Comparative Transcriptome Analyses Provide New Insights into the Evolution of Divergent Thermal Resistance in Two Eel Gobies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Cold Tolerance Capacity in the Two Eel Gobies
2.3. Transcriptomic Response to Cold Stress in the Two Eel Gobies
2.3.1. Cold Stress Experiments and Tissue Dissection
2.3.2. RNA Isolation, Library Preparation and Sequencing
2.3.3. Reads Mapping and Gene Expression Comparison
2.4. Identification of Cold Responsive Modules in the Two Eel Gobies
3. Results
3.1. Variation of Cold Tolerance between the Two Eel Gobies
3.2. Statistics of Transcriptome Sequences
3.3. Transcriptomic Response to Cold Stress in Two Eel Gobies
3.4. Distinct DEGs in the Two Eel Gobies under Cold Stresses
3.5. Cold Responsive Modules and Hub Genes Associated with Cold Tolerance
4. Discussion
4.1. Species Divergency in Capacity of Cold Tolerance
4.2. Common Processes Involved in the Cold Stress Response
4.3. Distinct DEGs Corresponding with Divergent Thermal Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikkink, K.L.; Reynolds, R.M.; Ituarte, C.M.; Cresko, W.A.; Phillips, P.C. Rapid evolution of phenotypic plasticity and shifting thresholds of genetic assimilation in the nematode Caenorhabditis Remanei. G3 Genes Genomes Genet. 2014, 4, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Tomiolo, S.; Ward, D. Species migrations and range shifts: A synthesis of causes and consequences. Perspect. Plant Ecol. Evol. Syst. 2018, 33, 62–77. [Google Scholar] [CrossRef]
- Narum, S.R.; Campbell, N.R. Transcriptomic response to heat stress among ecologically divergent populations of redband trout. BMC Genom. 2015, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Gibert, P.; Moreteau, B.; Pétavy, G.; Karan, D.; David, J.R. Chill-coma tolerance, a major climatic adaptation among Drosophila species. Evolution 2001, 55, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; MacMillan, H.A. The integrative physiology of insect chill tolerance. Annu. Rev. Physiol. 2017, 79, 187–208. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Wang, H.; Li, H.; Qu, C.; Wen, J.; Zhang, X.; Zhu, T.; Nie, C.; Li, X.; et al. Genomic and transcriptomic analyses reveal genetic adaptation to cold conditions in the chickens. Genomics 2022, 114, 110485. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Wang, J.; Huang, N.; Jiang, Q.; Ju, Z.; Yang, C.; Wei, X.; Xiao, Y.; Zhang, Y.; et al. Selection signature and CRISPR/Cas9-mediated gene knockout analyses reveal ZC3H10 involved in cold adaptation in Chinese native cattle. Genes 2022, 13, 1910. [Google Scholar] [CrossRef] [PubMed]
