Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Callus Induction and Stress Treatment
2.3. Histological Observation
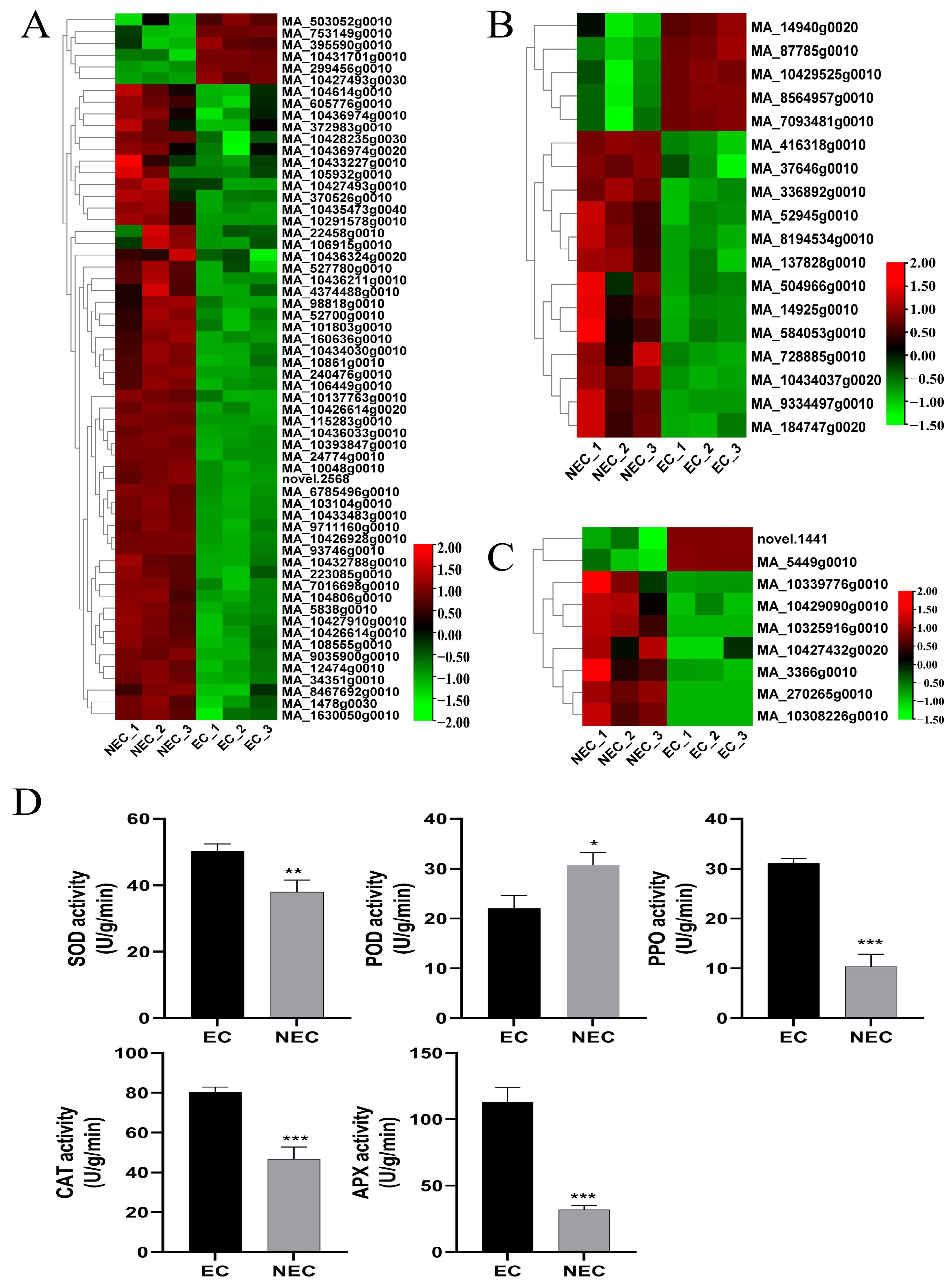
2.4. Physiological Determinations
2.5. RNA-seq
2.6. Identification of Differentially Expressed Genes (DEGs) and Enrichment Analysis
2.7. Quantitative Real-Time PCR (qRT-PCR)
3. Results
3.1. Morphological and Histological Analysis of EC and NEC
3.2. The Identification, GO Classification, and KEGG Enrichment Analysis of DEGs
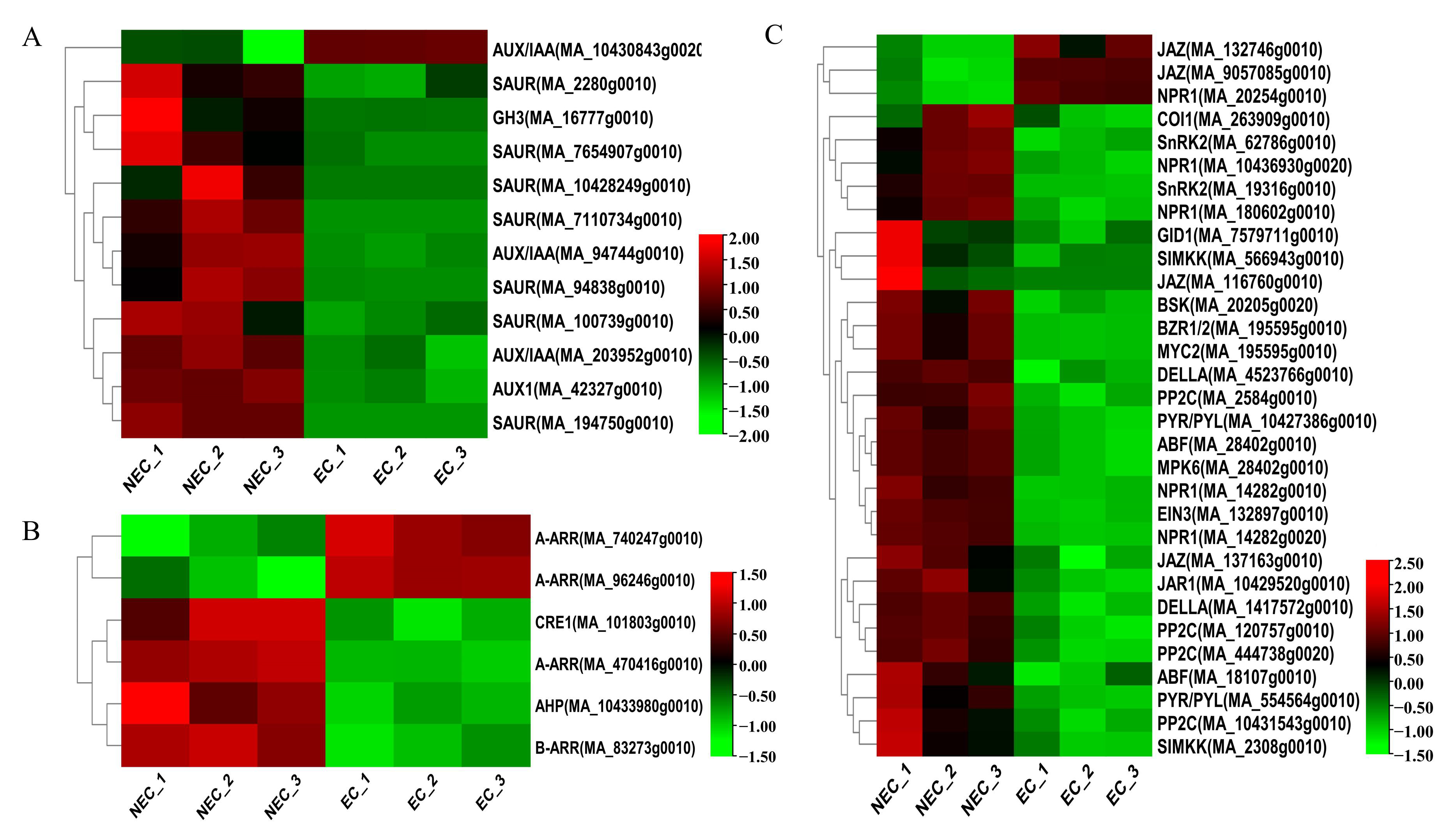
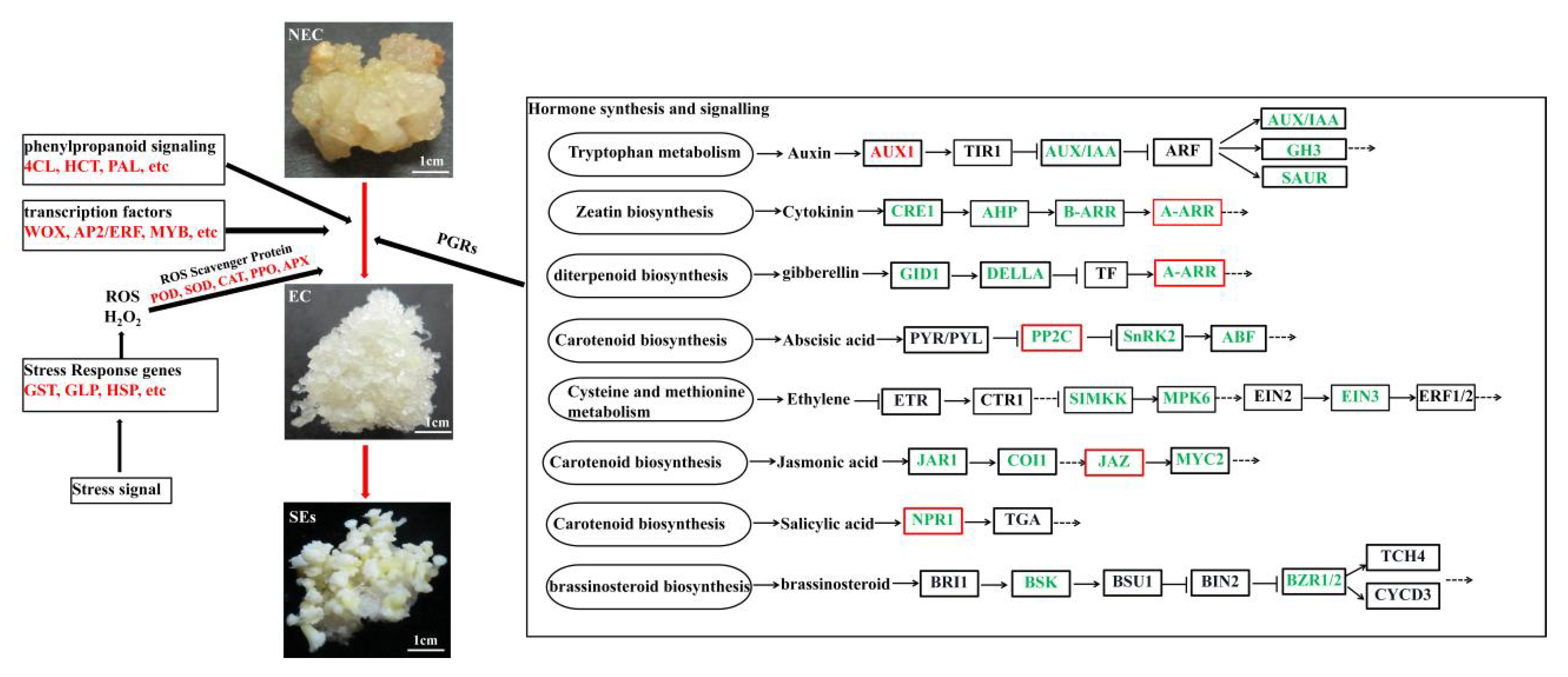
3.3. Identification and Expression Pattern Analysis of Important DEGs in the Plant Hormone Pathway
3.4. Identification and Expression Pattern Analysis of Important DEGs in the Phenylpropanoid Metabolism Pathway
3.5. Identification and Expression Profile Analysis of Putative Decisive DEGs Related to Stress Responses
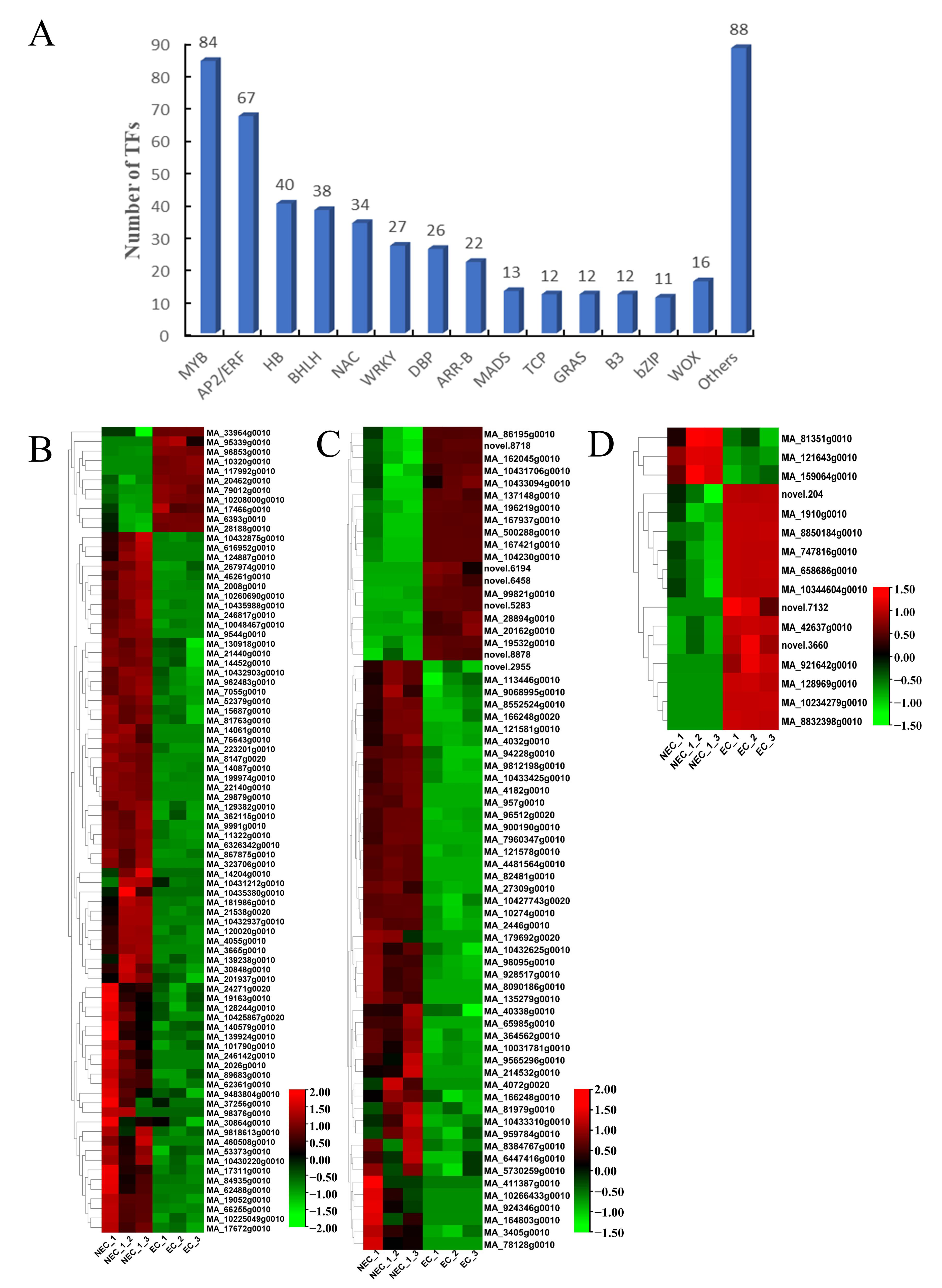
3.6. Identification and Expression Profile Analysis of Expression of Decisive Transcription Factors (TFs) Associated with SE
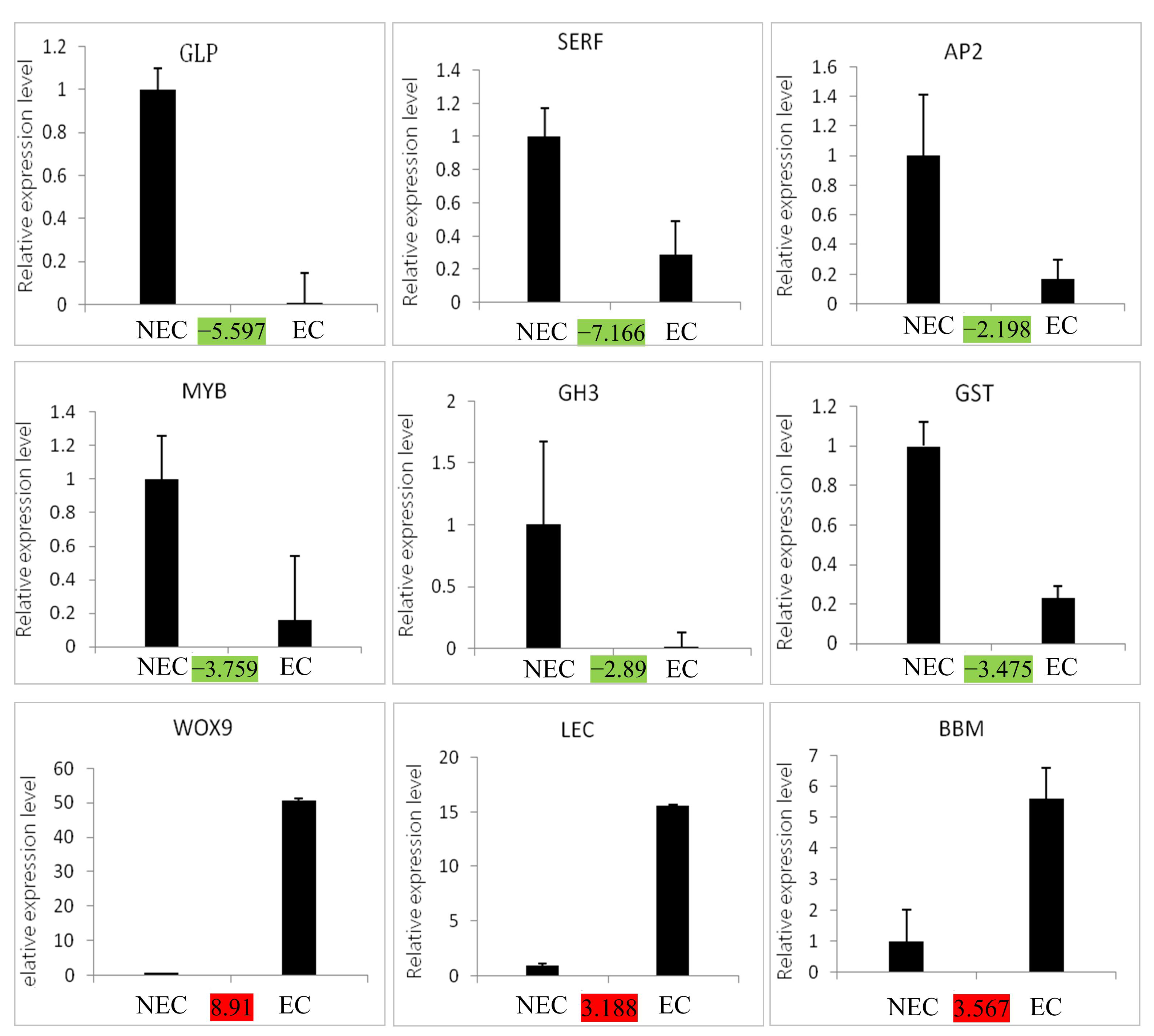
3.7. Validation of the Expression Level of Key Candidate DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.D.; Li, W.D.; Zheng, Y. A study on taxonomy of P. mongolica in inner Mongolia. Bull. Bot. Res. 1994, 14, 59–69. (In Chinese) [Google Scholar]
- Ha, B.R.; Bai, Y.E.; Hou, W.F.; Qing, S. Research progress and prospect of Picea mongolica of species endemic to sandy land. North. Hortic. 2021, 14, 152–157. (In Chinese) [Google Scholar]
- Nystedt, B.; Street, N.R.; Wetterbom, A.; Zuccolo, A.; Lin, Y.C.; Scofield, D.G.; Vezzi, F.; Delhomme, N.; Giacomello, S.; Alexeyenko, A.; et al. The norway spruce genome sequence and conifer genome evolution. Nature 2013, 497, 579–584. [Google Scholar] [CrossRef]
- Mordhorst, A.P.; Toonen, M.A.J.; Vries, S.C.D.; Meinke, D. Plant embryogenesis. Crit. Rev. Plant Sci. 1997, 16, 535–576. [Google Scholar] [CrossRef]
- Yin, M.H.; Hong, S.R. A simple cryopreservation protocol of Dioscorea bulbifera L. embryogenic calli by encapsulation-vitrifcation. Plant Cell Tissue Organ Cult. 2010, 101, 349–358. [Google Scholar]
- Kessel-Domini, A.; Pérez-Brito, D.; Guzmán-Antonio, A.; Barredo-Pool, F.A.; Mijangos-Cortés, J.O.; Iglesias-Andreu, L.G.; Cortés-Velázquez, A.; Canto-Flick, A.; Avilés-Viñas, S.A.; Rodríguez-Llanes, Y.; et al. Indirect somatic embryogenesis: An efficient and genetically reliable clonal propagation system for Ananas comosus L. Merr. Hybrid “MD2”. Agriculture 2022, 12, 713. [Google Scholar] [CrossRef]
