Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Flow Cytometry
2.3. Definition of Lymphocyte Subpopulations
- Early differentiated CD4 or CD8 lymphocytes that expressed:
- CD31, CD4+CD31+, and CD8+CD31+, defined as Recent Thymic Emigrants (RTE);
- CD45RA together with CCR7, CD4+CD45RA+CCR7+, and CD8+CD45RA+CCR7+, defined as naïve CD4 or CD8 lymphocytes;
- CD28 and not CD57, CD4CD28+CD57-, and CD8CD28+CD57-;
- CD45RA and not CD57, CD4CD45RA+CD57-, and CD8CD45RA+CD57-;
- Neither CD45RA nor CD57, CD4CD45RA-CD57-, and CD8CD45RA-CD57-.
- 2.
- In the group of memory cells, CD4 or CD8 lymphocytes that expressed:
- CCR7 and not CD45RA, CD4+CD45RA-CCR7+, and CD8+CD45RA-CCR7+, are defined as central memory (CM);
- Neither CCR7 nor CD45RA, CD4+CD45RA-CCR7- and CD8+CD45RA-CCR7-, are defined as effector memory (EM).
- 3.
- In the group of senescent/advanced differentiated cells:
- T cells expressing CD45RA and not CCR7, CD4+CD45RA+CCR7-, and CD8+CD45RA+CCR7-, are defined as EMRA;
- T cells lacking the CD28 molecule, CD4CD28-, and CD8CD28-, and their subtypes according to the presence of CD57, CD4+CD28-CD57-, CD4+CD28-CD57+, and CD8+CD28-CD57-, CD8+CD28-CD57+;
- T cells expressing both CD45RA and CD57, CD4CD45RA+CD57+, and CD8CD45RA+CD57+.
2.4. Statistical Analysis
3. Results
3.1. Characteristics of SLE Patients
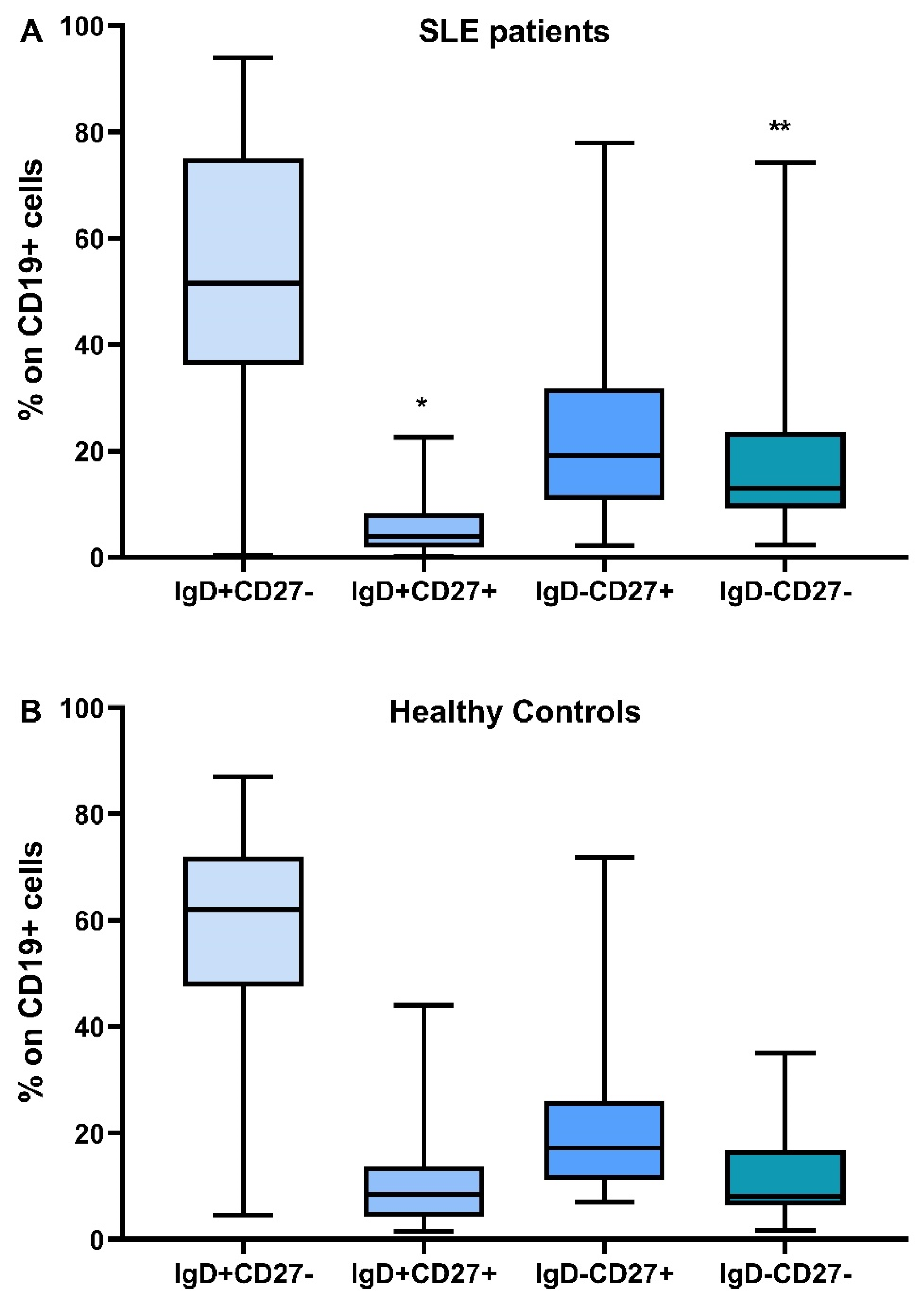
3.2. Phenotypic Analysis of B Lymphocytes in Patients with SLE and in HC
3.3. Phenotypic Analysis of T Lymphocytes in SLE Patients and HC
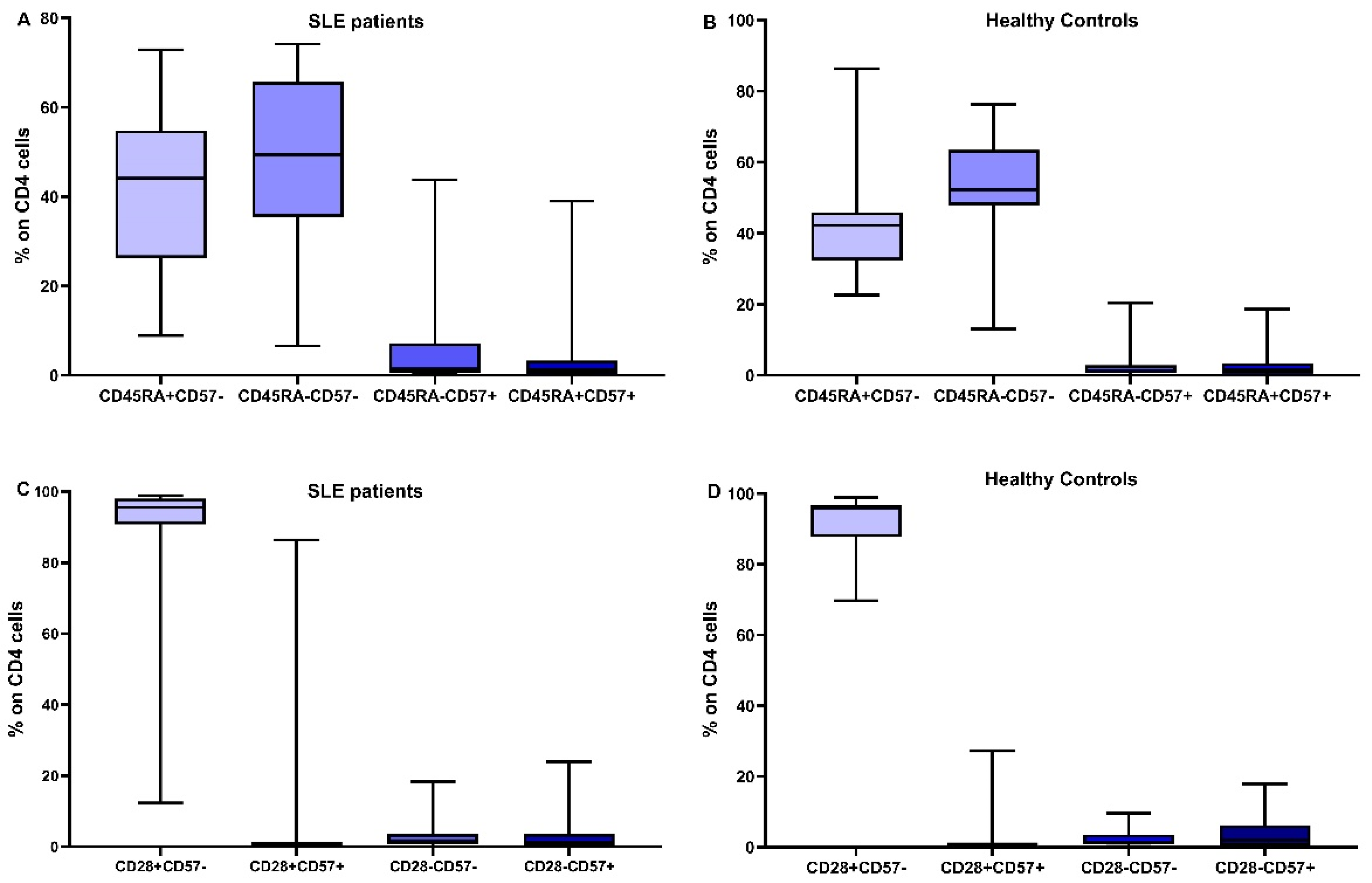
3.4. Distribution of CD4 and CD8 Subtypes according to Their Differentiation Status
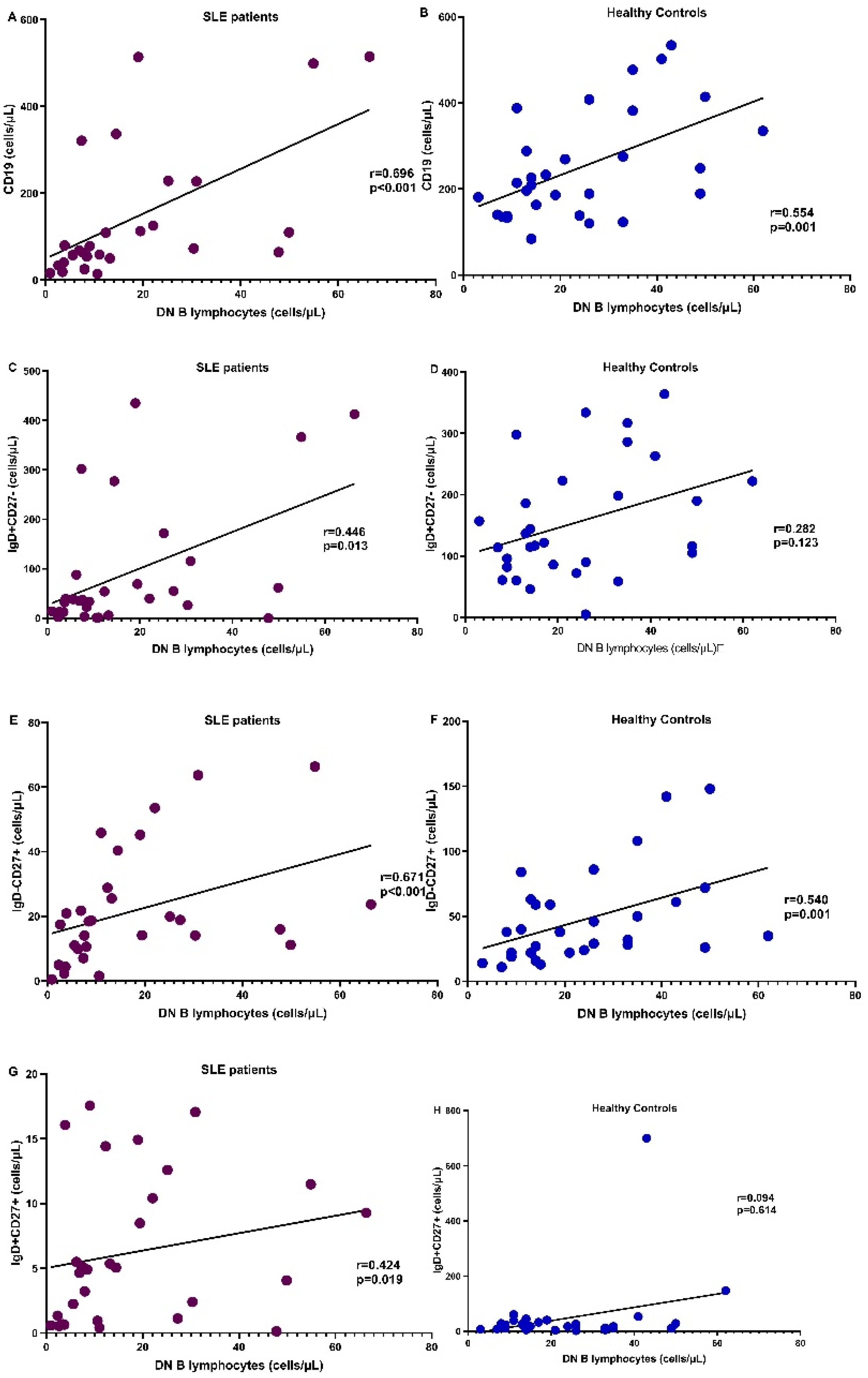
3.5. Correlation of DN B Cells with B Lymphocyte Subpopulations
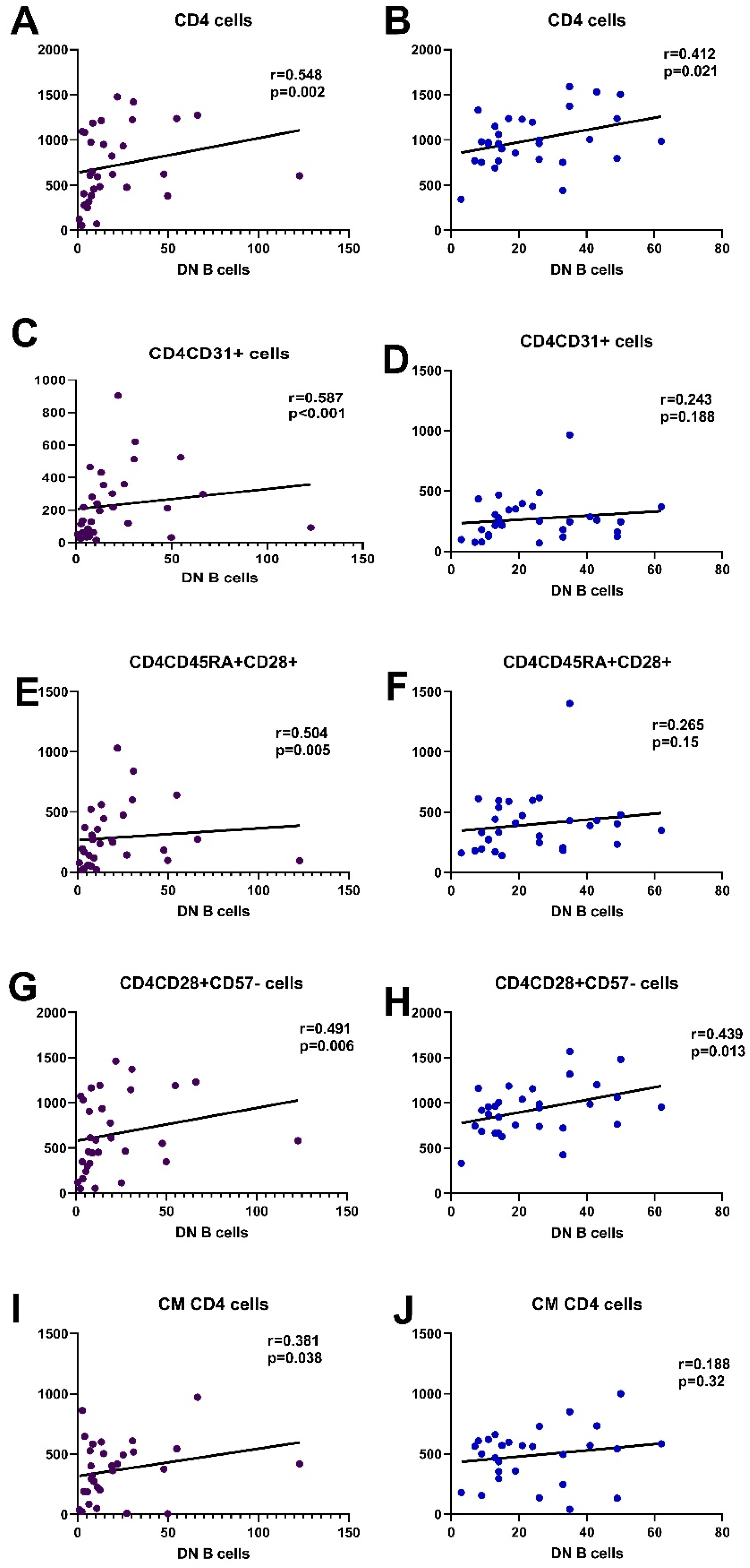
3.6. Correlation of DN B Cells with T Lymphocyte Subpopulations
3.6.1. Correlation with CD4 Cells
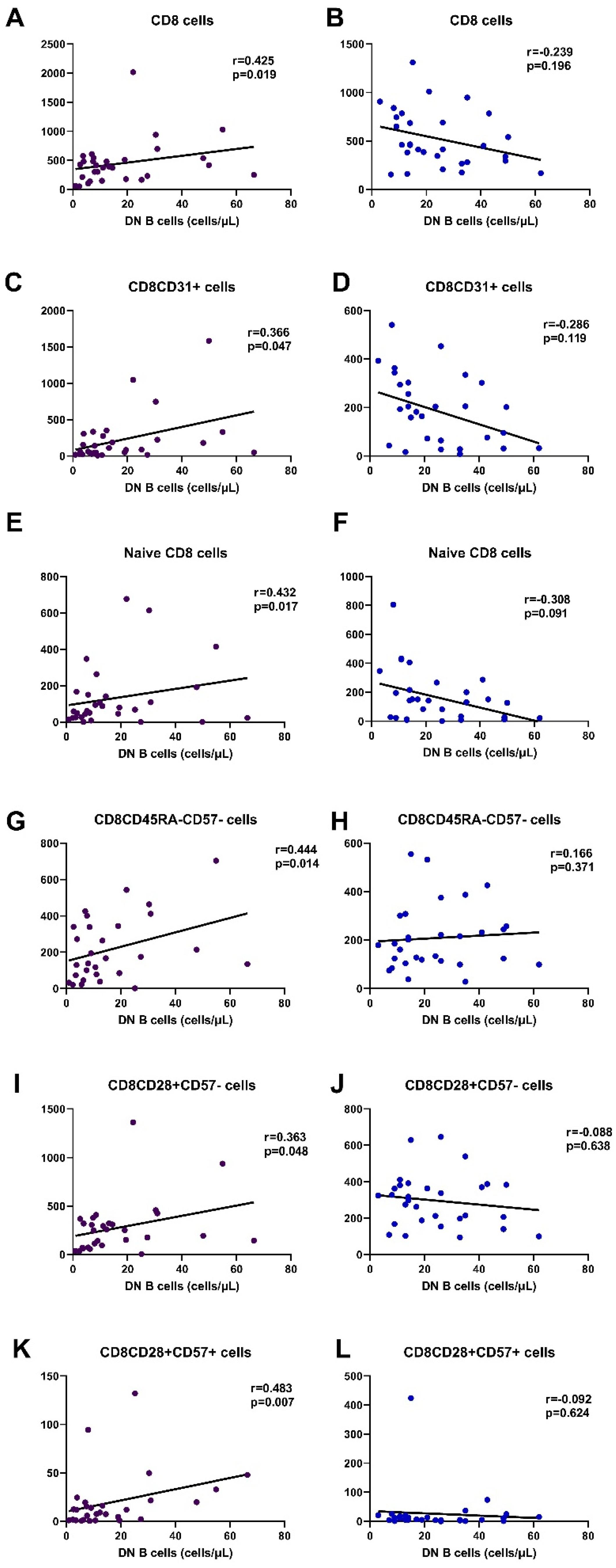