- Pirri, F.; Ometto, L.; Fuselli, S.; Fernandes, F.A.N.; Ancona, L.; Perta, N.; Di Marino, D.; Le Bohec, C.; Zane, L.; Trucchi, E. Selection-driven adaptation to the extreme Antarctic environment in the Emperor penguin. Heredity 2022, 129, 317–326. [Google Scholar] [CrossRef]
- Donelson, J.; Sunday, J.; Figueira, W.; Gaitán-Espitia, J.D.; Hobday, A.; Johnson, C.; Leis, J.; Ling, S.; Marshall, D.; Pandolfi, J.; et al. Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180186. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Storey, K.B.; Dong, Y.W. Adaptations to the mudflat: Insights from physiological and transcriptional responses to thermal stress in a burrowing bivalve Sinonovacula constricta. Sci. Total Environ. 2020, 710, 136280. [Google Scholar] [CrossRef]
- Ma, K.; Li, Y.; Liu, X.; Song, W.; Zhou, J.; Gong, X.; Wang, M.; Li, C.; Liu, J.; Tu, Q. Bacteria rather than fungi mediate the chemodiversity of dissolved organic matter in a mudflat intertidal zone. Sci. Total Environ. 2023, 893, 164835. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Yadav, R.; Rajput, V.; Dharne, M.S.; Rastogi, G. Metagenomic analysis reveals genetic insights on biogeochemical cycling, xenobiotic degradation, and stress resistance in mudflat microbiome. J. Environ. Manag. 2021, 292, 112738. [Google Scholar] [CrossRef] [PubMed]
- Phartyal, S.; Rosbakh, S.; Poschlod, P. Seed germination of mudflat species responds differently to prior exposure to hypoxic (flooded) environments. Seed Sci. Res. 2020, 30, 268–274. [Google Scholar] [CrossRef]
- Dong, Y.W.; Yu, S.S.; Wang, Q.L.; Dong, S.L. Physiological responses in a variable environment: Relationships between metabolism, hsp and thermotolerance in an intertidal-subtidal species. PLoS ONE 2011, 6, e26446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, Y. Living on the upper intertidal mudflat: Different behavioral and physiological responses to high temperature between two sympatric cerithidea snails with divergent habitat-use strategies. Mar. Environ. Res. 2020, 159, 105015. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Staton, S.C.; Velotta, J.P.; Winchell, K.M. Selection on adaptive and maladaptive gene expression plasticity during thermal adaptation to urban heat islands. Nat. Commun. 2021, 12, 6195. [Google Scholar] [CrossRef] [PubMed]
- Kassahn, K.S.; Crozier, R.H.; Pörtner, H.O.; Caley, M.J. Animal performance and stress: Responses and tolerance limits at different levels of biological organisation. Biol. Rev. Camb. Philos. Soc. 2009, 84, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. The physiology of global change: Linking patterns to mechanisms. Ann. Rev. Mar. Sci. 2012, 4, 39–61. [Google Scholar] [CrossRef]