- Manjkhola, S.; Dhar, U.; Joshi, M. Organogenesis, embryogenesis, and synthetic seed production in Arnebia euchroma-a critically endangered medicinal plant of the Himalaya. Cell Dev. Biol. Plant 2005, 41, 244–248. [Google Scholar] [CrossRef]
- Li, B.Q.; Feng, C.H.; Hu, L.Y.; Wang, M.R.; Wang, Q.C. Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann. Appl. Biol. 2016, 168, 142–150. [Google Scholar] [CrossRef]
- Pathi, K.M.; Tula, S.; Tuteja, N. High frequency regeneration via direct somatic embryogenesis and efcient Agrobacterium-mediated genetic transformation of tobacco. Plant Signal Behav. 2013, 8, e24354. [Google Scholar] [CrossRef]
- Durzan, D.J.; Chalupa, V.; Mia, A.J. Growth and metabolism of cells and tissues of jack pine (Pinus banksiana). 1. The establishment and some characteristics of a proliferated callus from jack pine seedlings. Can J. Bot. 1976, 54, 437–445. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Bernier-Cardou, M.; Cyr, D.R.; Sutton, B.C.S. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. Vitr. Cell Dev. Biol. Plant 2000, 36, 279–286. [Google Scholar] [CrossRef]
- Pullman, G.S.; Namjoshi, K.; Zhang, Y. Somatic embryogenesis in loblolly pine (Pinus taeda L.): Improving culture initiation rates. Plant Cell Rep. 2003, 22, 85–95. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Bernier-Cardou, M.; Klimaszewska, K. Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep. 2006, 25, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.K.; Liao, C.K.; Ho, Y.L. Maturation of somatic embryos in two embryogenic cultures of Picea morrisonicola Hayata as affected by alternation of endogenous IAA content. Plant Cell Tiss. Org. 2008, 93, 257–268. [Google Scholar] [CrossRef]
- Li, C.H.; Liu, B.G.; Kim, T.D.; Moon, H.K.; Choi, Y.E. Somatic embryogenesis and plant regeneration in elite genotypes of Picea koraiensis. Plant Biotechnol. Rep. 2008, 2, 259–265. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, S.N.; Fang, C.; Wang, Y.B.; Yi, S.J.; Ning, D.L. Somatic embryogensis in mature zygotic embryos of Picea likiangensis. Biologia 2010, 62, 853–858. [Google Scholar] [CrossRef]
- Hakman, I.; Fowke, L.C.; Von-Arnold, S.; Erikssona, T. The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway Spruce). Plants 1985, 38, 53–59. [Google Scholar] [CrossRef]
- Harry, I.S.; Thorpe, T.A. Somatic embryogensis and plant regeneration from mature zygotic embryos of red spruce. Int. J. Plant Sci. 1991, 152, 446–452. [Google Scholar]
- Yan, J.; Peng, P.; Duan, G.Z.; Lin, T.; Bai, Y.E. Multiple analyses of various factors affecting the plantlet regeneration of Picea mongolica (H. Q. Wu) W.D. Xu from somatic embryos. Sci. Rep. 2021, 11, 6694. [Google Scholar] [CrossRef]