3.6.2. Correlation with CD8 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, U.; Rajewsky, K.; Küppers, R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 1998, 188, 1679–1689. [Google Scholar] [CrossRef]
- Shi, Y.; Agematsu, K.; Ochs, H.D.; Sugane, K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin. Immunol. 2003, 108, 128–137. [Google Scholar] [CrossRef]
- Borst, J.; Hendriks, J.; Xiao, Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005, 17, 275–281. [Google Scholar] [CrossRef]
- Beckers, L.; Somers, V.; Fraussen, J. IgD−CD27− double negative (DN) B cells: Origins and functions in health and disease. Immunol. Lett. 2023, 255, 67–76. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Hu, F. Double-negative (DN) B cells: An under-recognized effector memory B cell subset in autoimmunity. Clin. Exp. Immunol. 2021, 205, 119–127. [Google Scholar] [CrossRef]
- Rodríguez-Bayona, B.; Ramos-Amaya, A.; Pérez-Venegas, J.J.; Rodríguez, C.; Brieva, J.A. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res Ther. 2010, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Fraussen, J.; Marquez, S.; Takata, K.; Beckers, L.; Montes Diaz, G.; Zografou, C.; Van Wijmeersch, B.; Villar, L.M.; O’Connor, K.C.; Kleinstein, S.H.; et al. Phenotypic and Ig Repertoire Analyses Indicate a Common Origin of IgD-CD27- Double Negative B Cells in Healthy Individuals and Multiple Sclerosis Patients. J. Immunol. 2019, 203, 1650–1664. [Google Scholar] [CrossRef] [PubMed]
- Lioulios, G.; Fylaktou, A.; Xochelli, A.; Sampani, E.; Tsouchnikas, I.; Giamalis, P.; Daikidou, D.V.; Nikolaidou, V.; Papagianni, A.; Theodorou, I.; et al. Clustering of End Stage Renal Disease Patients by Dimensionality Reduction Algorithms According to Lymphocyte Senescence Markers. Front. Immunol. 2022, 13, 841031. [Google Scholar] [CrossRef] [PubMed]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 2018, 49, 725–739.e6. [Google Scholar] [CrossRef]
- Szelinski, F.; Lino, A.C.; Dörner, T. B cells in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2022, 34, 125–132. [Google Scholar] [CrossRef]
