Acceleration of the Deamination of Cytosine through Photo-Crosslinking
Abstract
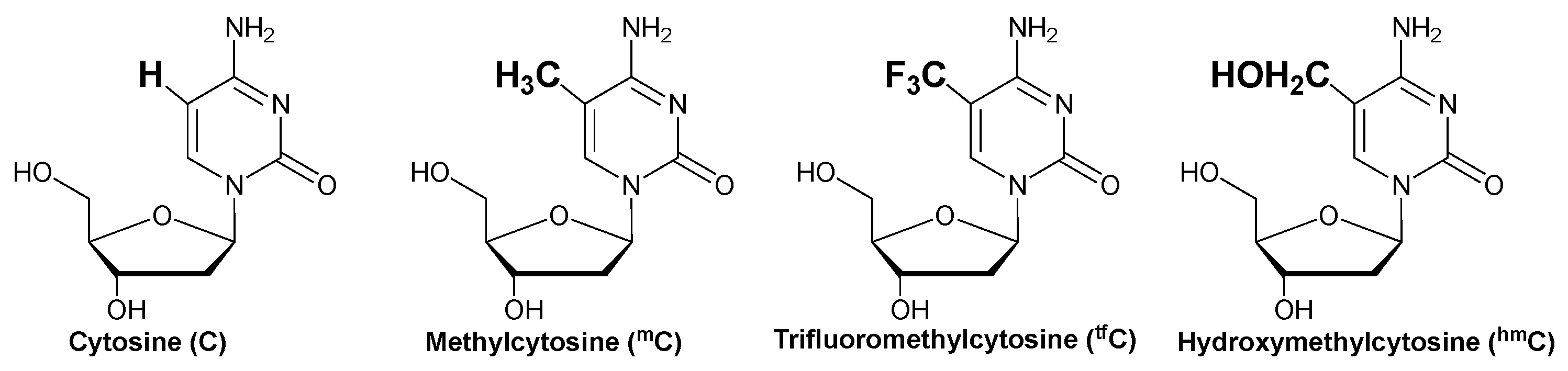
:1. Introduction
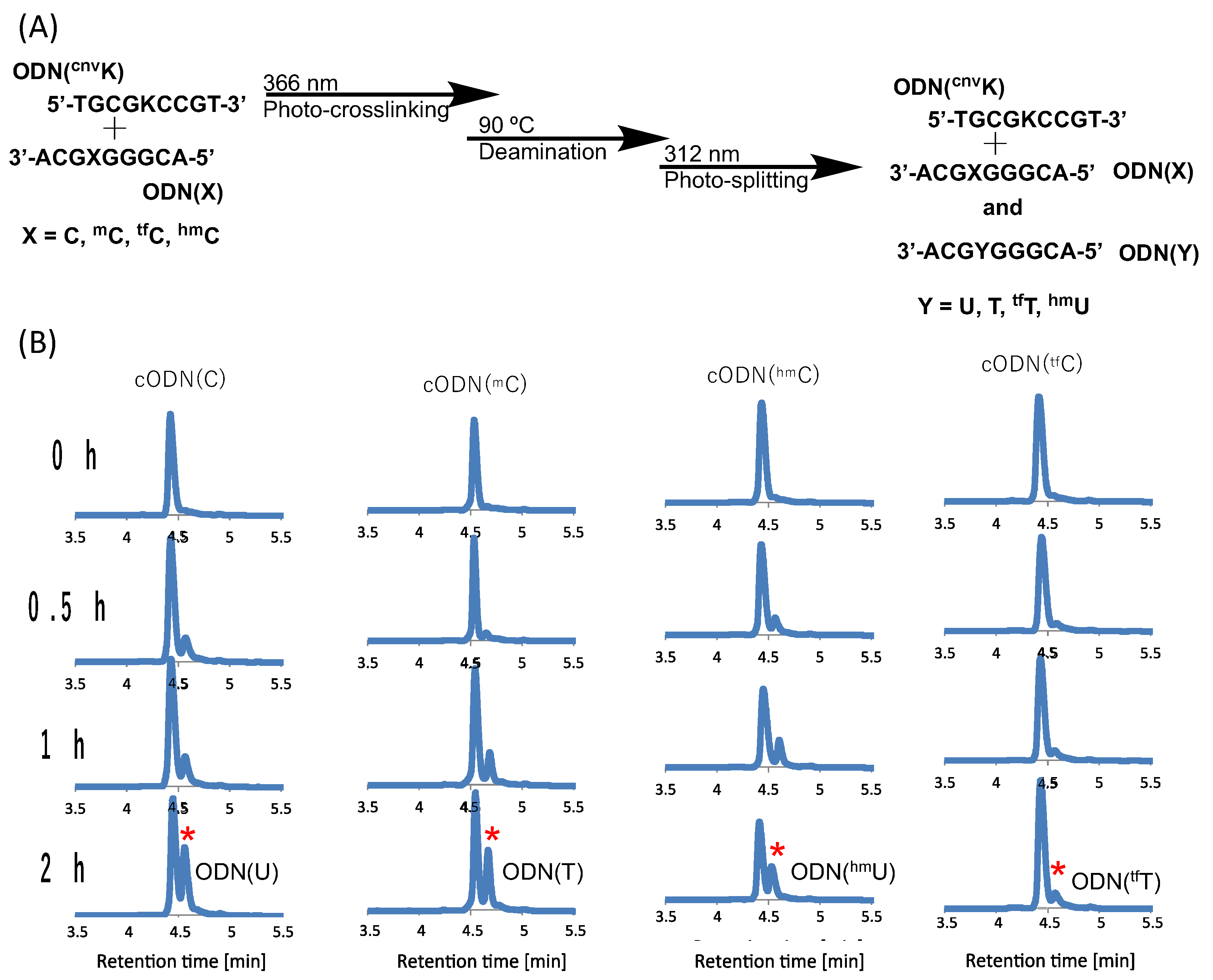
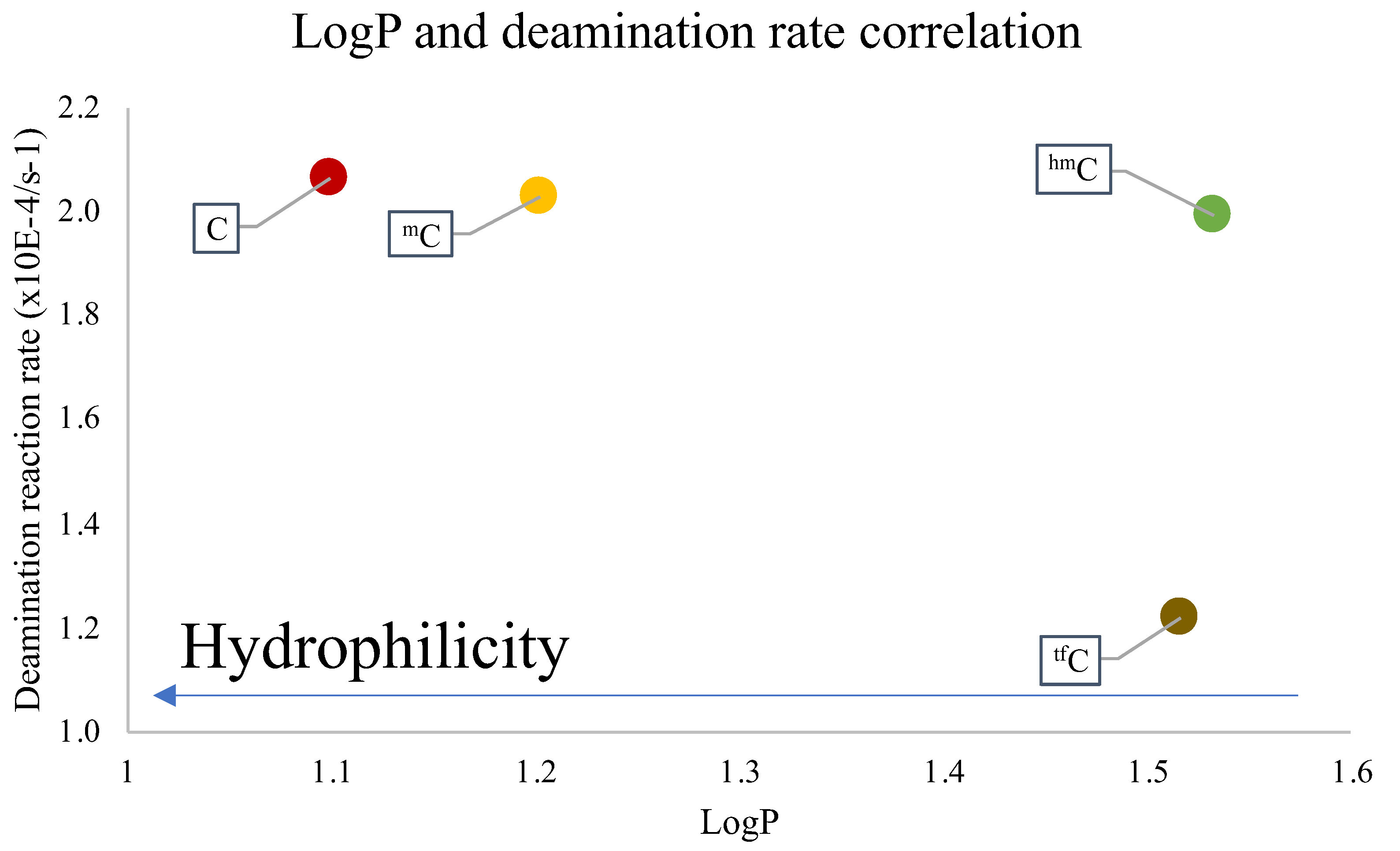
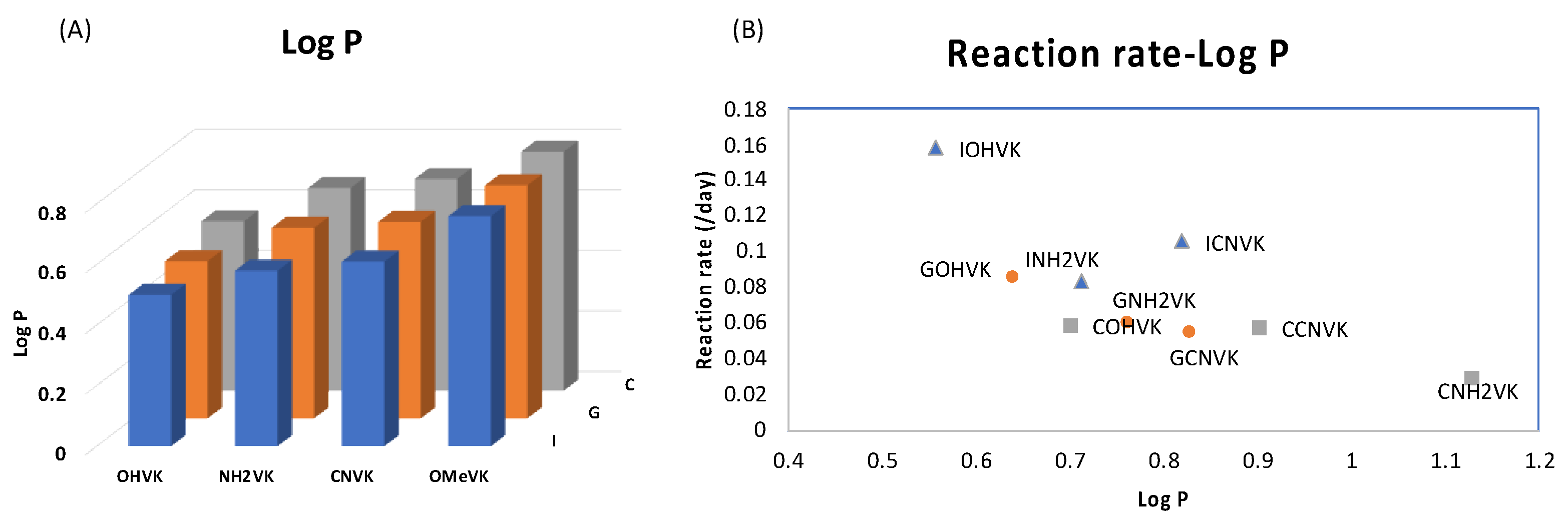
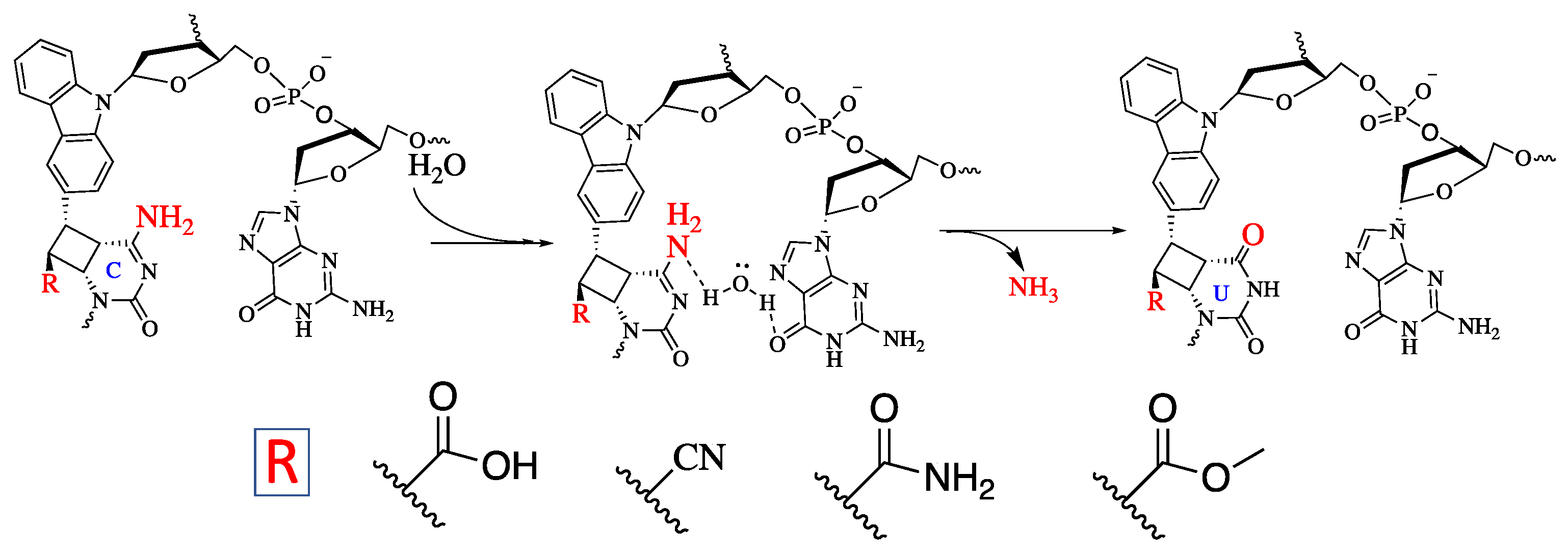
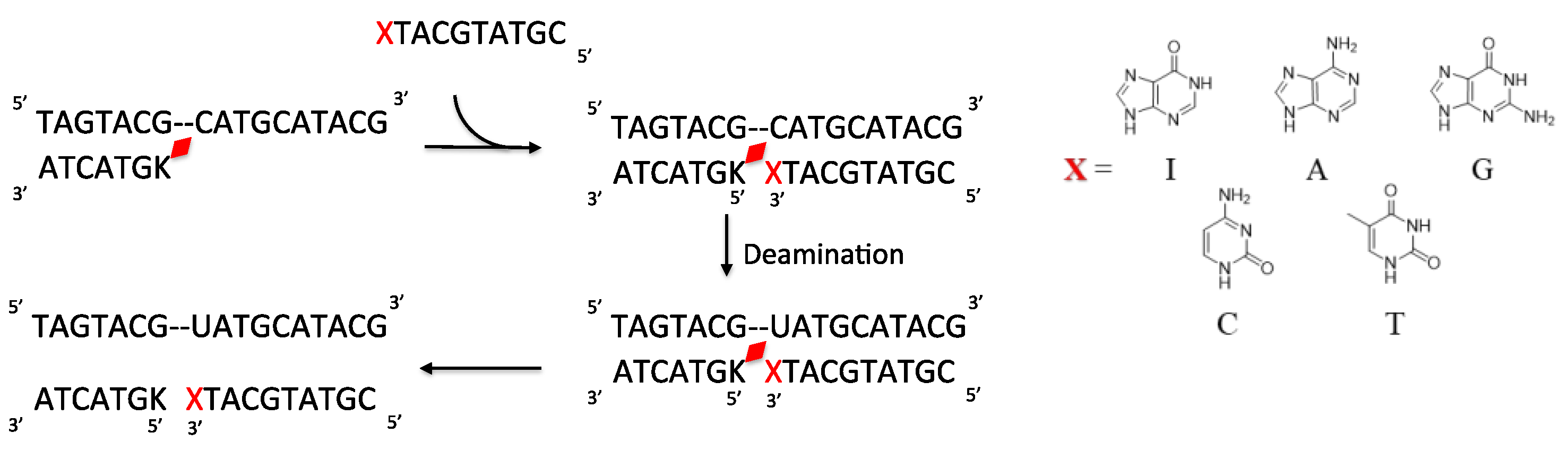
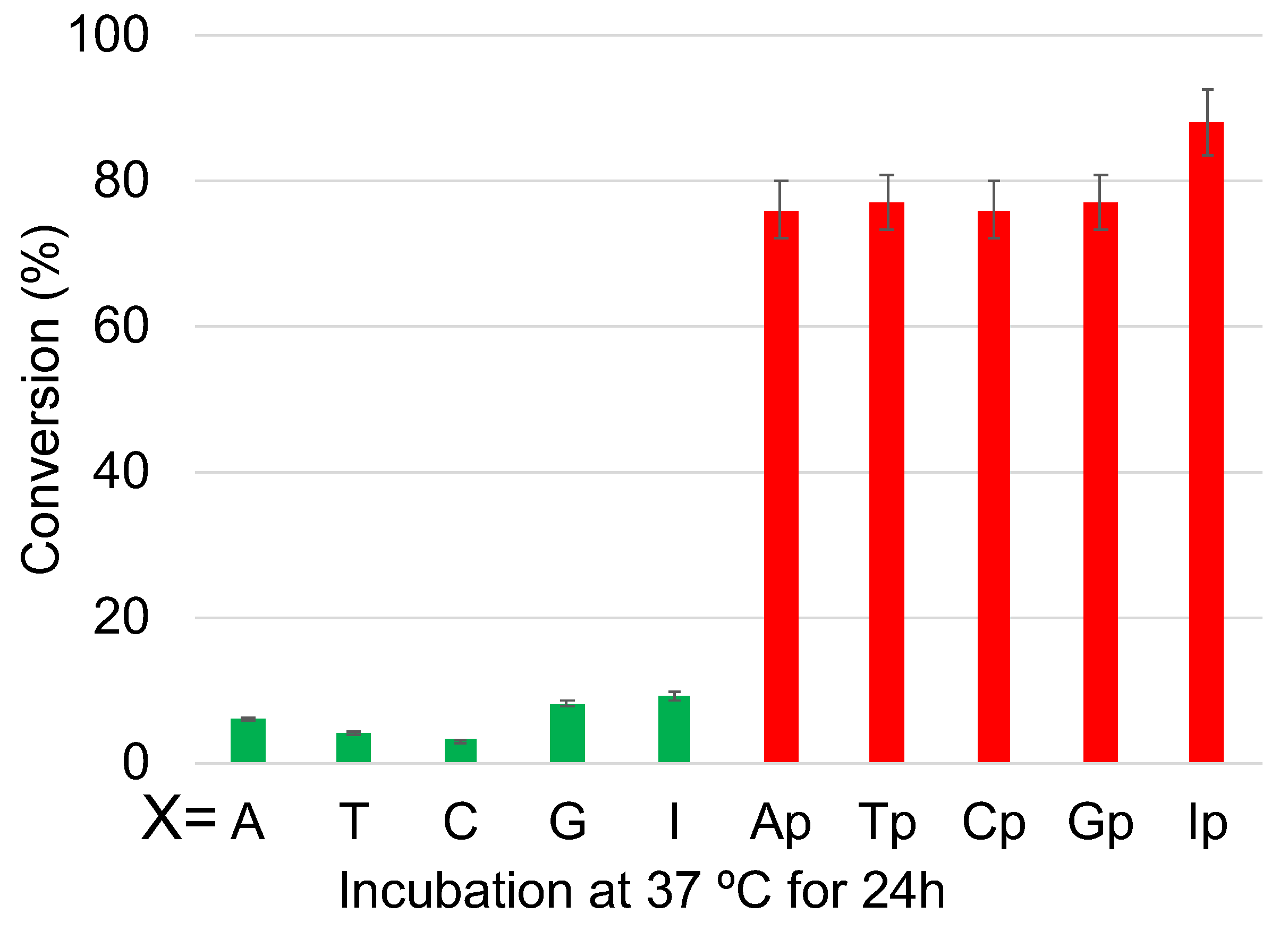
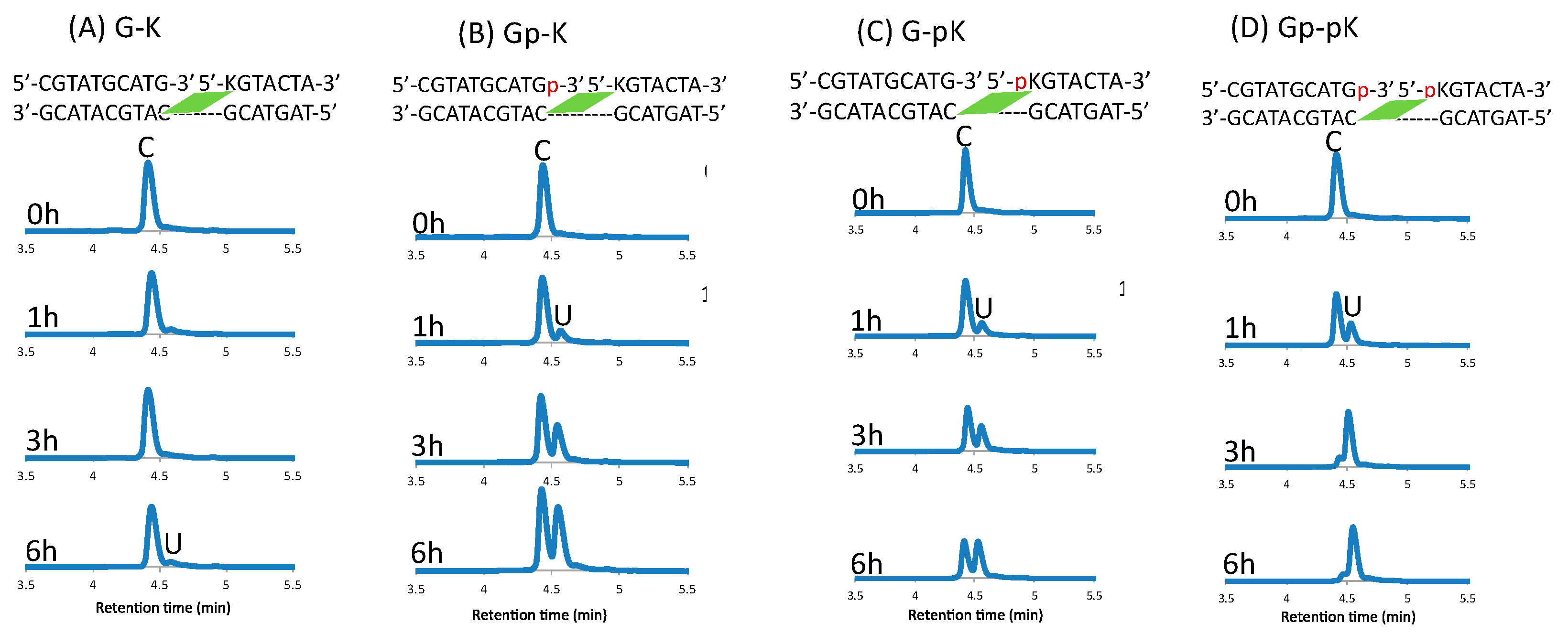
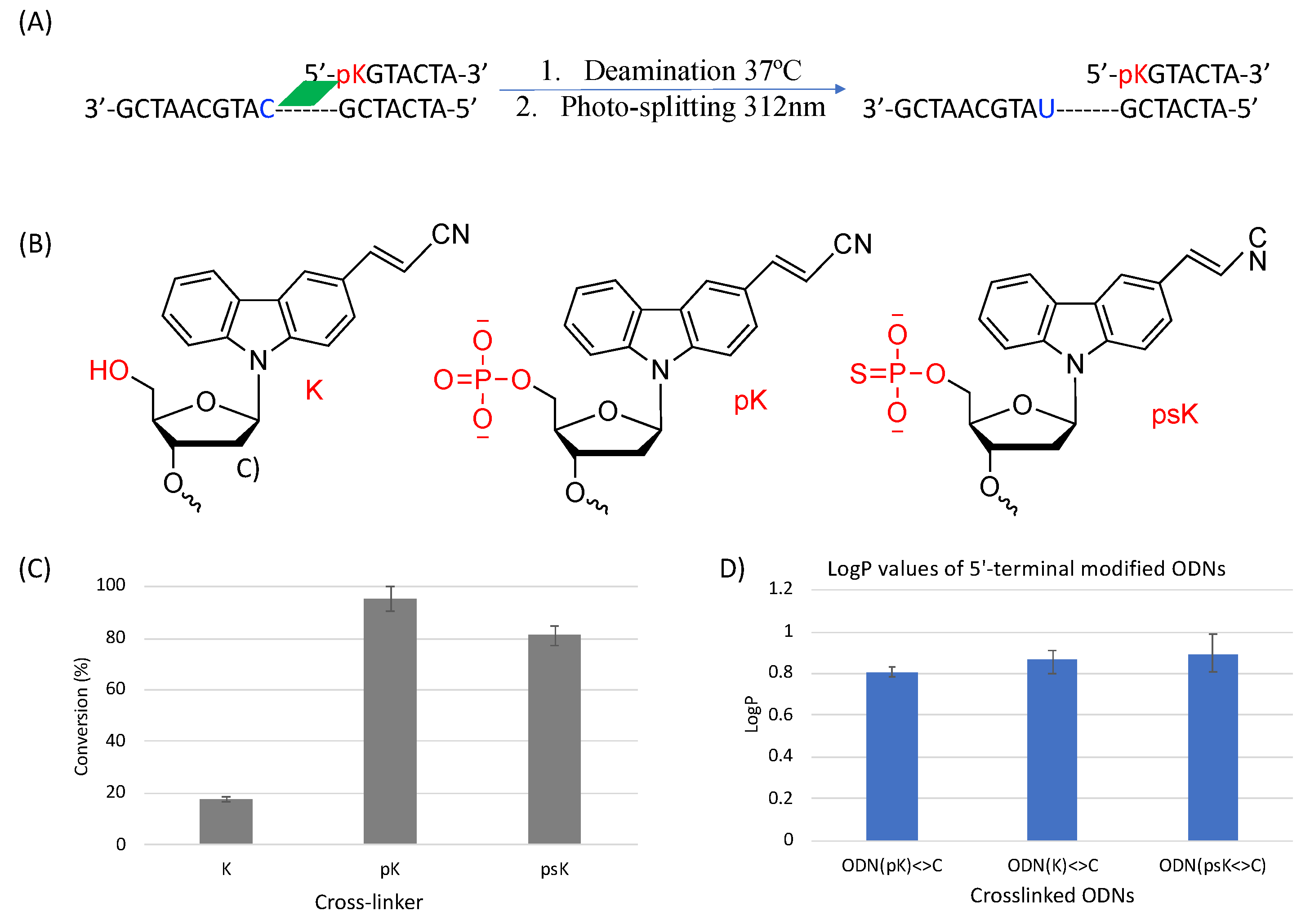
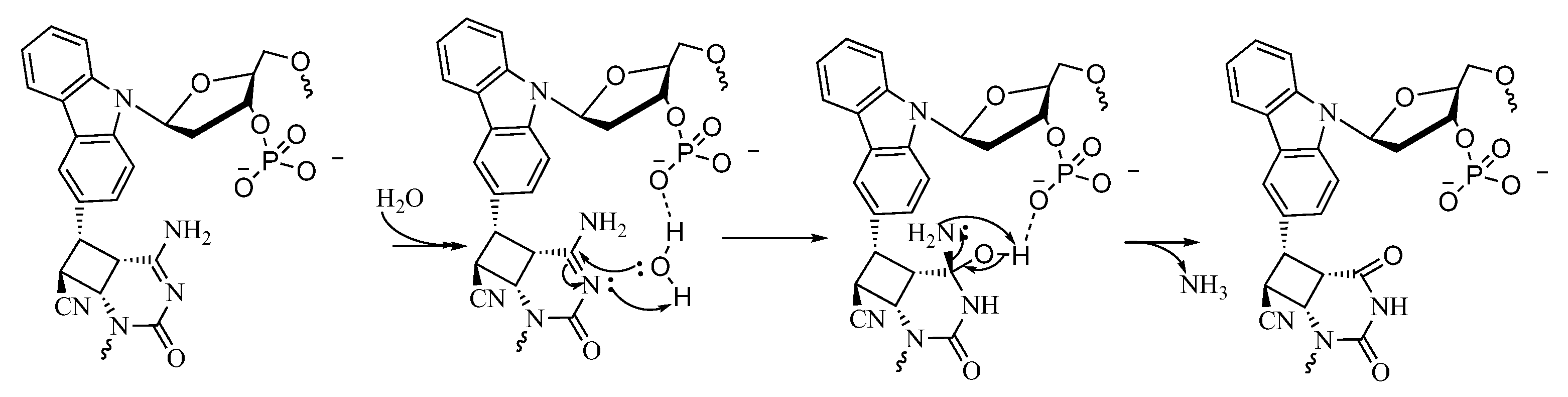
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. ODN Synthesis
3.3. Isolation of the Photo-Cross-Linked dsDNA
3.4. Deamination
3.5. Photo-Splitting and UPLC Analysis
3.6. Partition Coefficient (LogP)
3.7. Melting Point Analysis
3.8. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikebe, S.; Tanaka, M.; Ozawa, T. Point mutations of mitochondrial genome in Parkinson’s disease. Mol. Brain Res. 1995, 28, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, H.; Oshima, A.; Fukuhara, Y.; Shimmoto; Nagao, Y.; Bishop, D.F.; Desnick, R.J.; Suzuki, Y. Identification of point mutations in the alpha-galactosidase A gene in classical and atypical hemizygotes with Fabry disease. Am. J. Hum. Genet. 1990, 47, 784–789. [Google Scholar] [PubMed]