- Camille, S.; Loïc, L.; Bertrand, D. Genetic and molecular control of somatic embryogenesis. Plants 2021, 10, 1467. [Google Scholar]
- Robert, H.S.; Grones, P.; Stepanova, A.N.; Robles, L.M.; Lokerse, A.S.; Alonso, J.M.; Weijers, D.; Friml, J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. Expression profifiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017, 36, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin Synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Cham, Switzerland, 2016; pp. 171–182. [Google Scholar]
- Membré, N.; Berna, A.; Neutelings, G.; David, A.; David, H.; Staiger, D.; Sáez-Vásquez, J.; Raynal, M.; Delseny, M.; Bernier, F. cDNA sequence, genomic organization and differential expression of three Arabidopsis genes for germin/oxalate oxidase-like proteins. Plant Mol. Biol. 1997, 35, 459–469. [Google Scholar] [CrossRef]
- Thompson, E.W.; Lane, B.G. Relation of protein synthesis in imbibing wheat embryos to the cell-free translational capacities of bulk mRNA from dry and imbibing embryos. J. Bio. Chem. 1980, 255, 5965–5970. [Google Scholar] [CrossRef]
- Kim, H.J.; Pesacreta, T.C.; Triplett, B.A. Cotton-fiber germin-like protein. II: Immunolocalization, purification, and functional analysis. Planta 2004, 218, 525–535. [Google Scholar] [CrossRef]
- Mathieu, M.; Lelu-Walter, M.A.; Blervacq, A.S.; David, H.; Hawkins, S.; Neutelings, G. Germin-like genes are expressed during somatic embryogenesis and early development of conifers. Plant Mol. Biol. 2006, 61, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Marrs, K.A.; Casey, E.S.; Capitant, S.A.; Bouchard, R.A.; Dietrich, P.S.; Mettler, I.J.; Sinibaldi, R.M. Characterization of two maize hsp90 heat shock protein genes and expression during heat shock, embryogenesis and pollen development. Dev. Genet. 1993, 14, 27–41. [Google Scholar] [CrossRef]
- Reddy, R.; Chaudhary, S.; Patil, P.; Krishna, P. The 90 kDa heat shock protein (hsp90) is expressed throughout Brassica napus seed development and germination. Plants 1998, 131, 131–137. [Google Scholar] [CrossRef]
- Haralampidis, K.; Milioni, D.; Rigas, S.; Hatzopoulos, P. Combinatorial interaction of cis elements specifies the expression of the Arabidopsis AtHsp90-1 gene. Plant Physiol. 2002, 129, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Constantinos, P.; Konstantinos, K.; Despina, S.; Polydefkis, H. Tight regulation of expression of two Arabidopsis cytosolic Hsp90 genes during embryo development. J. Exp. Bot. 2005, 56, 633–644. [Google Scholar]
