Ductal Hyperkeratinization and Acinar Renewal Abnormality: New Concepts on Pathogenesis of Meibomian Gland Dysfunction
Abstract
:1. Introduction
2. Physiology of MG
2.1. MG Stem Cells
2.2. Differentiation and Proliferation of Meibocytes
2.3. Regeneration of MGs
3. Meibography and Function of MGs
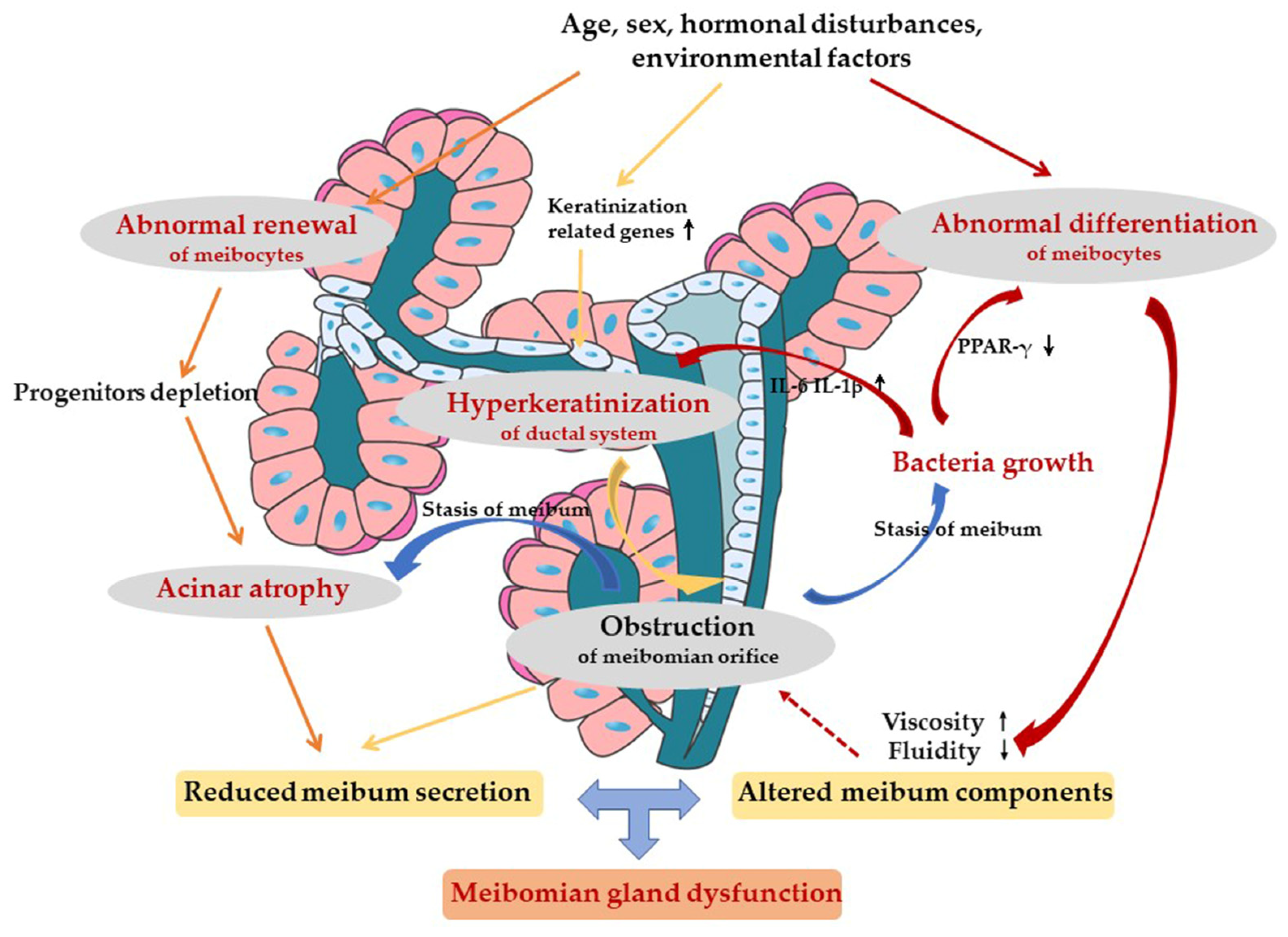
4. Pathological Mechanism of MGD
4.1. Ductal-Centric Theory
4.2. Meibocyte-Centric Theory
5. Endogenous and External Factors for MGD
6. Our Perspectives for MGD
7. Prospective Future Research and Therapeutic Strategies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butovich, I.A. Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 2017, 163, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, B.L.; Larke, J.R. Meibomian gland dysfunction: Some clinical, biochemical and physical observations. Ophthalmic Physiol. Opt. 1990, 10, 144–148. [Google Scholar] [CrossRef]
- Eom, Y.; Choi, K.E.; Kang, S.Y.; Lee, H.K.; Kim, H.M.; Song, J.S. Comparison of meibomian gland loss and expressed meibum grade between the upper and lower eyelids in patients with obstructive meibomian gland dysfunction. Cornea 2014, 33, 448–452. [Google Scholar] [CrossRef]
- Efron, N. 7-Meibomian gland dysfunction. In Contact Lens Complications, 4th ed.; Efron, N., Ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 78–91. [Google Scholar]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the asia dry eye society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Jester, J.V.; Parfitt, G.J.; Brown, D.J. Meibomian gland dysfunction: Hyperkeratinization or atrophy? BMC Ophthalmol. 2015, 15 (Suppl. 1), 156. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Parfitt, G.J.; Brown, D.J.; Jester, J.V. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp. Eye Res. 2017, 163, 37–45. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
- Knop, E.; Knop, N. Meibomian glands: Part IV. Functional interactions in the pathogenesis of meibomian gland dysfunction (MGD). Ophthalmologe 2009, 106, 980–987. [Google Scholar] [CrossRef]
- Fan, N.W.; Ho, T.C.; Lin, E.H.; Wu, C.W.; Chien, H.Y.; Tsao, Y.P. Pigment epithelium-derived factor peptide reverses mouse age-related meibomian gland atrophy. Exp. Eye Res. 2019, 185, 107678. [Google Scholar] [CrossRef]
- Xie, H.T.; Sullivan, D.A.; Chen, D.; Hatton, M.P.; Kam, W.R.; Liu, Y. Biomarkers for progenitor and differentiated epithelial cells in the human meibomian gland. Stem Cells Transl. Med. 2018, 7, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Gao, H.; Xie, H.T.; Liu, S.T.; Huang, Y.K.; Zhang, M.C. Hyperkeratinization and proinflammatory cytokine expression in meibomian glands induced by staphylococcus aureus. Investig. Ophthalmol. Vis. Sci. 2021, 62, 11. [Google Scholar] [CrossRef]
