Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses
Abstract
:1. Introduction
2. Phenolic Acid Diversification
3. Phenolic Acid Generation in Plants

4. Phenolic Acids in Plants under Salt Stress Conditions
5. The Impact of Hydroxycinnamic Acids and Their Derivatives on Drought Stress
6. The Role of Hydroxycinnamic Acids and Their Derivatives in Heavy Metal Tolerance
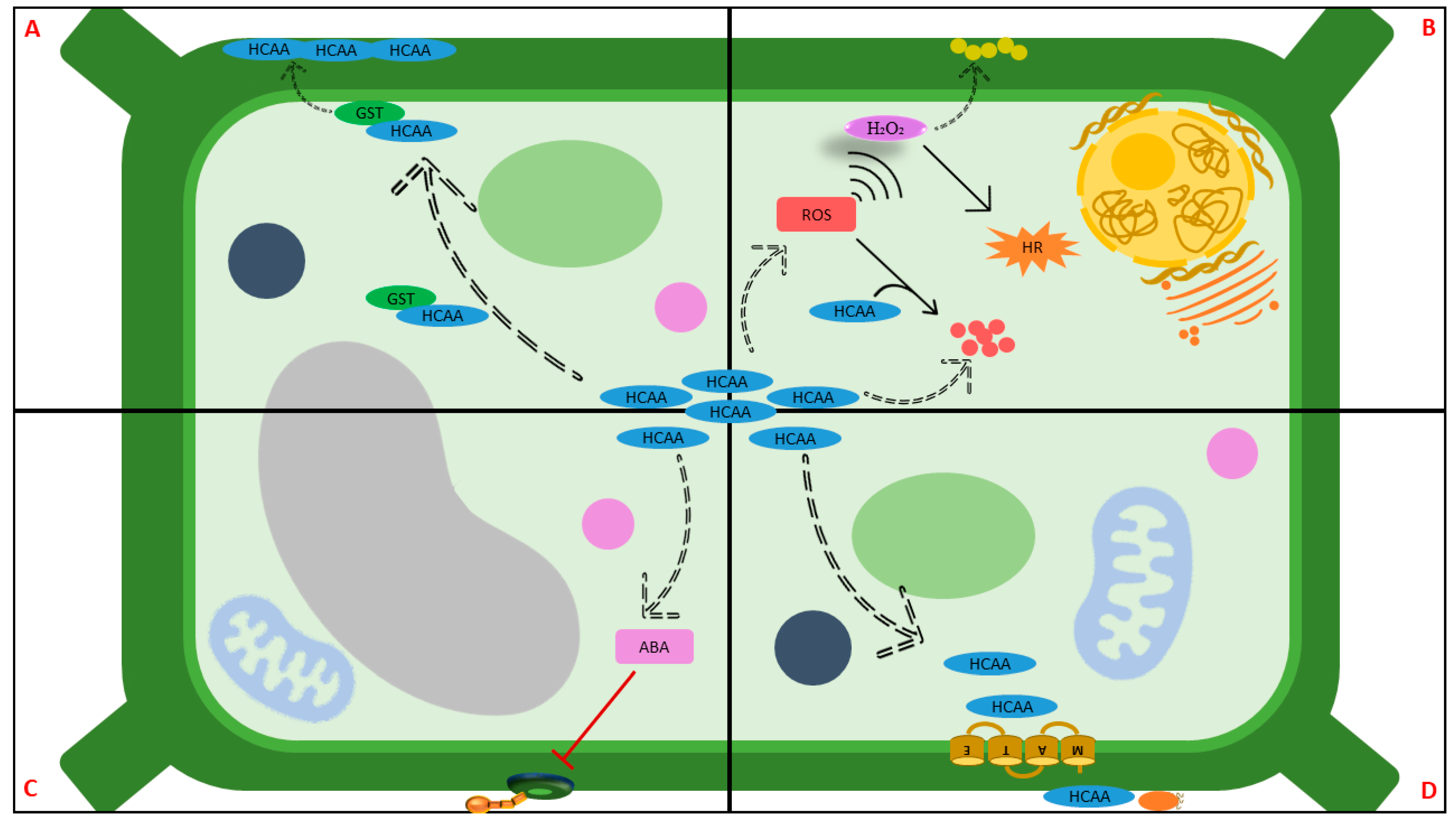
7. The Role of Hydroxycinnamic Acids and Their Derivatives in Plant Disease Resistance
8. Role of Phenolic Acids in Plant Growth under Temperature Stress
9. Integrating Proteomic Approaches to Improving Stress Tolerance
10. Future Perspectives
11. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.N.; Robinson, R.W.; Buultjens, A.; Al Harun, M.A.Y.; Shampa, S.H. Role of allelopathy of Phragmites australis in its invasion processes. J. Exp. Mar. Bio. Ecol. 2017, 486, 237–244. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Haider, M.S.; Jaskani, M.J.; Fang, J. Overproduction of ROS: Underlying molecular mechanism of scavenging and redox signaling. Biocontrol Agents Second. Metab. Appl. Immun. Plant Growth Prot. 2021, 347–382. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2020, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Elbasheir, E.; Ndiko, L. Antioxidant responses are associated with differences in drought tolerance between maize and sorghum. In Proceedings of the 4th Tunisian-South African Mini-Symposium on «Biodiversity in Forage and Feed Crops for Sustainable Productivity under Environmental Constraints», Borj Cedria, Tunisia, 18–19 March 2021; pp. 1–12. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Pratyusha, S.; Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Qiao, W.; Fan, L.M. Nitric oxide signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2008, 50, 1238–1246. [Google Scholar] [CrossRef]
- Kısa, D.; Kayır, Ö.; Sağlam, N.; Şahin, S.; Öztürk, L.; Elmastaş, M. Changes of Phenolic Compounds in Tomato Associated with The Heavy Metal Stress. Res. Artic. Araştırma Makal. Bartın Univ. Int. J. Nat. Appl. Sci. 2019, 2, 35–43. [Google Scholar]
- Nkomo, M.; Gokul, A.; Ndimba, R.; Badiwe, M.; Keyster, M.; Klein, A. Piperonylic acid alters growth, mineral content accumulation and reactive oxygen species-scavenging capacity in chia seedlings. AoB Plants 2022, 14, plac025. [Google Scholar] [CrossRef]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. Plant Phenolics Sustain. Agric. 2020, 1, 517–532. [Google Scholar] [CrossRef]