- Vrinten, P.L.; Nakamura, T.; Kasha, K.J. Characterization of cDNAs expressed in the early stages of microspore embryogenesis in barley (Hordeum vulgare) L. Plant Mol. Biol. 1999, 41, 455–463. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Zhu, L.; Tu, L.; Guo, X.; Nie, Y. Isolation and characterization of genes associated to cotton somatic embryogenesis by suppression subtractive hybridization and macroarray. Plant Mol. Biol. 2006, 60, 167–183. [Google Scholar] [CrossRef]
- Thibaud-Nissen, F.O.; Shealy, R.T.; Khanna, A.; Vodkin, L.O. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol. 2003, 32, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Lotan, T.; Ohto, M.A.; Yee, K.M.; West, M.; Harada, J.J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 1998, 93, 1195–1205. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, Y.; Ji, H.; Burnie, W.; Perry, S.E. Gene regulation by the AGL15 transcription factor reveals hormone interactions in somatic embryogenesis. Plant Physiol. 2016, 172, 2374–2387. [Google Scholar] [CrossRef]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.M.; Hattori, J.; Liu, C.M.; Lammeren, A.A.M.; Miki, B.L.A.; et al. Ectopic expression of BABY BOOM triggers aconversion from vegetative to embryonic growth. Plant Cell. 2002, 14, 1737–1749. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR:a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Karami, O.; Aghavaisi, B.; Pour, A.M. Molecular aspects of somatic-to-embryogenic transition in plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef]
- Kumar, S.; Ruggles, A.; Logan, S.; Mazarakis, A.; Tyson, T.; Bates, M.; Grosse, C.; Reed, D.; Li, Z.G.; Grimwood, J.; et al. Comparative transcriptomics of non-embryogenic and embryogenic callus in semi-recalcitrant and non-recalcitrant upland cotton lines. Plants 2021, 10, 1775. [Google Scholar] [CrossRef]
- Li, W.; Wei, L.; Parris, S.; West, M.; Saski, C.A. Transcriptomic profiles of non-embryogenic and embryogenic callus cells in a highly regenerative upland cotton line (Gossypium hirsutum L.). BMC Dev. Biol. 2020, 20, 25. [Google Scholar]
- Ci, H.T.; Li, C.Y.; Aung, T.t.; Wang, S.L.; Yun, C.; Wang, F.; Ren, X.X.; Zhang, X.X. A comparative transcriptome analysis reveals the molecular mechanisms that underlie somatic embryogenesis in Peaonia ostii ‘Fengdan’. Int. J. Mol. Sci. 2022, 23, 10595. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.H.; Dong, Y.; Du, C.M.; Shi, Y.B.; Teng, Y. Transcriptomic and physiological analyses reveal the acquisition of somatic embryogenesis potential in Agapanthus praecox. Sci. Hortic. 2022, 305, 111362. [Google Scholar] [CrossRef]