- Moysidou, E.; Lioulios, G.; Xochelli, A.; Nikolaidou, V.; Christodoulou, M.; Mitsoglou, Z.; Stai, S.; Fylaktou, A.; Papagianni, A.; Stangou, M. Different Types of Chronic Inflammation Engender Distinctive Immunosenescent Profiles in Affected Patients. Int. J. Mol. Sci. 2022, 23, 14688. [Google Scholar] [CrossRef]
- Lioulios, G.; Mitsoglou, Z.; Fylaktou, A.; Xochelli, A.; Christodoulou, M.; Stai, S.; Moysidou, E.; Konstantouli, A.; Nikolaidou, V.; Papagianni, A.; et al. Exhausted but Not Senescent T Lymphocytes Predominate in Lupus Nephritis Patients. Int. J. Mol. Sci. 2022, 23, 13928. [Google Scholar] [CrossRef]
- Sampani, E.; Vagiotas, L.; Daikidou, D.V.; Nikolaidou, V.; Xochelli, A.; Kasimatis, E.; Lioulios, G.; Dimitriadis, C.; Fylaktou, A.; Papagianni, A.; et al. End stage renal disease has an early and continuous detrimental effect on regulatory T cells. Nephrology 2022, 27, 281–287. [Google Scholar] [CrossRef]
- Shang, Q.; Yip, G.W.; Tam, L.S.; Zhang, Q.; Sanderson, J.E.; Lam, Y.Y.; Li, C.M.; Wang, T.; Li, E.K.; Yu, C.M. SLICC/ACR damage index independently associated with left ventricular diastolic dysfunction in patients with systemic lupus erythematosus. Lupus 2012, 21, 1057–1062. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Zheng, J.; Zhu, L.; Ju, B.; Zhang, J.; Luo, J.; Wang, Y.; Lv, X.; Pu, D.; He, L.; Wang, J. Peripheral immunophenotypes associated with the flare in the systemic lupus erythematosus patients with low disease activity state. J. Clin. Immunol. 2022, 245, 109166. [Google Scholar] [CrossRef] [PubMed]
- Liossis, S.C.; Staveri, C. The Role of B Cells in Scleroderma Lung Disease Pathogenesis. Front. Med. 2022, 9, 936182. [Google Scholar] [CrossRef]
- Wang, Y.; Lloyd, K.A.; Melas, I.; Zhou, D.; Thyagarajan, R.; Lindqvist, J.; Hansson, M.; Svärd, A.; Mathsson-Alm, L.; Kastbom, A.; et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci. Rep. 2019, 9, 19995. [Google Scholar] [CrossRef] [PubMed]