- Xu, C.; Ju, X.; Song, D.; Huang, F.; Tang, D.; Zou, Z.; Zhang, C.; Joshi, T.; Jia, L.; Xu, W.; et al. An association analysis between psychophysical characteristics and genome-wide gene expression changes in human adaptation to the extreme climate at the Antarctic Dome Argus. Mol. Psychiatry 2015, 20, 536–544. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Plastic and adaptive gene expression patterns associated with temperature stress in Arabidopsis thaliana. Heredity 2007, 99, 143–150. [Google Scholar] [CrossRef]
- Schoville, S.D.; Barreto, F.S.; Moy, G.W.; Wolff, A.; Burton, R.S. Investigating the molecular basis of local adaptation to thermal stress: Population differences in gene expression across the transcriptome of the copepod Tigriopus californicus. BMC Evol. Biol. 2012, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.R.; Zenger, K.R.; Jerry, D.R. Next-generation transcriptome profiling reveals insights into genetic factors contributing to growth differences and temperature adaptation in Australian populations of barramundi (Lates calcarifer). Mar. Genom. 2013, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Brix, K.V. Evolution of salinity tolerance from transcriptome to physiological system. Mol. Ecol. 2013, 22, 3656–3658. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lshimatsu, A.; Fu, C.; Yin, W.; Li, G.; Chen, H.; Wu, Q.; Li, B. Cryptic species and historical biogeography of eel gobies (Gobioidei: Odontamblyopus) along the northwestern Pacific coast. Zool. Sci. 2010, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.; Liu, T.; Liu, Y.; Wang, Y.; Liu, J.; Liu, B.; Gong, L.; Liu, L. Climate adaptation and drift shape the genomes of two eel-goby sister species endemic to contrasting latitude. Animals 2023, 13, 3240. [Google Scholar] [CrossRef] [PubMed]
- Murdy, E.O.; Shibukawa, K. Odontamblyopus rebecca, a new species of amblyopine goby from Vietnam with a key to known species of the genus (Gobiidae: Amblyopinae). Zootaxa 2003, 138, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.; Song, N.; Yanagimoto, T.; Zhou, Z.; Yang, J.; Gao, T. Identification of Odontamblyopus lacepedii via morphology and DNA barcoding. Biochem. Syst. Ecol. 2016, 66, 281–285. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, S.; Xu, S.; Xiong, P.; Cao, Y.; Chen, Z.; Li, M. Comparison of environmental DNA metabarcoding and bottom trawling for detecting seasonal fish communities and habitat preference in a highly disturbed estuary. Ecol. Indic. 2023, 146, 109754. [Google Scholar] [CrossRef]
- Zhang, C.G.; Li, Y.H.; Zhong, Y.C. Investigation on fish eggs, larvae and juveniles in Beitang River Estuary. Acta. Oceanol. Sin. 1981, 6, 856–866. (In Chinese) [Google Scholar]