- Qu, J.Y.; Xiao, Y.T.; Zhang, Y.Y.; Xie, H.T.; Zhang, M.C. Hedgehog signaling pathway regulates the proliferation and differentiation of rat meibomian gland epithelial cells. Investig. Ophthalmol. Vis. Sci. 2021, 62, 33. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Wang, W.; Lin, P.; Huang, Y. Composition and diversity of bacterial community on the ocular surface of patients with meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4774–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nattis, A.; Perry, H.D.; Rosenberg, E.D.; Donnenfeld, E.D. Influence of bacterial burden on meibomian gland dysfunction and ocular surface disease. Clin. Ophthalmol. 2019, 13, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Chen, X.; Ma, Y.; Lin, X.; Yu, X.; He, S.; Luo, C.; Xu, W. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int. Ophthalmol. 2020, 40, 3049–3058. [Google Scholar] [CrossRef]
- Obata, H. Anatomy and histopathology of human meibomian gland. Cornea 2002, 21, S70–S74. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Garreis, F.; Paulsen, F. Pathophysiology of meibomian glands—An overview. Ocul. Immunol. Inflamm. 2021, 29, 803–810. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Madigan, M.C.; Stapleton, F.; Willcox, M.; Golebiowski, B. Human meibomian gland epithelial cell culture models: Current progress, challenges, and future directions. Ocul. Surf. 2022, 23, 96–113. [Google Scholar] [CrossRef]
- McCulley, J.P.; Shine, W.E. The lipid layer of tears: Dependent on meibomian gland function. Exp. Eye Res. 2004, 78, 361–365. [Google Scholar] [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.G.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. CLEAR—Anatomy and physiology of the anterior eye. Cont. Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Korb, D.R.; Blackie, C.A.; Knop, N. The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Dev. Ophthalmol. 2010, 45, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, X.; Xie, H.T.; Hatton, M.P.; Liu, X.; Liu, Y. Expression of extracellular matrix components in the meibomian gland. Front. Med. 2022, 9, 981610. [Google Scholar] [CrossRef] [PubMed]
- Olami, Y.; Zajicek, G.; Cogan, M.; Gnessin, H.; Pe’er, J. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Res. 2001, 33, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Treet, J.; Sun, T. Label-retaining cells (LRCs) are preferentially located in the ductal Epithelium of the meibomian gland: Implications on the mucocutaneous junctional (MCJ) Epithelium of the eyelid. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3781. [Google Scholar]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef]