- Claes, N.; Fraussen, J.; Vanheusden, M.; Hellings, N.; Stinissen, P.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J. Immunol. 2016, 197, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, G.R.; Hijikata, A.; Kitamura, H.; Ohara, O.; Wang, J.Y.; Cooper, M.D. Discriminating gene expression profiles of memory B cell subpopulations. J. Exp. Med. 2008, 205, 1807–1817. [Google Scholar] [CrossRef]
- Buchta, C.; Bishop, G. TRAF5 negatively regulates TLR signaling in B lymphocytes. J. Immunol. 2014, 192, 145–150. [Google Scholar] [CrossRef]
- You, X.; Zhang, R.; Shao, M.; He, J.; Chen, J.; Liu, J.; Zhang, X.; Liu, X.; Jia, R.; Sun, X.; et al. Double Negative B Cell Is Associated with Renal Impairment in Systemic Lupus Erythematosus and Acts as a Marker for Nephritis Remission. Front. Med. 2020, 7, 85. [Google Scholar] [CrossRef]
- Kajihara, A.; Morita, T.; Kato, Y.; Konaka, H.; Murakami, T.; Yamaguchi, Y.; Koyama, S.; Takamatsu, H.; Nishide, M.; Maeda, Y.; et al. The proliferative activity levels of each immune cell population evaluated by mass cytometry are linked to the clinical phenotypes of systemic lupus erythematosus. Int. Immunol. 2023, 35, 27–41. [Google Scholar] [CrossRef]
- Sachinidis, A.; Xanthopoulos, K.; Garyfallos, A. Age-Associated B Cells (ABCs) in the Prognosis, Diagnosis and Therapy of Systemic Lupus Erythematosus (SLE). Mediterr. J. Rheumatol. 2020, 31, 311–318. [Google Scholar] [CrossRef]
- Sosa-Hernández, V.A.; Romero-Ramírez, S.; Cervantes-Díaz, R.; Carrillo-Vázquez, D.A.; Navarro-Hernandez, I.C.; Whittall-García, L.P.; Absalón-Aguilar, A.; Vargas-Castro, A.S.; Reyes-Huerta, R.F.; Juárez-Vega, G.; et al. CD11c+ T-bet+CD21hi B Cells Are Negatively Associated with Renal Impairment in Systemic Lupus Erythematosus and Act as a Marker for Nephritis Remission. Front. Immunol. 2022, 13, 892241. [Google Scholar] [CrossRef] [PubMed]