- Wang, Q.L.; Dong, Y.W.; Qin, C.X.; Yu, S.X.; Dong, S.L.; Wang, F. Effects of rearing temperature on growth, metabolism and thermal tolerance of juvenile sea cucumber, Apostichopus japonicus Selenka: Critical thermal maximum (CTmax) and hsps gene expression. Aquac. Res. 2013, 44, 1550–1559. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Chrétien, E.; Chapman, L.J. Divergence in aerobic scope and thermal tolerance is related to local thermal regime in two populations of introduced Nile perch (Lates niloticus). J. Fish. Biol. 2020, 97, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Currie, R.J.; Bennett, W.A.; Beitinger, T.L. Critical thermal minima and maxima of three freshwater game-fish species acclimated to constant temperatures. Environ. Biol. Fishes 1998, 51, 187–200. [Google Scholar] [CrossRef]
- Hazell, S.P.; Bale, J.S. Low temperature thresholds: Are chill coma and CT(min) synonymous? J. Insect. Physiol. 2011, 57, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Ospina, A.F.; Mora, C. Effect of body size on reef fish tolerance to extreme low and high temperatures. Environ. Biol. Fishes 2004, 70, 339–343. [Google Scholar] [CrossRef]
- Becker, C.D.; Genoway, R.G. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ. Biol. Fishes 1979, 4, 245–256. [Google Scholar] [CrossRef]
- Madeira, D.; Mendonça, V.; Dias, M.; Roma, J.; Costa, P.M.; Diniz, M.S.; Vinagre, C. Physiological and biochemical thermal stress response of the intertidal rock goby Gobius paganellus. Ecol. Indic. 2014, 46, 232–239. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.J.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold. Spring Harb. Protoc. 2010, 2010, pdb-prot5439. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Team, R.D.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. Computing 2009, 14, 12–21. [Google Scholar]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. Mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R. J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Liu, S.; Li, M.; Yao, R.; Niu, S.; Yuan, J.; Wang, H.; Hu, J.; Bao, Z.; et al. Transcriptome and network analyses reveal key pathways and genes involved in response to carotenoid deposition in scallop muscle. Front. Mar. Sci. 2023, 10, 1158325. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Dou, M.M.; Liu, Z.Q.; Jiao, D.C.; Li, Z.N.; Chen, J.J.; Li, J.; Yao, Y.; Li, L.F.; Li, Y.H.; et al. Screening prognosis-related lncRNAs based on WGCNA to establish a new risk score for predicting prognosis in patients with Hepatocellular carcinoma. J. Immunol. Res. 2021, 2021, 5518908. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Li, X.; Ou, Y. Identifying prognosis and metastasis-associated genes associated with Ewing sarcoma by weighted gene co-expression network analysis. Oncol. Lett. 2019, 18, 3527–3536. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Z.; Shen, Z.; Zhang, C.; Liu, H. Regional differences in thermal adaptation of a cold-water fish Rhynchocypris oxycephalus revealed by thermal tolerance and transcriptomic responses. Sci. Rep. 2018, 8, 11703. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.N.; Pu, J.J.; Tao, K.X.; Zhao, X.X.; Yang, Q.Q. Genome-wide identification and characterization of the HSP gene superfamily in apple snails (Gastropoda: Ampullariidae) and expression analysis under temperature stress. Int. J. Biol. Macromol. 2022, 222, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Ma, C.S.; Yan, Y.; Renault, D.; Colinet, H. The interspecific variations in molecular responses to various doses of heat and cold stress: The case of cereal aphids. J. Insect. Physiol. 2023, 147, 104520. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Tan, X.; Lu, Y.; Wu, Z.; Li, J.; Xu, D.; Zhang, P.; You, F. Network of microRNA-transcriptional factor-mRNA in cold response of turbot Scophthalmus maximus. Fish. Physiol. Biochem. 2019, 45, 583–597. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Guo, X.; Wei, W.; Li, Z.; Zhou, L.; Wang, Z.; Gui, J. Comparative transcriptomes and metabolomes reveal different tolerance mechanisms to cold stress in two different catfish species. Aquaculture 2022, 560, 738543. [Google Scholar] [CrossRef]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish. Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Liu, R.; Long, Y.; Liu, R.; Song, G.; Li, Q.; Yan, H.; Cui, Z. Understanding the function and mechanism of zebrafish Tmem39b in regulating cold resistance. Int. J. Mol. Sci. 2022, 23, 11442. [Google Scholar] [CrossRef]
- Lyu, L.; Wen, H.; Li, Y.; Li, J.; Zhao, J.; Zhang, S.; Song, M.; Wang, X. Deep transcriptomic analysis of black rockfish (Sebastes schlegelii) provides new insights on responses to acute temperature stress. Sci. Rep. 2018, 8, 9113. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Z.; Yang, Y.; Wang, J.; Yang, T.; Chen, X.; Liang, L.; Mu, W. Histology, physiology, and glucose and lipid metabolism of Lateolabrax maculatus under low temperature stress. J. Therm. Biol. 2022, 104, 103161. [Google Scholar] [CrossRef]
- Verleih, M.; Borchel, A.; Krasnov, A.; Rebl, A.; Korytář, T.; Kühn, C.; Goldammer, T. Impact of thermal stress on kidney-specific gene expression in farmed regional and imported rainbow trout. Mar. Biotechnol. 2015, 17, 576–592. [Google Scholar] [CrossRef]