- Maskin, S.L.; Testa, W.R. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br. J. Ophthalmol. 2018, 102, 59–68. [Google Scholar] [CrossRef]
- Parfitt, G.J.; Lewis, P.N.; Young, R.D.; Richardson, A.; Lyons, J.G.; Di Girolamo, N.; Jester, J.V. Renewal of the holocrine meibomian glands by label-retaining, unipotent epithelial progenitors. Stem Cell Rep. 2016, 7, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Tchegnon, E.; Liao, C.P.; Ghotbi, E.; Shipman, T.; Wang, Y.; McKay, R.M.; Le, L.Q. Epithelial stem cell homeostasis in Meibomian gland development, dysfunction, and dry eye disease. JCI Insight 2021, 6, 20. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, X.; Huang, A.J.; Reneker, L.W. Spontaneous acinar and ductal regrowth after meibomian gland atrophy induced by deletion of FGFR2 in a mouse model. Ocul. Surf. 2022, 26, 300–309. [Google Scholar] [CrossRef]
- Kim, S.W.; Xie, Y.; Nguyen, P.Q.; Bui, V.T.; Huynh, K.; Kang, J.S.; Brown, D.J.; Jester, J.V. PPARγ regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul. Surf. 2018, 16, 463–469. [Google Scholar] [CrossRef]
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Paugh, J.R.; Jester, J.V. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Nien, C.J.; Paugh, J.R.; Massei, S.; Wahlert, A.J.; Kao, W.W.; Jester, J.V. Age-related changes in the meibomian gland. Exp. Eye Res. 2009, 89, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Jun, I.; Choi, Y.J.; Kim, B.R.; Seo, K.Y.; Kim, T.I. Activation of ADRB2/PKA signaling pathway facilitates lipid synthesis in meibocytes, and beta-blocker glaucoma drug impedes PKA-induced lipid synthesis by inhibiting ADRB2. Int. J. Mol. Sci. 2022, 23, 9478. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.R.; Liu, Y.; Ding, J.; Sullivan, D.A. Do Cyclosporine A, an IL-1 receptor antagonist, uridine triphosphate, rebamipide, and/or bimatoprost regulate human meibomian gland epithelial cells? Investig. Ophthalmol. Vis. Sci. 2016, 57, 4287–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, I.; Kim, B.R.; Park, S.Y.; Lee, H.; Kim, J.; Kim, E.K.; Seo, K.Y.; Kim, T.I. interleukin-4 stimulates lipogenesis in meibocytes by activating the STAT6/PPARγ signaling pathway. Ocul. Surf. 2020, 18, 575–582. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Z.; Wan, L.; Xue, J.; Chen, C.; Yu, B.; Zhang, Z.; Yang, L.; Xie, L. Periplocin ameliorates mouse age-related meibomian gland dysfunction through up-regulation of Na/K-ATPase via SRC pathway. Biomed. Pharmacother. 