- Weijers, D.; Schlereth, A.; Ehrismann, J.S. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell. 2006, 10, 265–270. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Liu, Z.; Zhang, Z.; XuHan, X.; Lin, Y.; Lai, Z. Global scale transcriptome analysis reveals differentially expressed genes involve in early somatic embryogenesis in Dimocarpus longan Lour. BMC Genom. 2020, 21, 4. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Xu, X.Q.; Xu, X.P.; Li, Y.; Zhao, P.C.; Chen, X.H.; Shen, X.; Zhang, Z.H.; Chen, Y.K.; Liu, S.C.; et al. Genome-wide identification, evolution analysis of cytochrome P450 monooxygenase multigene family and their expression patterns during the early somatic embryogenesis in Dimocarpus longan Lour. Gene 2022, 826, 146453. [Google Scholar] [CrossRef]
- Wang, Y.L.; He, S.J.; Long, Y.; Zhang, X.L.; Zhang, X.X.; Hu, H.M.; Li, Z.L.; Hou, F.X.; Ge, F.; Gao, S.B.; et al. Genetic variations in ZmSAUR15 contribute to the formation of immature embryo-derived embryonic calluses in maize. Plant J. 2022, 109, 980–991. [Google Scholar] [CrossRef]
- Weijers, D.; Sauer, M.; Meurette, O.; Friml, J.R.; Ljung, K.; Sandberg, G.; Hooykaas, P.; Offringa, R. Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell. 2005, 17, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Liu, H.H.; Duan, C.Y.; Zhang, B.K.; Wei, G.; Zhang, Y.; Li, S. Arabidopsis JANUS Regulates Embryonic Pattern Formation through Pol II-Mediated Transcription of WOX2 and PIN7. iScience 2019, 19, 1179–1188. [Google Scholar] [CrossRef]
- Inoue, T.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar] [CrossRef]
- Schaller, G.E.; Kieber, J.J.; Shiu, S.H. Two-Component Signaling Elements and Histidyl-Aspartyl Phosphorelays. Arab. Book 2008, 6, e0112. [Google Scholar] [CrossRef]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Igielski, R.; Kępczyńska, E. Gene expression and metabolite profiling of gibberellin biosynthesis during induction of somatic embryogenesis in Medicago truncatula Gaertn. PLoS ONE 2017, 12, e0182055. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Bai, B. Establishment of embryonic shoot-root axis is involved in auxin and cytokinin response during Arabidopsis somatic embryogenesis. Front. Plant Sci. 2015, 5, 792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Zhu, H.G.; Tian, W.G.; Zhu, S.H.; Xiong, X.P.; Sun, Y.Q.; Zhu, Q.H.; Sun, J. De novo transcriptome analysis reveals insights into dynamic homeostasis regulation of somatic embryogenesis in upland cotton (G. hirsutum L.). Plant Mol. Biol. 2016, 92, 279–292. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.J.; Ao, K.; Peng, Y.J.; Zhang, Y.X.; Li, X.; Zhang, Y.L. Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef]
