroviruses and of TRIM28 and SETDB1 in children with food allergy. Clin. Transl. Allergy 2022, 12, e12124. [Google Scholar]
- Ruprecht, K.; Obojes, K.; Wengel, V.; Gronen, F.; Kim, K.S.; Perron, H.; Schneider-Schaulies, J.; Rieckmann, P. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: Implications for multiple sclerosis. J. Neurovirol. 2006, 12, 65–71. [Google Scholar] [CrossRef]
- Brudek, T.; Lühdorf, P.; Christensen, T.; Hansen, H.J.; Møller-Larsen, A. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV-6 and VZV. J. Neuroimmunol. 2007, 187, 147–155. [Google Scholar]
- Tai, A.K.; Luka, J.; Ablashi, D.; Huber, B.T. HHV-6A Infection induces expression of HERV-K18-encoded superantigen. J. Clin. Virol. 2009, 46, 47–48. [Google Scholar] [CrossRef]
- Hsiao, F.C.; Tai, A.K.; Deglon, A.; Sutkowski, N.; Longnecker, R.; Huber, B.T. EBV LMP-2A employs a novel mechanism to transactivate the HERV-K18 superantigen through its ITAM. Virology 2009, 385, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Poddighe, L.; Mei, A.; Uleri, E.; Sotgiu, S.; Serra, C.; Manetti, R.; Dolei, A. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PLoS ONE 2012, 7, e44991. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Yaiw, K.C.; Göttesdorfer, I.; Leib-Mösch, C.; Söderberg-Nauclér, C. Human cytomegalovirus (HCMV) induces human endogenous retrovirus (HERV) transcription. Retrovirology 2013, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Van der Kuyl, A.C. HIV infection and HERV expression: A review. Retrovirology 2012, 9, 6. [Google Scholar] [CrossRef]
- Vincendeau, M.; Göttesdorfer, I.; Schreml, J.M.; Wetie, A.G.; Mayer, J.; Greenwood, A.D.; Helfer, M.; Kramer, S.; Seifarth, W.; Hadian, K.; et al. Modulation of human endogenous retrovirus (HERV) transcription during persistent and de novo HIV-1 infection. Retrovirology 2015, 12, 27. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Wang, X.; Liu, Y.; Wang, M.; Zhu, F. HBV X Protein induces overexpression of HERV-W env through NF-kappaB in HepG2 cells. Virus Genes 2017, 53, 797–806. [Google Scholar] [CrossRef]
- Tovo, P.A.; Garazzino, S.; Daprà, V.; Alliaudi, C.; Silvestro, E.; Calvi, C.; Montanari, P.; Galliano, I.; Bergallo, M. Chronic HCV infection is associated with overexpression of human endogenous retroviruses that persists after drug-induced viral clearance. Int. J. Mol. Sci. 2020, 21, 3980. [Google Scholar] [CrossRef]
- Schmidt, N.; Domingues, P.; Golebiowski, F.; Patzina, C.; Tatham, M.H.; Hay, R.T.; Hale, B.G. An influenza virus-triggered SUMO switch orchestrates co-opted endogenous retroviruses to stimulate host antiviral immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 17399–17408. [Google Scholar] [CrossRef]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the pathogenic HERV-W envelope expression in T lymphocytes in association with the respiratory outcome of COVID-19 patients. EBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef]
- Marston, J.L.; Greenig, M.; Singh, M.; Bendall, M.L.; Duarte, R.R.R.; Feschotte, C.; Iñiguez, L.P.; Nixon, D.F. SARS-CoV-2 infection mediates differential expression of human endogenous retroviruses and long interspersed nuclear elements. JCI Insight 2021, 6, e147170. [Google Scholar] [CrossRef]
- Temerozo, J.R.; Fintelman-Rodrigues, N.; Dos Santos, M.C.; Hottz, E.D.; Sacramento, C.Q.; de Paula Dias da Silva, A.; Mandacaru, S.C.; Dos Santos Moraes, E.C.; Trugilho, M.R.O.; Gesto, J.S.M.; et al. Human endogenous retrovirus K in the respiratory tract is associated with COVID-19 physiopathology. Microbiome 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Manghera, M.; Ferguson-Parry, J.; Lin, R.; Douville, R.N. NF-κB and IRF1 induce endogenous retrovirus K expression via interferon-stimulated response elements in its 5′ long terminal repeat. J. Virol. 2016, 90, 9338–9349. [Google Scholar] [CrossRef] [PubMed]
- Srinivasachar Badarinarayan, S.; Sauter, D. Switching sides: How endogenous retroviruses protect us from viral infections. J. Virol. 2021, 95, e02299-20. [Google Scholar] [CrossRef]