2022, 146, 112487. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, H.; Zhang, B.; Zhang, Z.; Chen, R.; Yang, L. Inhibition of Gli1 suppressed hyperglycemia-induced meibomian gland dysfunction by promoting pparγ expression. Biomed. Pharmacother. 2022, 151, 113109. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.; Varmaghani, M.; Zarei-Ghanavati, S.; Heravian Shandiz, J.; Azimi Khorasani, A. Global prevalence of meibomian gland dysfunction: A systematic review and meta-analysis. Ocul. Immunol. Inflamm. 2021, 29, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kam, W.R.; Liu, Y.; Ding, J.; Li, Y.; Sullivan, D.A. Comparative influence of differentiation and proliferation on gene expression in human meibomian gland epithelial cells. Exp. Eye Res. 2021, 205, 108452. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Inoue, K. Clinic-based study on meibomian gland dysfunction in Japan. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Randon, M.; Aragno, V.; Abbas, R.; Liang, H.; Labbé, A.; Baudouin, C. In vivo confocal microscopy classification in the diagnosis of meibomian gland dysfunction. Eye 2019, 33, 754–760. [Google Scholar] [CrossRef]
- Yokoi, N.; Komuro, A.; Yamada, H.; Maruyama, K.; Kinoshita, S. A newly developed video-meibography system featuring a newly designed probe. Jpn. J. Ophthalmol. 2007, 51, 53–56. [Google Scholar] [CrossRef]
- Robin, J.B.; Jester, J.V.; Nobe, J.; Nicolaides, N.; Smith, R.E. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology 1985, 92, 1423–1426. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Amano, S. A newly developed noninvasive and mobile pen-shaped meibography system. Cornea 2013, 32, 242–247. [Google Scholar] [CrossRef]
- Srinivasan, S.; Menzies, K.; Sorbara, L.; Jones, L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom. Vis. Sci. 2012, 89, 788–794. [Google Scholar] [CrossRef]
- Ifrah, R.; Quevedo, L.; Gantz, L. Repeatability and reproducibility of Cobra HD fundus camera meibography in young adults with and without symptoms of dry eye. Ophthalmic Physiol. Opt. 2023, 43, 183–194. [Google Scholar] [CrossRef]
- Gulmez Sevim, D.; Gumus, K.; Unlu, M. Reliable, noncontact imaging tool for the evaluation of meibomian gland function: Sirius meibography. Eye Contact Lens 2020, 46 (Suppl. 2), S135–S140. [Google Scholar] [CrossRef]
- Ngo, W.; Srinivasan, S.; Schulze, M.; Jones, L. Repeatability of grading meibomian gland dropout using two infrared systems. Optom. Vis. Sci. 2014, 91, 658–667. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Q.; Luo, Z.; Li, S.; Wang, B.; Zhong, J.; Peng, L.; Xiao, P.; Yuan, J. Quantitative analysis of morphological and functional features in meibography for meibomian gland dysfunction: Diagnosis and grading. eClinicalMedicine 2021, 40, 101132. [Google Scholar] [CrossRef] [PubMed]