- Li, J.; Uversky, V.N.; Fink, A.L. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry 2001, 40, 11604–11613. [Google Scholar] [CrossRef] [PubMed]
- De Vries, D.D.; van Engelen, B.G.; Gabreëls, F.J.; Ruitenbeek, W.; van Oost, B.A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome. Ann. Neurol. 1993, 34, 410–412. [Google Scholar] [CrossRef]
- Lake, N.J.; Compton, A.G.; Rahman; Thorburn, D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016, 79, 190–203. [Google Scholar] [CrossRef]
- Baertling, F.; Rodenburg, R.J.; Schaper, J.; Smeitink, J.; Koopman, W.J.H.; Mayatepek, E.; Morava, E.; Distelmaier, F. A guide to diagnosis and treatment of Leigh syndrome. J. Neurol. Neurosurg. Psychiatry 2014, 85, 257–265. [Google Scholar] [CrossRef]
- Cascalho, M. Advantages and disadvantages of cytidine deamination. J. Immunol. 2004, 172, 6513–6518. [Google Scholar] [CrossRef]
- Lamont, P.J.; Surtees, R.; Woodward, C.E.; Leonard, J.V.; Wood, N.V.; Harding, A.E. Clinical and laboratory findings in referrals for mitochondrial DNA analysis. Arch. Dis. Child. 1998, 79, 22–27. [Google Scholar] [CrossRef]
- Watanabe, M.; Nobuta, A.; Tanaka, J.; Asaka, M. An effect of K-ras gene mutation on epidermal growth factor receptor signal transduction in PANC-1 pancreatic carcinoma cells. Int. J. Cancer 1996, 67, 264–268. [Google Scholar] [CrossRef]