- Zaid, A.; Ahmad, B.; Wani, S.H. Medicinal and aromatic plants under abiotic stress: A crosstalk on phytohormones’ perspective. In Plant Growth Regulators; Springer: Berlin/Heidelberg, Germany, 2021; pp. 115–132. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Bhat, B.A.; Mir, W.R.; Sheikh, B.A.; Rather, M.A.; Dar, T.U.H.; Mir, M.A. In vitro and in silico evaluation of antimicrobial properties of Delphinium cashmerianum L., a medicinal herb growing in Kashmir, India. J. Ethnopharmacol. 2022, 291, 115046. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Bujor, O.C.; Talmaciu, I.A.; Volf, I.; Popa, V.I. Biorefining to recover aromatic compounds with biological properties. TAPPI J. 2015, 14, 187–193. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Prabhu, S.; Molath, A.; Choksi, H.; Kumar, S.; Mehra, R. Classifications of polyphenols and their potential application in human health and diseases. Int. J. Physiol. Nutr. Phys. Educ. 2021, 6, 293–301. [Google Scholar] [CrossRef]
- Delgado, A.; Gonçalves, S.; Romano, A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods 2023, 12, 840. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–205. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.E.F.; et al. Hydroxycinnamic Acids: Natural Sources, Biosynthesis, Possible Biological Activities, and Roles in Islamic Medicine. Stud. Nat. Prod. Chem. 2017, 55, 269–292. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arabidopsis Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Vashisth, D.; Misra, A.; Akhtar, M.Q.; Jalil, S.U.; Shanker, K.; Gupta, M.M.; Rout, P.K.; Gupta, A.K.; Shasany, A.K. RNAi down-regulation of cinnamate-4-hydroxylase increases artemisinin biosynthesis in Artemisia annua. Sci. Rep. 2016, 6, 26458. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Block, A.; Widhalm, J.R.; Fatihi, A.; Cahoon, R.E.; Wamboldt, Y.; Elowsky, C.; Mackenzie, S.A.; Cahoon, E.B.; Chapple, C.; Dudareva, N.; et al. The Origin and Biosynthesis of the Benzenoid Moiety of Ubiquinone (Coenzyme Q) in Arabidopsis. Plant Cell 2014, 26, 1938–1948. [Google Scholar] [CrossRef]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA. 2012, 109, 16383–16388. [Google Scholar] [CrossRef]