- Morozov, V.A.; Dao Thi, V.L.; Denner, J. The transmembrane protein of the human endogenous retrovirus—K (HERV-K) modulates cytokine release and gene expression. PLoS ONE 2013, 8, e70399. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, C.; Harper, F.; Pierron, G.; Heidmann, T.; Dewannieux, M. The HERV-K human endogenous retrovirus envelope protein antagonizes Tetherin antiviral activity. J. Virol. 2014, 88, 13626–13637. [Google Scholar] [CrossRef]
- Tolosa, J.M.; Parsons, K.S.; Hansbro, P.M.; Smith, R.; Wark, P.A. The placental protein syncytin-1 impairs antiviral responses and exaggerates inflammatory responses to influenza. PLoS ONE 2015, 10, e0118629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.R.; Fredericks, W.J.; Jensen, D.E.; Speicher, D.W.; Huang, X.P.; Neilson, E.G.; Rauscher, F.J., 3rd. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996, 10, 2067–2078. [Google Scholar] [CrossRef]
- Schultz, D.C.; Ayyanathan, K.; Negorev, D.; Maul, G.G.; Rauscher, F.J., III. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Nisole, S.; Stoye, J.P.; Saïb, A. TRIM family proteins: Retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005, 3, 799–808. [Google Scholar] [CrossRef]
- Adoue, V.; Binet, B.; Malbec, A.; Fourquet, J.; Romagnoli, P.; van Meerwijk, J.P.M.; Amigorena, S.; Joffre, O.P. The histone methyltransferase SETDB1 controls T helper cell lineage integrity by repressing endogenous retroviruses. Immunity 2019, 50, 629–644.e8. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yu, J.; Chang, M.; Zhang, M.; Zhou, D.; Cammas, F.; Sun, S.C. TRIM28 mediates chromatin modifications at the TCRα enhancer and regulates the development of T and natural killer T cells. Proc. Natl. Acad. Sci. USA 2012, 109, 20083–20088. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, U.; Burbage, M.; Zueva, E.; Goudot, C.; Esnault, C.; Ye, M.; Carpier, J.M.; Burgdorf, N.; Hoyler, T.; Suarez, G.; et al. Critical role for TRIM28 and HP1β/γ in the epigenetic control of T cell metabolic reprograming and effector differentiation. Proc. Natl. Acad. Sci. USA 2019, 116, 25839–25849. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, P.; Jaworska, A.M.; Wlodarczyk, N.A.; Mackiewicz, A.A. Melanoma stem cell-like phenotype and significant suppression of immune response within a tumor are regulated by TRIM28 protein. Cancers 2020, 12, 2998. [Google Scholar] [CrossRef]
- Kamitani, S.; Ohbayashi, N.; Ikeda, O.; Togi, S.; Muromoto, R.; Sekine, Y.; Ohta, K.; Ishiyama, H.; Matsuda, T. KAP1 regulates type I interferon/STAT1-mediated IRF-1 gene expression. Biochem. Biophys. Res. Commun. 2008, 370, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Deng, H.; Li, X.; Wu, X.; Tang, Q.; Chang, T.H.; Peng, H.; Rauscher, F.J., 3rd; Ozato, K.; Zhu, F. Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7. J. Immunol. 2011, 187, 4754–4763. [Google Scholar] [CrossRef]
- Cuellar, T.L.; Herzner, A.M.; Zhang, X.; Goyal, Y.; Watanabe, C.; Friedman, B.A.; Janakiraman, V.; Durinck, S.; Stinson, J.; Arnott, D. Silencing of retrotransposons by SETDB1 inhibits the interferon response in acute myeloid leukemia. J. Cell Biol. 2017, 216, 3535–3549. [Google Scholar] [CrossRef] [Green Version]
- Bergallo, M.; Galliano, I.; Pirra, A.; Daprà, V.; Licciardi, F.; Montanari, P.; Coscia, A.; Bertino, E.; Tovo, P.-A. Transcriptional activity of human endogenous retriviruses is higher at birth in inversed correlation with gestationale age. Infect. Genet. Evol. 2019, 68, 273–279. [Google Scholar] [CrossRef]
- Balestrieri, E.; Cipriani, C.; Matteucci, C.; Benvenuto, A.; Coniglio, A.; Argaw-Denboba, A.; Toschi, N.; Bucci, I.; Miele, M.T.; Grelli, S.; et al. Children with autism spectrum disorder and their mothers share abnormal expression of selected endogenous retroviruses families and cytokines. Front. Immunol. 2019, 10, 2244. [Google Scholar] [CrossRef]
- Tovo, P.A.; Davico, C.; Marcotulli, D.; Vitiello, B.; Daprà, V.; Calvi, C.; Montanari, P.; Carpino, A.; Galliano, I.; Bergallo, M. Enhanced expression of human endogenous retroviruses, TRIM28 and SETDB1 in autism spectrum disorder. Int. J. Mol. Sci. 2022, 23, 5964. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgenb, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- NIH. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules. 2019. Available online: https://osp.od.nih.gov/wp-content/uploads/2019_NIH_Guidelines.htm (accessed on 25 April 2019).