- Den, S.; Shimizu, K.; Ikeda, T.; Tsubota, K.; Shimmura, S.; Shimazaki, J. Association between meibomian gland changes and aging, sex, or tear function. Cornea 2006, 25, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Amano, S. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea 2010, 29, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Kuchiba, A.; Yamaguchi, T.; Amano, S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009, 116, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Balal, S.; Ahmad, S. Meibomian gland dysfunction, dropout and distress: Emerging therapies. Eye 2020, 34, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Rastad, H.; Emamian, M.H.; Fotouhi, A. Meibomian gland dysfunction and its determinants in Iranian adults: A population-based study. Cont. Lens Anterior Eye 2017, 40, 213–216. [Google Scholar] [CrossRef]
- Liu, S.; Richards, S.M.; Lo, K.; Hatton, M.; Fay, A.; Sullivan, D.A. Changes in gene expression in human meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2727–2740. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, G.J.; Xie, Y.; Geyfman, M.; Brown, D.J.; Jester, J.V. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging 2013, 5, 825–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korb, D.R.; Henriquez, A.S. Meibomian gland dysfunction and contact lens intolerance. J. Am. Optom. Assoc. 1980, 51, 243–251. [Google Scholar] [PubMed]
- Gupta, P.K.; Periman, L.M.; Lain, E.; Donnenfeld, E.; Hovanesian, J.; Kim, T.; Trattler, W.; Yeu, E.; Holland, E. Meibomian gland dysfunction: A dermatological perspective on pathogenesis and treatment outlook. Clin. Ophthalmol. 2021, 15, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.K.; Huang, Y.K.; Liu, X.; Zhang, M.C.; Xie, H.T. Organotypic culture of mouse meibomian gland: A novel model to study meibomian gland dysfunction in vitro. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villani, E.; Canton, V.; Magnani, F.; Viola, F.; Nucci, P.; Ratiglia, R. The aging Meibomian gland: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4735–4740. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, G.J.; Brown, D.J.; Jester, J.V. Transcriptome analysis of aging mouse meibomian glands. Mol. Vis. 2016, 22, 518–527. [Google Scholar]
- Sullivan, D.A.; Sullivan, B.D.; Ullman, M.D.; Rocha, E.M.; Krenzer, K.L.; Cermak, J.M.; Toda, I.; Doane, M.G.; Evans, J.E.; Wickham, L.A. Androgen influence on the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3732–3742. [Google Scholar]