- Agematsu, K.; Hokibara, S.; Nagumo, H.; Shinozaki, K.; Yamada, S.; Komiyama, A. Plasma cell generation from B-lymphocytes via CD27/CD70 interaction. Leuk Lymphoma 1999, 35, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hu, F.; Zhu, L.; Xu, C.; Zhu, H.; Li, Y.; Liu, H.; Li, C.; Liu, N.; Xu, L.; et al. CD70-mediated CD27 expression downregulation contributed to the regulatory B10 cell impairment in rheumatoid arthritis. Mol. Immunol. 2020, 119, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Claes, N.; Dhaeze, T.; Fraussen, J.; Broux, B.; Van Wijmeersch, B.; Stinissen, P.; Hupperts, R.; Hellings, N.; Somers, V. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: A 12-month follow-up study. PLoS ONE 2014, 9, e111115. [Google Scholar] [CrossRef]
- Moura, R.A.; Quaresma, C.; Vieira, A.R.; Gonçalves, M.J.; Polido-Pereira, J.; Romão, V.C.; Martins, N.; Canhão, H.; Fonseca, J.E. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PLoS ONE 2017, 12, e0182927. [Google Scholar] [CrossRef]
- Colonna-Romano, G.; Bulati, M.; Aquino, A.; Pellicanò, M.; Vitello, S.; Lio, D.; Candore, G.; Caruso, C. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev. 2009, 130, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Bulati, M.; Buffa, S.; Candore, G.; Caruso, C.; Dunn-Walters, D.K.; Pellicanò, M.; Wu, Y.C.; Colonna Romano, G. B cells and immunosenescence: A focus on IgG+IgD-CD27- (DN) B cells in aged humans. Ageing Res. Rev. 2011, 10, 274–284. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells 2021, 10, 3402. [Google Scholar] [CrossRef]
- Ma, K.; Du, W.; Wang, X.; Yuan, S.; Cai, X.; Liu, D.; Li, J.; Lu, L. Multiple Functions of B Cells in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 6021. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhuang, H.; Xu, Y.; Lee, P.; Li, Y.; Wilson, J.C.; Vidal, O.; Choi, H.S.; Sun, Y.; Yang, L.J.; et al. Maintenance of autoantibody production in pristane-induced murine lupus. Arthritis Res. Ther. 2015, 17, 384. [Google Scholar] [CrossRef]