- Huang, P.X.; Dong, Z.; Guo, P.R.; Zhang, X.; Qiu, Y.P.; Li, B.S.; Wang, Y.C.; Guo, H.W. Salicylic acid suppresses apical hook formation via NPR1-mediated repression of EIN3 and EIL1 in Arabidopsis. Plant Cell 2020, 32, 612–629. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.L.; Shen, X.L.; Liang, H.W.; Wang, Y.B.; He, Z.Q.; Zhang, D.C.; Chen, F.J. Comparative transcriptome analysis highlights the hormone effects on somatic embryogenesis in Catalpa bungei. Plant Reprod. 2019, 32, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; Ferdinando, M.D.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Zhang, C.H.; Ma, T.; Luo, W.C.; Xu, J.M.; Liu, J.Q.; Wan, D.S. Identification of 4CL genes in desert poplars and their changes in expression in response to salt stress. Genes 2015, 6, 901–917. [Google Scholar] [CrossRef]
- Wang, J.P.; Matthews, M.L.; Williams, C.M.; Shi, R.; Yang, C.; Tunlaya-Anukit, S.; Chen, H.C.; Li, Q.; Liu, J.; Lin, C.Y. Improving wood properties for wood utilization through multi-omics integration in lignin biosynthesis. Nat. Commun. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Lepelley, M.; Mahesh, V.; McCarthy, J.; Rigoreau, M.; Crouzillat, D.; Chabrillange, N.; de Kochko, A.; Campa, C. Characterization, high-resolution mapping and differential expression of three homologous PAL genes in Coffea canephora Pierre (Rubiaceae). Planta 2012, 236, 313–326. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agr. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Liu, B.B.; Shan, X.H.; Wu, Y.; Su, S.Z.; Li, S.; Liu, H.K.; Han, J.Y.; Yuan, Y.P. iTRAQ-Based quantitative proteomic analysis of embryogenic and non-embryogenic calli derived from a Maize (Zea mays L.) inbred line Y423. Int. J. Mol. Sci. 2018, 19, 4004. [Google Scholar] [CrossRef]
- Olivares-García, C.A.; Mata-Rosas, M.; Peña-Montes, C.; Quiroz-Figueroa, F.; Segura-Cabrera, A.; Shannon, L.M.; Loyola-Vargas, V.M.; Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Ibarra-Laclette, E.; et al. Phenylpropanoids are connected to cell wall fortifification and stress tolerance in avocado somatic embryogenesis. Int. J. Mol. Sci. 2020, 21, 5679. [Google Scholar] [CrossRef] [PubMed]
- Langhansova, L.; Konradova, H.; Vanek, T. Polyethylene glycol and abscisic acid improve maturation and regeneration of Panax ginseng somatic embryos. Plant Cell Rep. 2004, 22, 725–730. [Google Scholar] [CrossRef]