- Khandelwal, P.; Liu, S.; Sullivan, D.A. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol. Vis. 2012, 18, 1055–1067. [Google Scholar]
- Song, F.; Hao, S.; Gu, Y.; Yao, K.; Fu, Q. Research advances in pathogenic mechanisms underlying air pollution-induced ocular surface diseases. Adv. Ophthalmol. Pract. Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Wang, L.; Deng, Y. Malassezia species may play potential roles in the pathogenesis of meibomian gland dysfunction. Med. Hypotheses 2020, 144, 110137. [Google Scholar] [CrossRef]
- Dougherty, J.M.; McCulley, J.P. Bacterial lipases and chronic blepharitis. Investig. Ophthalmol. Vis. Sci. 1986, 27, 486–491. [Google Scholar]
- Machalińska, A.; Zakrzewska, A.; Adamek, B.; Safranow, K.; Wiszniewska, B.; Parafiniuk, M.; Machaliński, B. Comparison of morphological and functional meibomian gland characteristics between daily contact lens wearers and nonwearers. Cornea 2015, 34, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, H.; Xie, H.T.; Xu, K.K.; Shi, B.J.; Huang, Y.K. Changes in meibum lipid composition with ocular demodex infestation. Transl. Vis. Sci Technol. 2021, 10, 6. [Google Scholar] [CrossRef]
- Lam, P.Y.; Shih, K.C.; Fong, P.Y.; Chan, T.C.Y.; Ng, A.L.; Jhanji, V.; Tong, L. A review on evidence-based treatments for meibomian gland dysfunction. Eye Contact Lens 2020, 46, 3–16. [Google Scholar] [CrossRef]
- Thode, A.R.; Latkany, R.A.J.D. Current and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD). Drugs. 2015, 75, 1177–1185. [Google Scholar] [CrossRef]
- Kobayashi, A.; Ide, T.; Fukumoto, T.; Miki, E.; Tsubota, K.; Toda, I. Effects of a new eyelid shampoo on lid hygiene and eyelash length in patients with meibomian gland dysfunction: A comparative open study. J. Ophthalmol. 2016, 2016, 4292570. [Google Scholar] [CrossRef]
- Yeo, S.; Tan, J.H.; Acharya, U.R.; Sudarshan, V.K.; Tong, L. Longitudinal changes in tear evaporation rates after eyelid warming therapies in meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1974–1981. [Google Scholar] [CrossRef] [Green Version]
- Benitez Del Castillo, J.M.; Kaercher, T.; Mansour, K.; Wylegala, E.; Dua, H. Evaluation of the efficacy, safety, and acceptability of an eyelid warming device for the treatment of meibomian gland dysfunction. Clin. Ophthalmol. 2014, 8, 2019–2027. [Google Scholar] [CrossRef] [Green Version]