- WHO. Laboratory Biosafety Guidance Related to Coronavirus Disease (COVID-19): Interim Guidance. 2020. Available online: https://www.who.int/publications/i/item/laboratory-biosafety-guidance-related-to-coronavirus-disease-(covid-19) (accessed on 13 May 2020).
- Ioannidis, I.; McNally, B.; Willette, M.; Peeples, M.E.; Chaussabel, D.; Durbin, J.E.; Ramilo, O.; Mejias, A.; Flaño. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J. Virol. 2012, 86, 5422–5436. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Lee, N.R.; Lee, N.J.; Lee, J.K.; Quan, F.S.; Inn, K.S. Human respiratory syncytial virus NS 1 targets TRIM25 to suppress RIG-I ubiquitination and subsequent RIG-I-mediated antiviral signaling. Viruses 2018, 10, 10. [Google Scholar] [CrossRef]
- Ling, Z.; Tran, K.C.; Teng, M.N. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 2009, 83, 3734–3742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.; Zan, Y.; Du, N.; Yang, Y.; Tien, P. Human respiratory syncytial virus infection is inhibited by IFN-induced transmembrane proteins. J. Gen. Virol. 2015, 96, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Spann, K.M.; Tran, K.C.; Chi, B.; Rabin, R.L.; Collins, P.L. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 2004, 78, 4363.e9. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, P.; Tang, Y.; Pan, Z.; Zhao, D. Respiratory syncytial virus nonstructural proteins upregulate SOCS1 and SOCS3 in the different manner from endogenous IFN signaling. J. Immunol. Res. 2015, 2015, 738547. [Google Scholar] [CrossRef] [Green Version]
- Melendi, G.A.; Coviello, S.; Bhat, N.; Zea-Hernandez, J.; Ferolla, F.M.; Polack, F.P. Breastfeeding is associated with the production of type I interferon in infants infected with influenza virus. Acta Paediatr. 2010, 99, 1517–1521. [Google Scholar] [CrossRef]
- Cormier, S.A.; Shrestha, B.; Saravia, J.; Lee, G.I.; Shen, L.; DeVincenzo, J.P.; Kim, Y.I.; You, D. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J. Virol. 2014, 88, 9350–9360. [Google Scholar] [CrossRef]
- Marr, N.; Wang, T.I.; Kam, S.H.; Hu, Y.S.; Sharma, A.A.; Lam, A.; Markowski, J.; Solimano, A.; Lavoie, P.M.; Turvey, S.E. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J. Immunol. 2014, 192, 948–957. [Google Scholar] [CrossRef]
- Corneli, H.M.; Zorc, J.J.; Mahajan, P.; Shaw, K.N.; Holubkov, R.; Reeves, S.D.; Ruddy, R.M.; Malik, B.; Nelson, K.A.; Bregstein, J.S.; et al. Bronchiolitis study group of the pediatric emergency care applied research network (pecarn). A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N. Engl. J. Med. 2007, 357, 331–339. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Bialy, L.M.; Vandermeer, B.; Tjosvold, L.; Plint, A.C.; Patel, H.; Johnson, D.; Klassen, T.P.; Hartling, L. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst. Rev. 2013, 6, CD004878. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. American Academy of Pediatrics. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence. Bronchiolitis in Children: Diagnosis and Management. In NICE Guidelines; 2015. London, United Kingdom. Available online: https://www.nice.org.uk/guidance/ng9 (accessed on 21 July 2022).