- Storici, F.; Lewis, L.K.; Resnick, M.A. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 2001, 19, 773–776. [Google Scholar] [CrossRef]
- Puchta, H.; Fauser, F. Gene targeting in plants: 25 years later. Int. J. Dev. Biol. 2013, 57, 629–637. [Google Scholar] [CrossRef]
- Tan, W.S.; Carlson, D.F.; Walton, M.W.; Fahrenkrug, S.C.; Hackett, P.B. Precision editing of large animal genomes. Adv. Genet. 2012, 80, 37–97. [Google Scholar] [PubMed]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Papathanasiou, S.; Markoulaki, S.; Blaine, L.; Leibowitz, M.; Zhang, C.Z.; Jaenisch, R.; Pellman, D. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat. Commun. 2021, 12, 5855. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- White, M.K.; Kaminski, R.; Young, W.B.; Roehm, P.C.; Khalili, K. CRISPR Editing Technology in Biological and Biomedical Investigation. J. Cell. Biochem. 2017, 117, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, J.; Zhou, R.; Garcia, S.; Iyer, S.; Lareau, C.; Aryee, M.; Joung, J. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 2019, 569, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.; Tsukahara, T. Double MS2 guided restoration of genetic code in amber (TAG), opal (TGA) and ochre (TAA) stop codon. Enzyme Microb. Technol. 2021, 149, 109851. [Google Scholar] [CrossRef]