- Arita, R.; Morishige, N.; Shirakawa, R.; Sato, Y.; Amano, S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul. Surf. 2015, 13, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, S.J.; Gaster, R.N.; Barbarino, S.C.; Cunningham, D.N. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin. Ophthalmol. 2017, 11, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghai, Z.H.; Kode, A.; Saslow, J.G.; Nakhla, T.; Farhath, S.; Stahl, G.E.; Eydelman, R.; Strande, L.; Leone, P.; Rahman, I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007, 62, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughty, M.J. On the prescribing of oral doxycycline or minocycline by UK optometrists as part of management of chronic meibomian gland dysfunction (MGD). Cont. Lens Anterior Eye 2016, 39, 2–8. [Google Scholar] [CrossRef]
- Haque, R.M.; Torkildsen, G.L.; Brubaker, K.; Zink, R.C.; Kowalski, R.P.; Mah, F.S.; Pflugfelder, S.C. Multicenter open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea 2010, 29, 871–877. [Google Scholar] [CrossRef]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kim, S.H.; McKay, J.W. Topical azithromycin therapy for meibomian gland dysfunction: Clinical response and lipid alterations. Cornea 2010, 29, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balci, O.; Gulkilik, G. Assessment of efficacy of topical azithromycin 1.5 per cent ophthalmic solution for the treatment of meibomian gland dysfunction. Clin. Exp. Optom. 2018, 101, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Steven, P.; Augustin, A.J.; Geerling, G.; Kaercher, T.; Kretz, F.; Kunert, K.; Menzel-Severing, J.; Schrage, N.; Schrems, W.; Krösser, S.; et al. Semifluorinated alkane eye drops for treatment of dry eye disease due to meibomian gland disease. J. Ocul. Pharmacol. Ther. 2017, 33, 678–685. [Google Scholar] [CrossRef]
- Schmidl, D.; Bata, A.M.; Szegedi, S.; Aranha Dos Santos, V.; Stegmann, H.; Fondi, K.; Krösser, S.; Werkmeister, R.M.; Schmetterer, L.; Garhöfer, G. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: A randomized controlled clinical trial. J. Ocul. Pharmacol. Ther. 2020, 36, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Delicado-Miralles, M.; Velasco, E.; Díaz-Tahoces, A.; Gallar, J.; Acosta, M.C.; Aracil-Marco, A. Deciphering the action of perfluorohexyloctane eye drops to reduce ocular discomfort and pain. Front. Med. 2021, 8, 709712. [Google Scholar] [CrossRef]
- Downie, L.E.; Watson, S.L.; Tan, J.; Stapleton, F.; Bosworth, C. A multicenter, double-masked, vehicle-controlled, randomized, parallel group clinical trial of AZR-MD-001 (AZR) in individuals with meibomian gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2021, 62, 1334. [Google Scholar]
- Beining, M.W.; Magnø, M.S.; Moschowits, E.; Olafsson, J.; Vehof, J.; Dartt, D.A.; Utheim, T.P. In-office thermal systems for the treatment of dry eye disease. Surv. Ophthalmol. 2022, 67, 1405–1418. [Google Scholar] [CrossRef]