- Rubtsova, K.; Rubtsov, A.V.; Thurman, J.M.; Mennona, J.M.; Kappler, J.W.; Marrack, P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Investig. 2017, 127, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Faustini, F.; Sippl, N.; Stålesen, R.; Chemin, K.; Dunn, N.; Fogdell-Hahn, A.; Gunnarsson, I.; Malmström, V. Rituximab in Systemic Lupus Erythematosus: Transient Effects on Autoimmunity Associated Lymphocyte Phenotypes and Implications for Immunogenicity. Front. Immunol. 2022, 13, 826152. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Tang, Y.; Lin, L.; Zhong, H.; Yang, H.; Zeng, Y.; Lv, J.; Li, X.; Lu, Y.; Xu, A. Low level of circulating basophil counts in biopsy-proven active lupus nephritis. Clin. Rheumatol. 2018, 37, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Dossybayeva, K.; Bexeitov, Y.; Mukusheva, Z.; Almukhamedova, Z.; Assylbekova, M.; Abdukhakimova, D.; Rakhimzhanova, M.; Poddighe, D. Analysis of Peripheral Blood Basophils in Pediatric Systemic Lupus Erythematosus. Diagnostics 2022, 12, 1701. [Google Scholar] [CrossRef] [PubMed]

| SLE | HC | p | |
|---|---|---|---|
| n | 30 | 31 | |
| Laboratory results | |||
| WCC (cells/μL) | 7200 ± 3350 | 6400 ± 1800 | NS |
| Neutrophils (%) | 69.6 ± 20.7 | 58.1 ± 10.35 | <0.0001 |
| Neutrophils (cells/μL) | 4600 ± 3500 | 3500 ± 1200 | 0.03 |
| Lymphocytes (%) | 23.1 ± 16.1 | 25.6 ± 9 | 0.03 |
| Lymphocytes (cells/μL) | 1400 ± 900 | 2100 ± 900 | 0.005 |
| NLR | 3 ± 4 | 1.8 ± 0.85 | <0.0001 |
| Ht (%) | 33 ± 9 | 39 ± 5 | 0.02 |
| Hb (mg/dL) | 11.5 ± 6 | 13 ± 5 | 0.03 |
| Platelets (103/μL) | 245 ± 96 | 270 ± 35 | NS |
| CRP | 0.5 ± 0.4 | 0.4 ± 0.6 | NS |
| Serum urea (mg/dL) | 36 ± 12 | 34 ± 5 | NS |
| Serum creatinine (mg/dL) | 0.9 ± 0.5 | 0.87 ± 6 | NS |
| Total protein (g/dL) | 7.5 ± 4 | 7.7 ± 3.2 | NS |
| Serum albumin (g/dL) | 4.2 ± 2.3 | 4.6 ± 2.5 | NS |
| SLE | HC | p | |
|---|---|---|---|
| n | 30 | 31 | |
| CD19 (%) | 7.9 (2.1–28.6) | 11.8 (5.4–24) | 0.012 |
| CD19 cells/μL | 75.4 (14.4–520.8) | 214 (84–576) | <0.001 |