- Fan, J.; Ding, Y.; Ren, C.; Song, Z.; Yuan, J.; Chen, Q.; Du, C.; Li, C.; Wang, X.; Shu, W. Cytosine and adenine deaminase base-editors induce broad and nonspecific changes in gene expression and splicing. Commun. Biol. 2021, 4, 882. [Google Scholar] [CrossRef]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; Paul, C.L. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 1992, 89, 1827–1831. [Google Scholar] [CrossRef]
- Yamana, K.; Yoshikawa, A.; Nakano, H. Synthesis of a new photoisomerizable linker for connecting two oligonucleotide segments. Tetrahedron Lett. 1996, 37, 637–640. [Google Scholar] [CrossRef]
- Lee, B.L.; Blake, K.R.; Miller, P.S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with synthetic DNA containing a promoter for T7 RNA polymerase. Nucleic Acids Res. 1988, 16, 10681–10697. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.; Gu, K.; Lohse, P.A. Psoralen photo-crosslinked mRNA-puromycin conjugates: A novel template for the rapid and facile preparation of mRNA-protein fusions. Nucleic Acids Res. 2000, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wakuda, R.; Fujioka, K.; Asanuma, H. Photoregulation of DNA transcription by using photoresponsive T7 promoters and clarification of its mechanism. FEBS J. 2010, 277, 1551–1561. [Google Scholar] [CrossRef]
- Iwase, R.; Namba, M.; Yamaoka, T.; Murakami, A. Gene regulation by decoy approach (I): Synthesis and properties of photo-crosslinked oligonucleotides. Nucleic Acids Symp. Ser. 1997, 37, 203–204. [Google Scholar]
- Fujimoto, K.; Konishi-Hiratsuka, K.; Sakamoto, T.; Yoshimura, Y. Site-specific photochemical RNA editing. Chem. Commun. 2010, 46, 7545–7547. [Google Scholar] [CrossRef]
- Fujimoto, K.; Konishi-Hiratsuka, K.; Sakamoto, T.; Yoshimura, Y. Site-Specific Cytosine to Uracil Transition by Using Reversible DNA Photo-crosslinking. ChemBioChem 2010, 11, 1661–1664. [Google Scholar] [CrossRef]
- Kreutzer, D.A.; Essigmann, J.M. Oxidized, deaminated cytosines are a source of C –> T transitions in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 3578–3582. [Google Scholar] [CrossRef]
- Sethi, S.; Ooe, M.; Sakamoto, T.; Fujimoto, K. Effect of nucleobase change on cytosine deamination through DNA photo-cross-linking reaction via 3-cyanovinylcarbazole nucleoside. Mol. BioSyst. 2017, 13, 1152–1156. [Google Scholar] [CrossRef]