- Jiang, H.; Lv, W.; Wang, Y.; Qian, Y.; Wang, C.; Sun, N.; Fang, C.; Irwin, D.M.; Gan, X.; He, S.; et al. Multi-omics investigation of freeze tolerance in the amur sleeper, an aquatic ectothermic vertebrate. Mol. Biol. Evol. 2023, 40, msad040. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.F.; Grosso, A.R.; Almeida-Val, V.M.F.; Coelho, M.M. Transcriptome profiling of two Iberian freshwater fish exposed to thermal stress. J. Therm. Biol. 2016, 55, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, M.; Liu, Y.; Wang, J.; Zhang, D.; Niu, H.; Jiang, S.; Wang, J.; Zhang, D.; Han, B.; et al. Transcriptome comparison reveals a genetic network regulating the lower temperature limit in fish. Sci. Rep. 2016, 6, 28952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, J.; Zhu, J.; Wang, Y.; Zhang, Y.; Li, Y.; Xu, S.; Yan, X.; Zhang, D. Transcriptome, antioxidant enzymes and histological analysis reveal molecular mechanisms responsive to long-term cold stress in silver pomfret (Pampus argenteus). Fish Shellfish. Immunol. 2022, 121, 351–361. [Google Scholar] [CrossRef]
- Vigh, L.; Los, D.A.; Horváth, I.; Murata, N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc. Natl. Acad. Sci. USA 1993, 90, 9090–9094. [Google Scholar] [CrossRef]
- Lee, S.H.; Dobrzyn, A.; Dobrzyn, P.; Rahman, S.M.; Miyazaki, M.; Ntambi, J.M. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J. Lipid. Res. 2004, 45, 1674–1682. [Google Scholar] [CrossRef]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.; Cong, R.; Qi, H.; Wang, W.; Zhang, G.; Li, L. Cis- and trans-variations of stearoyl-coA desaturase provide new insights into the mechanisms of diverged pattern of phenotypic plasticity for temperature adaptation in two congeneric oyster species. Mol. Biol. Evol. 2023, 40, msad015. [Google Scholar] [CrossRef]
- Schünke, M.; Wodtke, E. Cold-induced increase of Δ9- and Δ6- desaturase activities in endoplasmic membranes of carp liver. Biochim. Biophys. Acta. 1983, 734, 70–75. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Kuo, C.M. Stearoyl-CoA desaturase expression and fatty acid composition in milkfish (Chanos chanos) and grass carp (Ctenopharyngodon idella) during cold acclimation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 141, 95–101. [Google Scholar] [CrossRef]