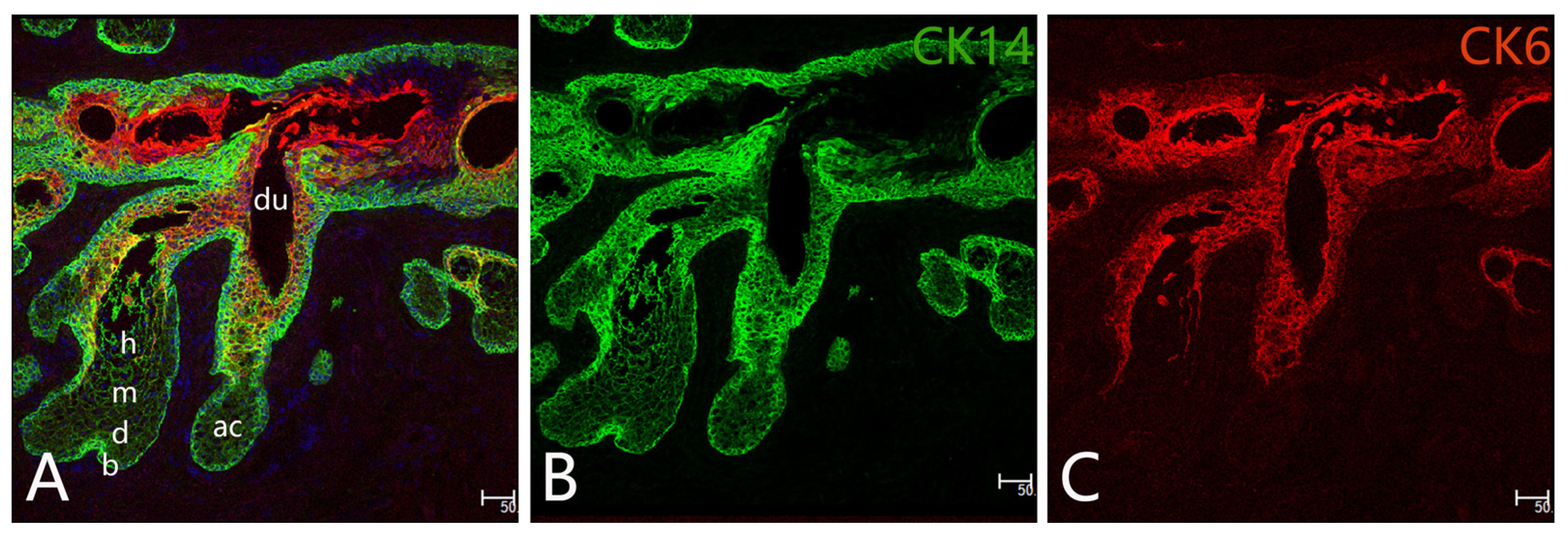

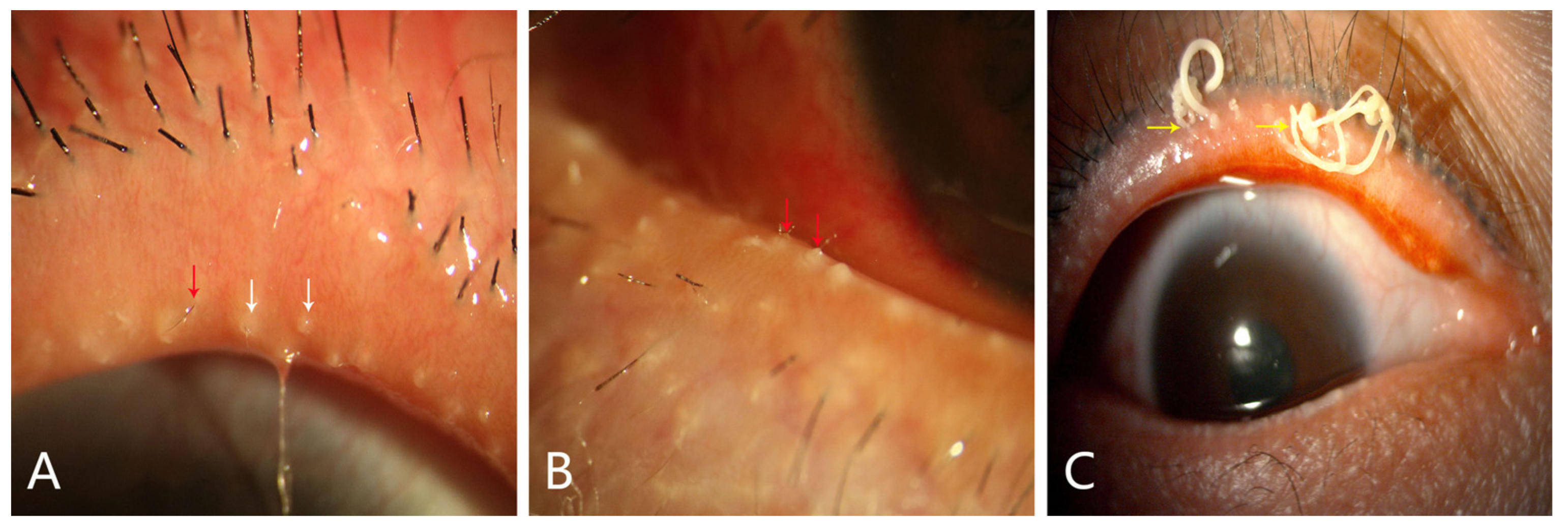
| Signaling Pathways | Experimental Model | Function |
|---|---|---|
| Hedgehog [16] | RMGECs | Proliferation and differentiation of acini |
| ADRB2/PKA [38] | HMGECs | Meibocyte differentiation and lipid synthesis |
| AKT [39] | HMGECs | Promote cell survival |
| STAT6/PPAR γ [40] | HMGECs | Meibocyte differentiation and lipid synthesis |
| SRC [41] | MOUSE | Meibocyte cell renewal and lipid synthesis |
| Shh [42] | MOUSE | Meibocyte differentiation and lipid synthesis |
| Author [Year] | Pathological Mechanism | Comments |
|---|---|---|
| Liu et al. [2011] [61] | Genes regulated | MGD is associated with the overexpression of keratinization-related genes |
| Knop et al. [2011] [11] | Hyperkeratinization of the ductal system | MGD is mainly caused by the hyperkeratinization of the meibomian duct and orifice, accompanied with increased viscosity in the meibum |
| Parfitt et al. [2011] [62] | Aged-related acinar atrophy | MGD most likely results from glandular atrophy caused by the loss of meibocyte progenitors rather than ductal hyperkeratinization and gland obstruction |
| Jester et al. [2015] [9] | Gland atrophy | Consistent results indicate MGD without evidence of hyperkeratinization, implying that gland atrophy may be a major cause |
| Hwang et al. [2017] [10] | Meibocyte differentiation and renewal of acinar cells | PPAR-signaling-pathway-mediated abnormalities in meibocyte differentiation and renewal are the main cause of MGD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-L.; Peng, X.; Liu, Y.; Wang, J.-S.; Ye, Y.-F.; Xu, K.-K.; Qu, J.-Y.; Chen, H.; Xie, H.-T.; Zhang, M.-C. Ductal Hyperkeratinization and Acinar Renewal Abnormality: New Concepts on Pathogenesis of Meibomian Gland Dysfunction. Curr. Issues Mol. Biol. 2023, 45, 1889-1901. https://doi.org/10.3390/cimb45030122
Du Y-L, Peng X, Liu Y, Wang J-S, Ye Y-F, Xu K-K, Qu J-Y, Chen H, Xie H-T, Zhang M-C. Ductal Hyperkeratinization and Acinar Renewal Abnormality: New Concepts on Pathogenesis of Meibomian Gland Dysfunction. Current Issues in Molecular Biology. 2023; 45(3):1889-1901. https://doi.org/10.3390/cimb45030122
Chicago/Turabian StyleDu, Ya-Li, Xi Peng, Yang Liu, Jia-Song Wang, You-Fan Ye, Kang-Kang Xu, Jing-Yu Qu, Hua Chen, Hua-Tao Xie, and Ming-Chang Zhang. 2023. "Ductal Hyperkeratinization and Acinar Renewal Abnormality: New Concepts on Pathogenesis of Meibomian Gland Dysfunction" Current Issues in Molecular Biology 45, no. 3: 1889-1901. https://doi.org/10.3390/cimb45030122