- Sethi, S.; Yasuharu, T.; Nakamura, S.; Fujimoto, K. Effect of substitution of photo-cross-linker in photochemical cytosine to uracil transition in DNA. Bioorg. Med. Chem. Lett. 2017, 27, 3905–3908. [Google Scholar] [CrossRef]
- Sethi, S.; Nakamura, S.; Fujimoto, K. Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA. Molecules 2018, 23, 828. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Shaw, B.R. Accelerated deamination of cytosine residues in UV-induced cyclobutane pyrimidine dimers leads to CC→TT transitions. Biochemistry 1886, 35, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Dammann, R.; Pfeifer, G.P. Sequence and time-dependent deamination of cytosine bases in UVB-induced cyclobutane pyrimidine dimers in vivo. J. Mol. Biol. 1998, 284, 297–311. [Google Scholar] [CrossRef]
- Cannistraro, V.J.; Pondugula, S.; Song, Q.; Taylor, J.S. Rapid deamination of cyclobutane pyrimidine dimer photoproducts at TCG sites in a translationally and rotationally positioned nucleosome in vivo. J. Biol. Chem. 2015, 290, 26597–26609. [Google Scholar] [CrossRef]
- Bird, A.P. CpG-rich islands and the function of DNA methylation. Nature 1986, 321, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Skirmantas, K.; Nathaniel, H. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929. [Google Scholar]