- Jesus, T.F.; Moreno, J.M.; Repolho, T.; Athanasiadis, A.; Rosa, R.; Almeida-Val, V.M.F.; Coelho, M.M. Protein analysis and gene expression indicate differential vulnerability of Iberian fish species under a climate change scenario. PLoS ONE 2017, 12, e0181325. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Liang, S.; Lin, G.; Chen, S.; Yang, G. Tissue-overlapping response of half-smooth tongue sole (Cynoglossus semilaevis) to thermostressing based on transcriptome profiles. Gene 2016, 586, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, R.; Wang, X.; Zhu, H.; Tian, Z. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.C.; Hsiao, Y.C.; Sun, H.S.; Chen, T.M.; Lee, S.J. MicroRNAs regulate gene plasticity during cold shock in zebrafish larvae. BMC Genom. 2016, 17, 922. [Google Scholar] [CrossRef] [PubMed]
- So, J.; Taleb, S.; Wann, J.; Strobel, O.; Kim, K.; Roh, H.C. Chronic cAMP activation induces adipocyte browning through discordant biphasic remodeling of transcriptome and chromatin accessibility. Mol. Metab. 2022, 66, 101619. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Liu, M.; Han, Z.; Gao, T. Comparative transcriptome reveals the thermal stress response differences between Heilongjiang population and Xinjiang population of Lota lota. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100960. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Chan, C.R.; Weng, C.F. Innate-immune responses of tilapia (Oreochromis mossambicus) exposure to acute cold stress. J. Cell. Physiol. 2019, 234, 16125–16135. [Google Scholar] [CrossRef]
- Negishi, K.; Aizawa, K.; Shindo, T.; Suzuki, T.; Sakurai, T.; Saito, Y.; Miyakawa, T.; Tanokura, M.; Kataoka, Y.; Maeda, M.; et al. An Myh11 single lysine deletion causes aortic dissection by reducing aortic structural integrity and contractility. Sci. Rep. 2022, 12, 8844. [Google Scholar] [CrossRef]
- Zhao, J.; Tao, C.; Chen, C.; Wang, Y.; Liu, T. Formation of thermogenic adipocytes: What we have learned from pigs. Fundam. Res. 2021, 1, 495–502. [Google Scholar] [CrossRef]
- Kim, J.S.; Han, H.S.; Seong, J.K.; Ko, Y.G.; Koo, S.H. Involvement of a novel cAMP signaling mediator for beige adipogenesis. Metabolism 2023, 143, 155536. [Google Scholar] [CrossRef]
- Bernardinelli, E.; Costa, R.; Scantamburlo, G.; To, J.; Morabito, R.; Nofziger, C.; Doerrier, C.; Krumschnabel, G.; Paulmichl, M.; Dossena, S. Mis-targeting of the mitochondrial protein LIPT2 leads to apoptotic cell death. PLoS ONE 2017, 12, e0179591. [Google Scholar] [CrossRef] [PubMed]
- Habarou, F.; Hamel, Y.; Haack, T.B.; Feichtinger, R.G.; Lebigot, E.; Marquardt, I.; Busiah, K.; Laroche, C.; Madrange, M.; Grisel, C.; et al. Biallelic mutations in LIPT2 cause a mitochondrial lipoylation defect associated with severe neonatal encephalopathy. Am. J. Hum. Genet 2017, 101, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hui, J.H.L.; Williams, G.A.; Cartwright, S.R.; Tsang, L.M.; Chu, K.H. Comparative transcriptomics across populations offers new insights into the evolution of thermal resistance in marine snails. Mar. Biol. 2016, 163, 92. [Google Scholar] [CrossRef]