| IgD+CD27- (%) | 51.5 (0.4–94) | 58.7 (4.5–86.9) | 0.34 |
| IgD+CD27- cells/μL | 37.71 (0.26–434.84) | 117 (5–364) | <0.001 |
| IgD+CD27+ (%) | 3.9 (0.2–22) | 8.4 (1.5–44) | 0.014 |
| IgD+CD27+ cells/μL | 5.12 (0.13–17.55) | 23 (2–700) | <0.001 |
| IgD-CD27+ (%) | 19.1 (2.2–78) | 17.9 (7.1–71.9) | 0.7 |
| IgD-CD27+ cells/μL | 18.58 (0.47–89.58) | 38 (11–258) | 0.001 |
| IgD-CD27- (%) | 12.9 (2.3–74.2) | 8 (1.7–35) | 0.04 |
| IgD-CD27- cells/μL | 10.84 (0.93–122.91) | 21 (3–202) | 0.007 |
| Ratio DN/[ (IgD+CD27-) + (IgD-CD27+) + (IgD+CD27+)] | 0.14 (0.02–2.9) | 0.08 (0.02–0.54) | p = 0.04 |
| SLE | HC | p | |
|---|---|---|---|
| n | 30 | 31 | |
| CD4 (cells/μL) | 651.2 (71.1–1478.2) | 986 (344–1591) | 0.004 |
| Early differentiated cells | |||
| CD4+CD31+ | 216.38 (16.3–904.7) | 250 (69–967) | 0.14 |
| CD4CD45RA+CD28+ | 267.97 (20.62–1030.31) | 388 (139–1402) | 0.02 |
| CD4CD45RA+CD57- | 254.03 (21.05–1077.61) | 401 (160–1373) | 0.035 |
| CD4CD45RA-CD57- | 290.67 (38.96–884.43) | 539 (173–991) | <0.001 |
| CD4CD28+CD57- | 610.7 (54.68–1461.94) | 958 (332–1569) | 0.004 |
| CD4CD28+CD57+ | 4.7 (0–806) | 7 (0–245) | 0.21 |
| Memory cells | |||
| CD4CD45RA-CCR7+ | 402.35 (38.7–972.4) | 563 (40–1001) | 0.046 |
| CD4CD45RA-CCR7- | 1.62 (0–73.49) | 11 (0–590) | 0.002 |
| Advanced differentiated/senescent cells | |||
| CD45RA+CCR7- | 7.29 (0–180.62) | 23 (0–487) | 0.027 |
| CD4CD28- | 20.12 (1.27–139.06) | 38 (3–299) | 0.04 |
| CD4CD28-CD57+ | 9.90 (0.46–73.8) | 23 (0–274) | 0.1 |
| CD45RA+CCR7-CD28- | 1.2 (0–82) | 2.5 (0–106) | 0.21 |
| SLE | HC | p | |
|---|---|---|---|
| n | 30 | 31 | |
| CD8 (cells/μL) | 414.8 (60.6–2017.8) | 454.5 (154–1310) | 0.26 |
| Early differentiated cells | |||
| CD8+CD31+ | 88.19 (8.2–1047) | 187.5 (8–541) | 0.26 |
| CD8CD45RA+CD28+ | 113.56 (1.81–753.7) | 212.5 (7–1257) | 0.17 |
| CD8CD45RA+CD57- | 63.65 (3.83–889.8) | 133 (8–552) | 0.17 |
| CD8CD45RA-CD57- | 194.52 (1.8–945.1) | 179 (28–555) | 0.99 |