- Cutrera, R.; Baraldi, E.; Indinnimeo, L.; Miraglia Del Giudice, M.; Piacentini, G.; Scaglione, F.; Ullmann, N.; Moschino, L.; Galdo, F.; Duse, M. Management of acute respiratory diseases in the pediatric population: The role of oral corticosteroids. Ital. J. Pediatr. 2017, 43, 31. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Lera, E.; Peremiquel-Trillas, P.; Martínez, L.; Barceló, I.; Andrés, C.; Rodrigo-Pendás, J.Á.; Antón, A.; Rodrigo, C. Management of hospitalized respiratory syncytial virus bronchiolitis in the pediatric ward in Spain: Assessing the impact of a new clinical practice protocol. Paediatr. Drugs 2022, 24, 63–71. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef]
- Finney, L.J.; Glanville, N.; Farne, H.; Aniscenko, J.; Fenwick, P.; Kemp, S.V.; Trujillo-Torralbo, M.B.; Loo, S.L.; Calderazzo, M.A.; Wedzicha, J.A.; et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J. Allergy Clin. Immunol. 2021, 147, 510–519.e5. [Google Scholar] [CrossRef]
- Hall, C.B.; Powell, K.R.; MacDonald, N.E.; Gala, C.; Menegus, M.E.; Suffin, S.C.; Cohen, H.J. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 1986, 315, 77–81. [Google Scholar] [CrossRef]
- Mordstein, M.; Neugebauer, E.; Ditt, V.; Jessen, B.; Rieger, T.; Falcone, V.; Sorgeloos, F.; Ehl, S.; Mayer, D.; Kochs, G.; et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 2010, 84, 5670–5677. [Google Scholar] [CrossRef]
- Liu, P.; Jamaluddin, M.; Li, K.; Garofalo, R.P.; Casola, A.; Brasier, A.R. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007, 81, 1401–1411. [Google Scholar] [CrossRef]
- Okabayashi, T.; Kojima, T.; Masaki, T.; Yokota, S.; Imaizumi, T.; Tsutsumi, H.; Himi, T.; Fujii, N.; Sawada, N. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011, 160, 360–366. [Google Scholar] [CrossRef]
- Bakre, A.A.; Harcourt, J.L.; Haynes, L.M.; Anderson, L.J.; Tripp, R.A. The central conserved region (CCR) of respiratory syncytial virus (RSV) G protein modulates host miRNA expression and alters the cellular response to infection. Vaccines 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, L.; Benveniste, E. Viral exploitation of host SOCS protein functions. J. Virol. 2010, 85, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Werder, R.B.; Lynch, J.P.; Simpson, J.C.; Zhang, V.; Hodge, N.H.; Poh, M.; Forbes-Blom, E.; Kulis, C.; Smythe, M.L.; Upham, J.W.; et al. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN-λ production. Sci. Transl. Med. 2018, 10, eaao0052. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, A.; Galen, B.T.; Ueki, I.F.; Sun, Y.; Mulenos, A.; Osafo-Addo, A.; Clark, B.; Joerns, J.; Liu, W.; Nadel, J.A.; et al. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium. Mucosal Immunol. 2018, 11, 958–967. [Google Scholar] [CrossRef]
- Mo, S.; Tang, W.; Xie, J.; Chen, S.; Ren, L.; Zang, N.; Xie, X.; Deng, Y.; Gao, L.; Liu, E. Respiratory syncytial virus activates Rab5a to suppress IRF1-dependent IFN-λ production, subverting the antiviral defense of airway epithelial cells. J. Virol. 2021, 95, e02333-20. [Google Scholar] [CrossRef]