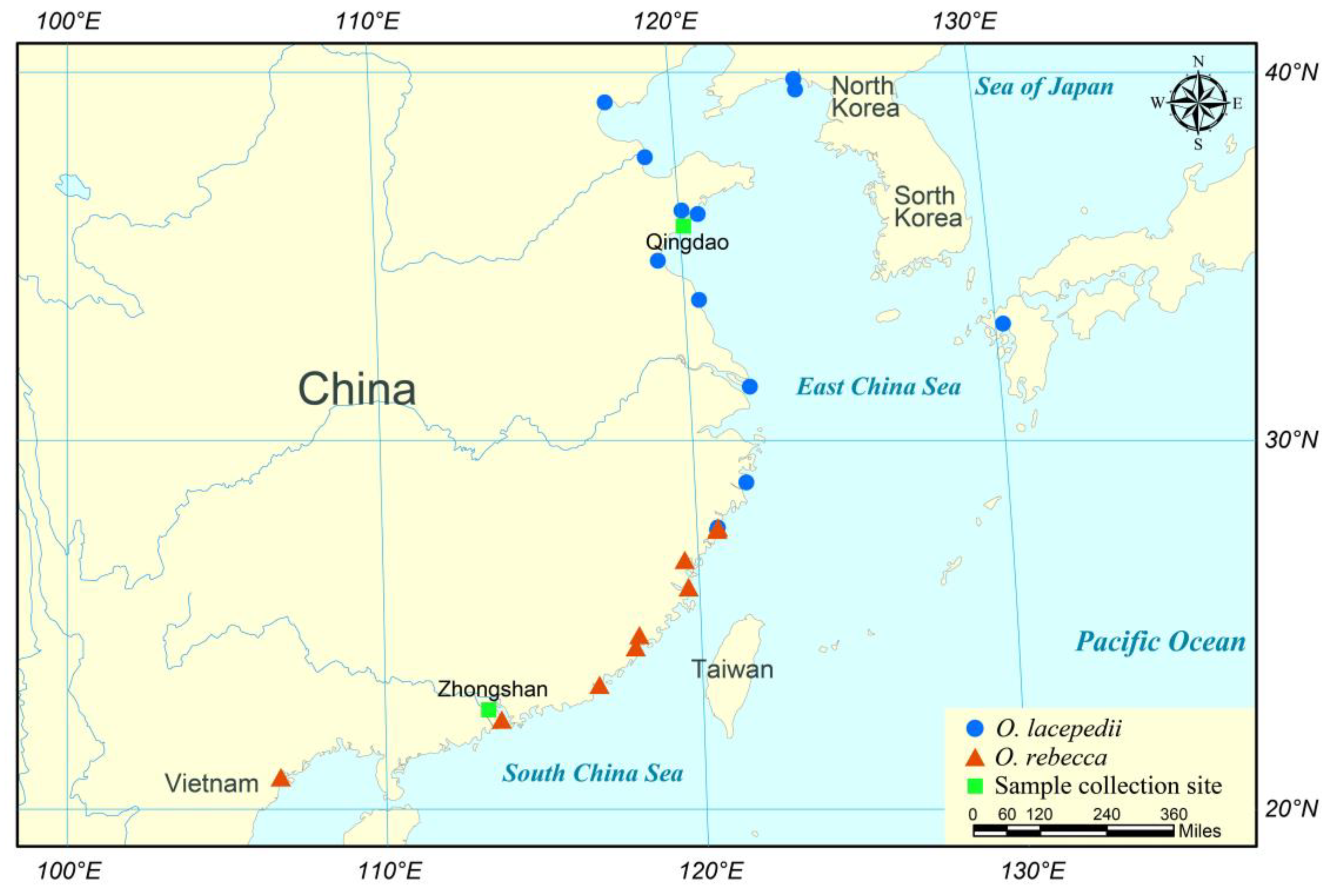

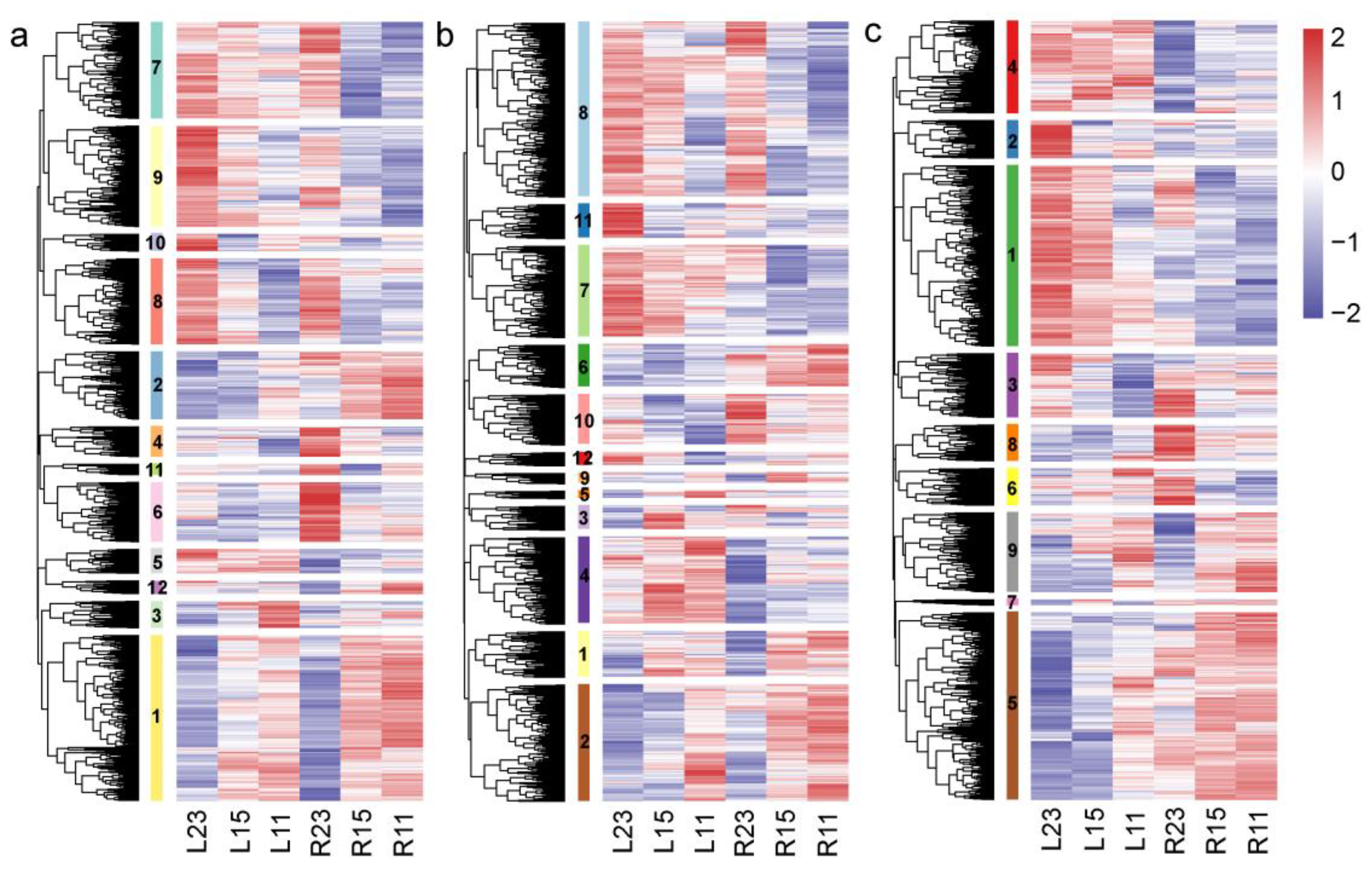

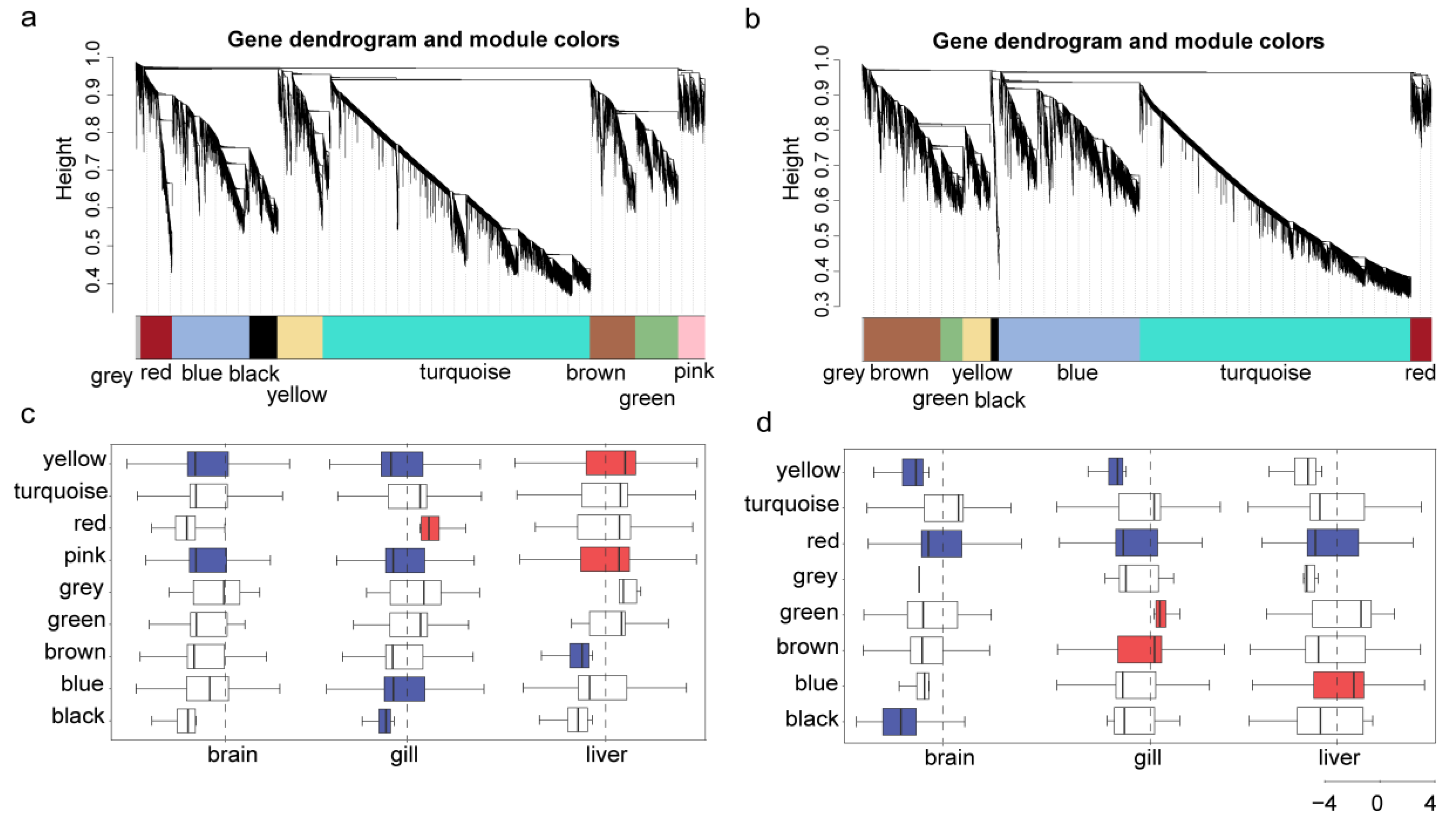
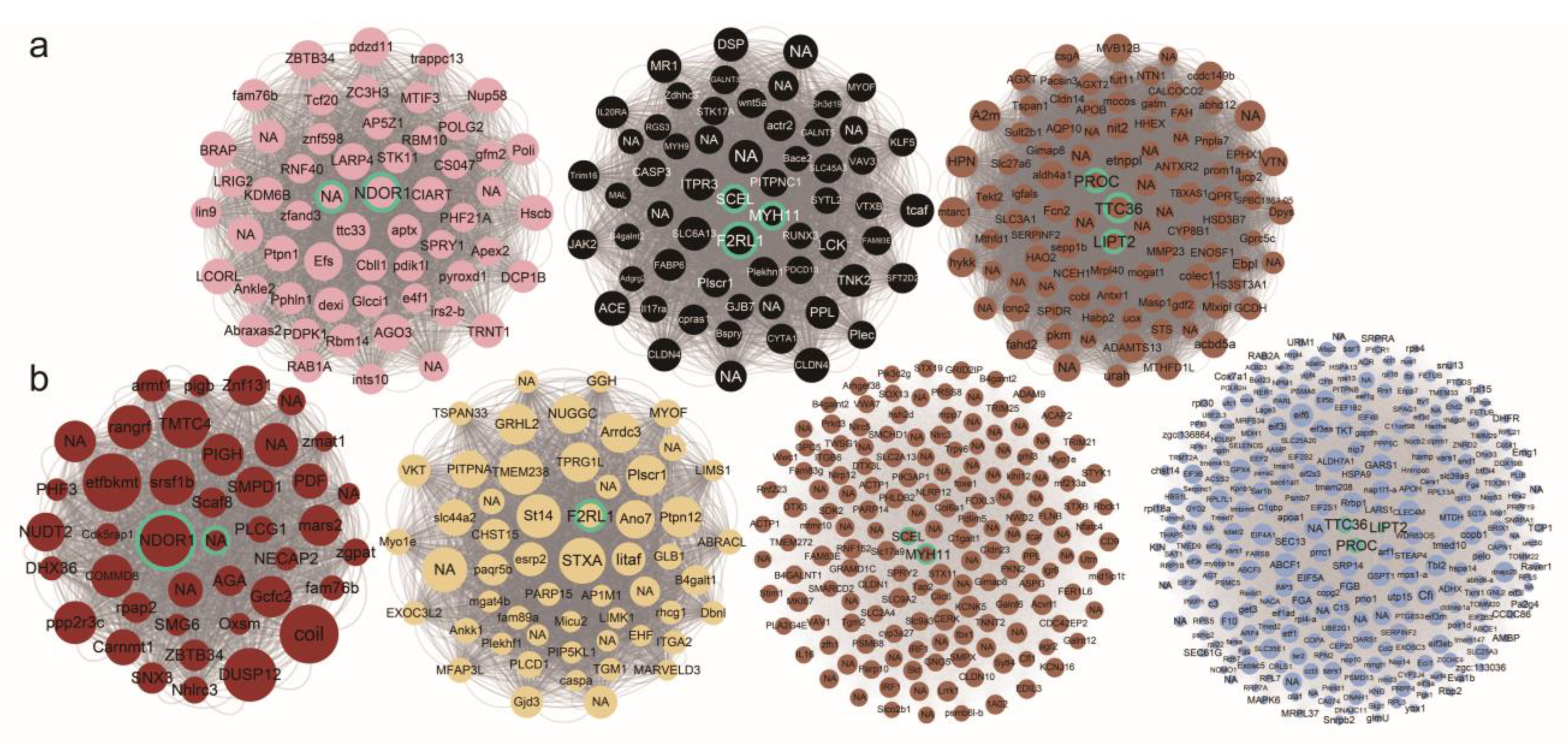
| Species | Sample Locations | Number | Body Mass (g) | CTmin (°C) |
|---|---|---|---|---|
| O. lacepedii | Qingdao, Shandong Province (120°19′ E, 36°16′ N; 3.0–26.7 °C) | 30 | 76.96 ± 14.09 | 0.61 ± 1.23 |
| O. rebecca | Zhongshan, Guangdong Province (113°36′ E, 22°30′ N; 16.9–29.9 °C) | 30 | 68.34 ± 9.48 | 9.57 ± 1.19 |
| GO ID | Description | GeneRatio | BgRatio | p Value | Count |
|---|---|---|---|---|---|
| GO:0016051 | carbohydrate biosynthetic process | 3/48 | 14/6996 | 1.05 × 10−4 | 3 |
| GO:0006629 | lipid metabolic process | 7/48 | 192/6996 | 2.98 × 10−4 | 7 |
| GO:0008610 | lipid biosynthetic process | 4/48 | 59/6996 | 6.73 × 10−4 | 4 |
| GO:0007623 | circadian rhythm | 3/48 | 10/6996 | 2.00 × 10−3 | 3 |
| GO:0048511 | rhythmic process | 3/48 | 10/6996 | 2.00 × 10−3 | 3 |
| GO:0048523 | negative regulation of cellular process | 3/48 | 69/6996 | 1.16 × 10−2 | 3 |
| GO:0008654 | phospholipid biosynthetic process | 2/48 | 25/6996 | 1.25 × 10−2 | 2 |
| GO:0048519 | negative regulation of biological process | 3/48 | 80/6996 | 1.72 × 10−2 | 3 |
| GO:0005975 | carbohydrate metabolic process | 4/48 | 162/6996 | 2.45 × 10−2 | 4 |
| GO:0006886 | intracellular protein transport | 3/48 | 107/6996 | 3.66 × 10−2 | 3 |
| GO:0048522 | positive regulation of cellular process | 2/48 | 49/6996 | 4.42 × 10−2 | 2 |
| GO:0048518 | positive regulation of biological process | 2/48 | 51/6996 | 4.75 × 10−2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, T.; Liu, Y.; Wang, Y.; Liu, L.; Gong, L.; Liu, B.; Lü, Z. Comparative Transcriptome Analyses Provide New Insights into the Evolution of Divergent Thermal Resistance in Two Eel Gobies. Curr. Issues Mol. Biol. 2024, 46, 153-170. https://doi.org/10.3390/cimb46010012
Liu J, Liu T, Liu Y, Wang Y, Liu L, Gong L, Liu B, Lü Z. Comparative Transcriptome Analyses Provide New Insights into the Evolution of Divergent Thermal Resistance in Two Eel Gobies. Current Issues in Molecular Biology. 2024; 46(1):153-170. https://doi.org/10.3390/cimb46010012
Chicago/Turabian StyleLiu, Jing, Tianwei Liu, Yantao Liu, Yuzhen Wang, Liqin Liu, Li Gong, Bingjian Liu, and Zhenming Lü. 2024. "Comparative Transcriptome Analyses Provide New Insights into the Evolution of Divergent Thermal Resistance in Two Eel Gobies" Current Issues in Molecular Biology 46, no. 1: 153-170. https://doi.org/10.3390/cimb46010012