- Touraev, A.; Indrianto, A.; Wratschko, I.; Vicente, O.; Heberle-Bors, E. Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex Plant Reprod. 1996, 9, 209–215. [Google Scholar] [CrossRef]
- Kiyosue, T.; Kamada, H.; Har, H. Induction of Somatic Embryogenesis by Salt Stress in Carrot. Plant Tissue Cult. Lett. 2010, 6, 162–164. [Google Scholar] [CrossRef]
- Ikeda-Iwai, M.; Umehara, M.; Satoh, S.; Kamada, H. Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. 2003, 34, 107–114. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, X.; Kai, G.; Deng, J.W.; Xu, J.; Gao, W.H.; Lindsey, K.; Zhang, X.L. ROS Homeostasis regulates somatic embryogenesis via the regulation of auxin signaling in cotton. Mol. Cell. Proteom. 2016, 15, 2108–2124. [Google Scholar] [CrossRef]
- Leonardo, J.; Dos, S.A.L.W.; Bueno, C.A.; Barbosa, H.R.; Floh, E.I.S. Proteomic analysis and polyamines, ethylene and reactive oxygen species levels of Araucaria angustifolia(Brazilian pine) embryogenic cultures with different embryogenic potential. Tree Physiol. 2014, 34, 94–104. [Google Scholar]
- Ma, L.; Xie, L.; Lin, G.; Jiang, S.; Chen, H.; Li, H.; Takáč, T.; Šamaj, J.; Xu, C. Histological changes and differences in activities of some antioxidant enzymes and hydrogen peroxide content during somatic embryogenesis of Musa AAA cv. Yueyoukang 1. Sci. Hortic. 2012, 144, 87–92. [Google Scholar] [CrossRef]
- Gallego, P.; Martin, L.; Blazquez, A.; Guerra, H.; Villalobos, N. Involvement of peroxidase activity in developing somatic embryos of Medicago arborea L. Identification of an isozyme peroxidase as biochemical marker of somatic embryogenesis. J. Plant Physiol. 2014, 171, 78–84. [Google Scholar] [CrossRef]
- Lin, Y.F.; Xiao, Q.; Hao, Q.W.; Qian, Z.J.; Li, X.X.; Li, P.; Li, H.; Lian, C. Genome-wide identification and functional analysis of the glutathione S-transferase (GST) family in Pomacea canaliculata. Int. J. Biol. Macromol. 2021, 193, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Cummins, I.; Cole, D.J.; Edwards, R. Glutathione-mediated detoxification systems in plants. Curr. Opin. Plant Biol. 1998, 1, 258–266. [Google Scholar] [CrossRef]
- Ganesan, M.; Jayabalan, N. Evaluation of haemoglobin (erythrogen):for improved somatic embryogenesis and plant regeneration in cotton (Gossypium hirsutum L. cv. SVPR 2). Plant Cell Rep. 2004, 23, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Dunstan, D.I. Expression of abundant mRNAs during somatic embryogenesis of white spruce [Picea glauca (Moench) Voss]. Planta 1996, 199, 459–466. [Google Scholar] [CrossRef]