- Salka, K.; Arroyo, M.; Chorvinsky, E.; Abutaleb, K.; Perez, G.F.; Wolf, S.; Xuchen, X.; Weinstock, J.; Gutierrez, M.J.; Pérez-Losada, M.; et al. Innate IFN-lambda responses to dsRNA in the human infant airway epithelium and clinical regulatory factors during viral respiratory infections in early life. Clin. Exp. Allergy 2020, 50, 1044–1054. [Google Scholar] [CrossRef]
- Hillyer, P.; Mane, V.P.; Chen, A.; Dos Santos, M.B.; Schramm, L.M.; Shepard, R.E.; Luongo, C.; Le Nouen, C.; Huang, L.; Yan, L.; et al. Respiratory syncytial virus infection induces a subset of types I and III interferons in human dendritic cells. Virology 2017, 504, 63–72. [Google Scholar] [CrossRef]
- Pierangeli, A.; Viscido, A.; Bitossi, C.; Frasca, F.; Gentile, M.; Oliveto, G.; Frassanito, A.; Nenna, R.; Midulla, F.; Scagnolari, C. Differential interferon gene expression in bronchiolitis caused by respiratory syncytial virus-A genotype ON1. Med. Microbiol. Immunol. 2020, 209, 23–28. [Google Scholar] [CrossRef]
- Fiegl, M.; Strasser-Wozak, E.; Geley, S.; Gsur, A.; Drach, J.; Kofler, R. Glucocorticoid-mediated immunomodulation: Hydrocortisone enhances immunosuppressive endogenous retroviral protein (p15E) expression in mouse immune cells. Clin. Exp. Immunol. 1995, 101, 259–264. [Google Scholar]
- Hsu, K.; Lee, Y.K.; Chew, A.; Chiu, S.; Lim, D.; Greenhalgh, D.G.; Cho, K. Inherently variable responses to glucocorticoid stress among endogenous retroviruses isolated from 23 mouse strains. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2594–2600. [Google Scholar]
- Pei, J.; Beri, N.R.; Zou, A.J.; Hubel, P.; Dorando, H.K.; Bergant, V.; Andrews, R.D.; Pan, J.; Andrews, J.M.; Sheehan, K.C.F.; et al. Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription. Cell Rep. 2021, 37, 109803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Ghildyal, R.; Hu, M.; Tran, K.C.; Starrs, L.M.; Mills, J.; Teng, M.N.; Jans, D.A. Respiratory syncytial virus matrix protein-chromatin association is key to transcriptional inhibition in infected cells. Cells 2021, 10, 2786. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Sechi, L.A.; Kelvin, D.J. Human endogenous retrovirus K (HML-2) in health and disease. Front. Microbiol. 2020, 11, 1690. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, P.; Li, S.; Zeng, J.; Tu, X.; Yan, Q.; Xiao, Z.; Pan, M.; Zhu, F. Syncytin-1, an endogenous retroviral protein, triggers the activation of CRP via TLR3 signal cascade in glial cells. Brain Behav. Immun. 2018, 67, 324–334. [Google Scholar] [CrossRef]
- Chen, S.; Bonifati, S.; Qin, Z.; St Gelais, C.; Kodigepalli, K.M.; Barrett, B.S.; Kim, S.H.; Antonucci, J.M.; Ladner, K.J.; Buzovetsky, O.; et al. SAMHD1 suppresses innate immune responses to viral infections and inflammatory stimuli by inhibiting the NF-kB. and interferon pathways. Proc. Natl. Acad. Sci. USA 2018, 115, E3798–E3807. [Google Scholar] [PubMed]