- Nakamura, S.; Yang, H.; Hirata, C.; Kersaudy, F.; Fujimoto, K. Development of 19F-NMR chemical shift detection of DNA B–Z equilibrium using 19F-NMR. Org. Biomol. Chem. 2017, 15, 5109–5111. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Fujimoto, K. Ultrafast Reversible Photocrosslinking Reaction: Toward in Situ DNA Manipulation. Org. Lett. 2008, 10, 3227–3230. [Google Scholar] [CrossRef]
- OECD. Test No. 117: Partition Coefficient (n-Octanol/Water), HPLC Method, OECD Guidelines for the Testing of Chemicals, Section 1; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Henczi, M.; Nagy, J.; Weaver, D.F. Determination of octanol-water partition coefficients by an HPLC method for anticonvulsant structure-activity studies. J. Pharm. Pharmacol. 1995, 47, 345–347. [Google Scholar] [CrossRef]

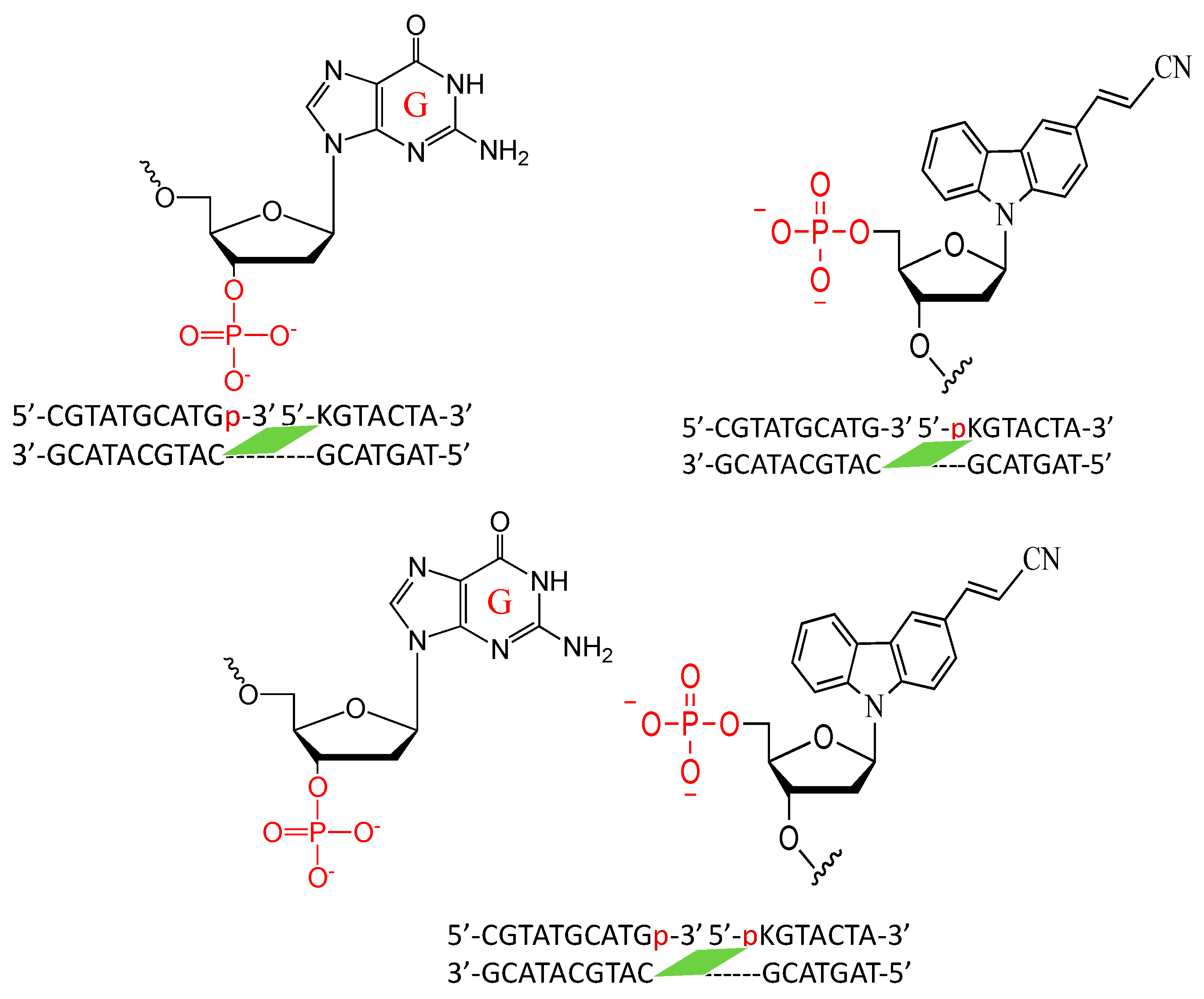


| ODN(X) X= | Sequence (5′→3′) | ODN(X) X= | Sequence (5→3′) |
|---|---|---|---|
| G | CGTATGCATG | Gp | CGTATGCATGp |
| C | CGTATGCATC | Cp | CGTATGCATCp |
| T | CGTATGCATT | Tp | CGTATGCATTp |
| A | CGTATGCATA | Ap | CGTATGCATAp |
| I | CGTATGCATI | Ip | CGTATGCATIp |
| ODN(K) | CNVKGTACTA | ODN (tC) | TAGTACGCATGCATACG |