| CD8CD28+CD57- | 249.45 (5.49–1362) | 298 (95–646) | 0.1 |
| CD8CD28+CD57+ | 12(0.4–132) | 8.5 (0–424) | 0.58 |
| Memory cells | |||
| CD8CD45RA-CCR7+ | 171.52 (2.5–1417) | 123 (1–941) | 0.14 |
| CD8CD45RA-CCR7- | 13.94 (0.59–92.37) | 25 (0–355) | 0.53 |
| Advanced differentiated/senescent cells | |||
| CD8CD45RA+CCR7- | 11.13 (0–279.6) | 49.5 (0–534) | 0.02 |
| CD8CD28- | 87.83 (4.56–1361.2) | 135 (36–633) | 0.14 |
| CD8CD28-CD57+ | 53.17 (0.83–571.04) | 71 (0–470) | 0.17 |
| CD45RA+CCR7-CD28- | 37.3 (2.1–263) | 197 (9–783) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moysidou, E.; Lioulios, G.; Christodoulou, M.; Xochelli, A.; Stai, S.; Iosifidou, M.; Iosifidou, A.; Briza, S.; Briza, D.I.; Fylaktou, A.; et al. Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations. Curr. Issues Mol. Biol. 2023, 45, 6667-6681. https://doi.org/10.3390/cimb45080421
Moysidou E, Lioulios G, Christodoulou M, Xochelli A, Stai S, Iosifidou M, Iosifidou A, Briza S, Briza DI, Fylaktou A, et al. Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations. Current Issues in Molecular Biology. 2023; 45(8):6667-6681. https://doi.org/10.3390/cimb45080421
Chicago/Turabian StyleMoysidou, Eleni, Georgios Lioulios, Michalis Christodoulou, Aliki Xochelli, Stamatia Stai, Myrto Iosifidou, Artemis Iosifidou, Sophia Briza, Dimitria Ioanna Briza, Asimina Fylaktou, and et al. 2023. "Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations" Current Issues in Molecular Biology 45, no. 8: 6667-6681. https://doi.org/10.3390/cimb45080421
APA StyleMoysidou, E., Lioulios, G., Christodoulou, M., Xochelli, A., Stai, S., Iosifidou, M., Iosifidou, A., Briza, S., Briza, D. I., Fylaktou, A., & Stangou, M. (2023). Increase in Double Negative B Lymphocytes in Patients with Systemic Lupus Erythematosus in Remission and Their Correlation with Early Differentiated T Lymphocyte Subpopulations. Current Issues in Molecular Biology, 45(8), 6667-6681. https://doi.org/10.3390/cimb45080421