- Ptaschinski, C.; Mukherjee, S.; Moore, M.L.; Albert, M.; Helin, K.; Kunkel, S.L.; Lukacs, N.W. RSV-Induced H3K4 demethylase KDM5B leads to regulation of dendritic cell-derived innate cytokines and exacerbates pathogenesis in vivo. PLoS Pathog. 2015, 11, e1004978. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Scheltema, N.M.; Qi, C.; Vedder, R.; Klein, L.B.C.; Nibbelke, E.E.; van der Ent, C.K.; Bont, L.J.; Koppelman, G.H. Infant RSV immunoprophylaxis changes nasal epithelial DNA methylation at 6 years of age. Pediatr. Pulmonol. 2021, 56, 3822–3831. [Google Scholar] [CrossRef]
- Van Tol, S.; Hage, A.; Giraldo, M.I.; Bharaj, P.; Rajsbaum, R. The TRIMendous role of TRIMs in virus-host interactions. Vaccines 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, D.; Dasso, M. Modification in reverse: The SUMO proteases. Trends Biochem. Sci. 2007, 32, 286–295. [Google Scholar] [CrossRef]
- Zheng, Q.; Cao, Y.; Chen, Y.; Wang, J.; Fan, Q.; Huang, X.; Wang, Y.; Wang, T.; Wang, X.; Ma, J.; et al. Senp2 regulates adipose lipid storage by de-SUMOylation of Setdb1. J. Mol. Cell Biol. 2018, 10, 258–266. [Google Scholar] [CrossRef]


| Name | Primer/Probe | Sequence |
|---|---|---|
| Type I interferon stimulated genes | ||
| IFI27 | Forward | TGTCATTGCGAGGTTCTACTAGCT |
| Reverse | CCCCTGGCATGGTTCTCTT | |
| Probe | 6FAM-CCTGCCCCTCGCCCTGCA-TAMRA | |
| IFI44L | Forward | GTGACTGGCCAAGCCGTAGT |
| Reverse | CACACAACATAAATGGCAGAGATTT | |
| Probe | 6FAM-TCTGATATCACCAGCATAACCGAGCGG-TAMRA | |
| IFIT1 | Forward | TGGCTGACTTCACCTAGCTCACT |
| Reverse | CATGGACTGGCCAGAACCA | |
| Probe | 6FAM-CGTAGCGCCACAGCCAGACTCCC-TAMRA | |
| ISG15 | Forward | TGGCGGGCAACGAATT |
| Reverse | GGGTGATCTGCGCCTTCA | |
| Probe | 6FAM-CCTGAGCAGCTCCATGTCGGTGTC-TAMRA | |
| RSAD2 | Forward | GAGGGCCAGATGAGACCAAA |
| Reverse | GTGAAGTGATAGTTGACGCTGGTT | |
| Probe | 6FAM-AGGACCCTCCTCTGCCCACCACC-TAMRA | |
| SIGLEC1 | Forward | AGGGAGACTGGGAAATGTAGTTTTTA |
| Reverse | ATTCCCAACAATGTCAAAAGTCTCA | |
| Probe | 6FAM-AGTCCAGAGGACATTTGGAATTGGAC-TAMRA | |
| Type III interferon | ||
| IFN-λ1 | Forward | GAGGCATCTGTCACCTTC |
| Reverse | GGTTGACGTTCTCAGACA | |
| Probe | 6FAM-ACCTCTTCCGCCTCCTCACG-BHQ1 | |
| IFN-λ2 | Forward | GCCACATAGCCCAGTTCAAG |
| Reverse | TCCTTCAGCAGAAGCGACTC | |
| Probe | 6FAM-CTGTCTCCACAGGAGCTGCAGGCC-BHQ1 | |
| IFN-λ3 | Forward | TCACCTTCAACCTCTTCC |
| Reverse | GAAGGGTCAGACACACAG | |
| Probe | 6FAM-TGGCAACACAATTCAGGTCTCG-BHQ1 | |
| IFN-λ4 | Forward | CCTTCTACAGGGAAGAGAC |
| Reverse | CTGGTAACCACACAAGGA | |
| Probe | 6FAM-CAGTTCTCCAGGAAGCCACGATA-BHQ1 | |
| HERV-K pol | Forward | CCACTGTAGAGCCTCCTAAACCC- |
| Reverse | TTGGTAGCGGCCACTGATTT | |
| Probe | 6FAM-CCCACACCGGTTTTTCTGTTTTCCAAGTTAA-TAMRA | |
| HERV-W pol | Forward | ACMTGGAYKRTYTTRCCCCAA |
| Reverse | GTAAATCATCCACMTAYYGAAGGAYMA | |
| Probe | 6FAM-TYAGGGATAGCCCYCATCTRTTTGGYCAGGCA-TAMRA | |
| HERV-H pol | Forward | TGGACTGTGCTGCCGCAA |
| Reverse | GAAGSTCATCAATATATTGAATAAGGTGAGA | |
| Probe | 6FAM- TTCAGGGACAGCCCTCGTTACTTCAGCCAAGCTC-TAMRA | |
| Syncytin 1 env | Forward | ACTTTGTCTCTTCCAGAATCG |
| Reverse | GCGGTAGATCTTAGTCTTGG | |
| Probe | 6FAM-TGCATCTTGGGCTCCAT-TAMRA | |
| Syncytin 2 env | Forward | GCCTGCAAATAGTCTTCTTT |