| ODN | pKa | Deamination Reaction Rate Constant (h−1) |
|---|---|---|
| ODN(pK<>C) | 6.5 | 0.1919 |
| ODN(psK<>C) | 5.0 | 0.0692 |
| ODN(K<>C) | - | 0.0078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, S.; Takashima, Y.; Nakamura, S.; Wan, L.; Honda, N.; Fujimoto, K. Acceleration of the Deamination of Cytosine through Photo-Crosslinking. Curr. Issues Mol. Biol. 2023, 45, 4687-4700. https://doi.org/10.3390/cimb45060298
Sethi S, Takashima Y, Nakamura S, Wan L, Honda N, Fujimoto K. Acceleration of the Deamination of Cytosine through Photo-Crosslinking. Current Issues in Molecular Biology. 2023; 45(6):4687-4700. https://doi.org/10.3390/cimb45060298
Chicago/Turabian StyleSethi, Siddhant, Yasuharu Takashima, Shigetaka Nakamura, Licheng Wan, Nozomi Honda, and Kenzo Fujimoto. 2023. "Acceleration of the Deamination of Cytosine through Photo-Crosslinking" Current Issues in Molecular Biology 45, no. 6: 4687-4700. https://doi.org/10.3390/cimb45060298
APA StyleSethi, S., Takashima, Y., Nakamura, S., Wan, L., Honda, N., & Fujimoto, K. (2023). Acceleration of the Deamination of Cytosine through Photo-Crosslinking. Current Issues in Molecular Biology, 45(6), 4687-4700. https://doi.org/10.3390/cimb45060298