- Pratyusha, D.S.; Sarada, D.V.L. MYB transcription factors-master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Niu, Q.W.; Teng, C.; Li, C.; Mu, J.Y.; Chua, N.H.; Zuo, J.R. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2009, 19, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jurgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.B.; Trontin, J.F.; Raschke, J.; Zoglauer, K.; Rupps, A. Constitutive overexpression of a conifer WOX2 homolog affects somatic embryo development in pinus pinaster and promotes somatic embryogenesis and organogenesis in Arabidopsis seedlings. Front. Plant Sci. 2022, 13, 838421. [Google Scholar] [CrossRef]
- Palovaara, J.; Hallberg, H.; Stasolla, C.; Hakman, I. Comparative expression pattern analysis of WUSCHEL-related homeobox 2(WOX2) and WOX8/9 in developing seeds and somatic embryos of the gymnosperm Picea abies. New. Phytol. 2010, 188, 122–135. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Song, Y.; Sheahan, M.B.; Rose, R.J. Transcriptional regulation of early embryo development in the model legume Medicago truncatula. Plant Cell Rep. 2014, 33, 349–362. [Google Scholar] [CrossRef]
- Hatzopoulos, P.; Fong, F.; Sung, Z.R. Abscisis acid regulation of DC8 a carrot embryonic gene. Plant Physiol. 1990, 94, 690–695. [Google Scholar] [CrossRef]
- Yang, H.; Saitou, T.; Komeda, Y.; Harada, H.; Kamada, H. Late embryogenesis abundant protein in Arabidopsis thaliana homologous to carrot ECP31. Physiol Plant. 2010, 98, 661–666. [Google Scholar] [CrossRef]
- Yang, H.; Saitou, T.; Komeda, Y.; Harada, H.; Kamada, H. Arabidopsis thaliana ECP 63 encoding a LEA protein is located in chromosome 4. Gene 1997, 184, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Kwong, L.W.; Yee, K.M.; Pelletier, J.; Lepiniec, L.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. USA 2001, 98, 11806. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Bao, W.; Sui, M.; Bai, Y. Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica. Curr. Issues Mol. Biol. 2023, 45, 5232-5247. https://doi.org/10.3390/cimb45070332
Wang Y, Wang H, Bao W, Sui M, Bai Y. Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica. Current Issues in Molecular Biology. 2023; 45(7):5232-5247. https://doi.org/10.3390/cimb45070332
Chicago/Turabian StyleWang, Yaping, Hao Wang, Wenquan Bao, Mingming Sui, and Yu´e Bai. 2023. "Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica" Current Issues in Molecular Biology 45, no. 7: 5232-5247. https://doi.org/10.3390/cimb45070332
APA StyleWang, Y., Wang, H., Bao, W., Sui, M., & Bai, Y. (2023). Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica. Current Issues in Molecular Biology, 45(7), 5232-5247. https://doi.org/10.3390/cimb45070332