| Reverse | ATAGGGGCTATTCCCATTAG | |
| Probe | 6FAM- TGATATCCGCCAGAAACCTCCC-TAMRA | |
| TRIM28 | Forward | GCCTCTGTGTGAGACCTGTGTAGA |
| Reverse | CCAGTAGAGCGCACAGTATGGT | |
| Probe | 6FAM-CGCACCAGCGGGTGAAGTACACC-TAMRA | |
| SETDB1 | Forward | GCCGTGACTTCATAGAGGAGTATGT |
| Reverse | GCTGGCCACTCTTGAGCAGTA | |
| Probe | 6FAM-TGCCTACCCCAACCGCCCCAT-TAMRA |
| Patients (n = 37) | |
|---|---|
| Median age (IQR) | 0.2 yrs (0.1–0.3) |
| Males (%) | 15 (40.5%) |
| Comorbidities, n (%) | 10 (27%) |
| Mean interval (+ SD) from symptom onset and sampling | 7.0 days (5.5) |
| Increased inflammatory markers n (%) | 10 (27%) |
| Leukocytosis, n (%) * | 3 (13%) |
| Lymphopenia, n (%) ** | 8 (21.6%) |
| Steroid treatment, n (%) | 12 (32.4%) |
| Oxygen treatment (%) | 31 (83.8%) |
| RSV+ Patients | Healthy Children | ||||
|---|---|---|---|---|---|
| Group A1 | Group A2 | Group B1 | Group B2 | Group B3 | Group B4 |
| On steroid treatment | No steroid treatment | Tested for interferon signatures | Tested for pol genes of HERV-H, -K, and –W | Tested for env genes of syncytin 1 and syncytin 2 | Tested for TRIM28 and SETDB1 |
| n. 12 | n. 25 | n. 46 | n. 29 | n. 30 | n. 40 |
| 7 males, median age 0.29, IQR 0.17–2.25 years | 16 males, median age 0.17, IQR 0.11–3.5 years | 27 males, median age 0.7, IQR 0.2–2.1 years | 17 males, median age 3.3, IQR 2.3–4.1 years | 17 males, median age 1.9, IQR 1.1–3.8 years | 23 males, median age 3.3, IQR 2.0–3.8 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovo, P.-A.; Garazzino, S.; Savino, F.; Daprà, V.; Pruccoli, G.; Dini, M.; Filisetti, G.; Funiciello, E.; Galliano, I.; Bergallo, M. Expressions of Type I and III Interferons, Endogenous Retroviruses, TRIM28, and SETDB1 in Children with Respiratory Syncytial Virus Bronchiolitis. Curr. Issues Mol. Biol. 2023, 45, 1197-1217. https://doi.org/10.3390/cimb45020079
Tovo P-A, Garazzino S, Savino F, Daprà V, Pruccoli G, Dini M, Filisetti G, Funiciello E, Galliano I, Bergallo M. Expressions of Type I and III Interferons, Endogenous Retroviruses, TRIM28, and SETDB1 in Children with Respiratory Syncytial Virus Bronchiolitis. Current Issues in Molecular Biology. 2023; 45(2):1197-1217. https://doi.org/10.3390/cimb45020079
Chicago/Turabian StyleTovo, Pier-Angelo, Silvia Garazzino, Francesco Savino, Valentina Daprà, Giulia Pruccoli, Maddalena Dini, Giacomo Filisetti, Elisa Funiciello, Ilaria Galliano, and Massimiliano Bergallo. 2023. "Expressions of Type I and III Interferons, Endogenous Retroviruses, TRIM28, and SETDB1 in Children with Respiratory Syncytial Virus Bronchiolitis" Current Issues in Molecular Biology 45, no. 2: 1197-1217. https://doi.org/10.3390/cimb45020079
APA StyleTovo, P. -A., Garazzino, S., Savino, F., Daprà, V., Pruccoli, G., Dini, M., Filisetti, G., Funiciello, E., Galliano, I., & Bergallo, M. (2023). Expressions of Type I and III Interferons, Endogenous Retroviruses, TRIM28, and SETDB1 in Children with Respiratory Syncytial Virus Bronchiolitis. Current Issues in Molecular Biology, 45(2), 1197-1217. https://doi.org/10.3390/cimb45020079