- Otto, M.; Wynands, B.; Marienhagen, J.; Blank, L.; Wierckx, N. Benzoate synthesis from glucose or glycerol using engineered Pseudomonas taiwanensis. Authorea Prepr. 2020, 15, 2000211. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Labiad, M.H.; Giménez, A.; Varol, H.; Tüzel, Y.; Egea-Gilabert, C.; Fernández, J.A.; Martínez-Ballesta, M.D.C. Effect of Exogenously Applied Methyl Jasmonate on Yield and Quality of Salt-Stressed Hydroponically Grown Sea Fennel (Crithmum maritimum L.). Agronomy 2021, 11, 1083. [Google Scholar] [CrossRef]
- Zvanarou, S.; Vágnerová, R.; Mackievic, V.; Usnich, S.; Smolich, I.; Sokolik, A.; Yu, M.; Huang, X.; Angelis, K.J.; Demidchik, V. Salt stress triggers generation of oxygen free radicals and DNA breaks in Physcomitrella patens protonema. Environ. Exp. Bot. 2020, 180, 104236. [Google Scholar] [CrossRef]
- Hayat, K.; Zhou, Y.; Menhas, S.; Hayat, S.; Aftab, T.; Bundschuh, J.; Zhou, P. Salicylic Acid Confers Salt Tolerance in Giant Juncao Through Modulation of Redox Homeostasis, Ionic Flux, and Bioactive Compounds: An Ionomics and Metabolomic Perspective of Induced Tolerance Responses. J. Plant Growth Regul. 2022, 41, 1999–2019. [Google Scholar] [CrossRef]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek-Sondi, B. Involvement of Phenolic Acids in Short-Term Adaptation to Salinity Stress is Species-Specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Nedved, E.L.; Kalatskaja, J.N.; Ovchinnikov, I.A.; Rybinskaya, E.I.; Kraskouski, A.N.; Nikalaichuk, V.V.; Hileuskaya, K.S.; Kulikouskaya, V.I.; Agabekov, V.E.; Laman, N.A. Growth Parameters and Antioxidant Activity in Cucumber Seedlings with the Application of Chitosan and Hydroxycinnamic Acids Conjugates under Salt Stress. Appl. Biochem. Microbiol. 2022, 58, 69–76. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jáuregui, O.; Codina, C.; Tiburcio, A.F.; Bastida, J.; Viladomat, F. Analysis of phenolic compounds by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry in senescent and water-stressed tobacco. Plant Sci. 2012, 182, 71–78. [Google Scholar] [CrossRef]
- Latif, F.; Ullah, F.; Mehmood, S.; Khattak, A.; Khan, A.U.; Khan, S.; Husain, I. Effects of salicylic acid on growth and accumulation of phenolics in Zea mays L. under drought stress. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2016, 66, 325–332. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y. Comparison of heavy metals in riverine and estuarine sediments in the lower Yangtze River: Distribution, sources, and ecological risks. Environ. Technol. Innov. 2023, 30, 103076. [Google Scholar] [CrossRef]
- Boquete, M.T.; Lang, I.; Weidinger, M.; Richards, C.L.; Alonso, C. Patterns and mechanisms of heavy metal accumulation and tolerance in two terrestrial moss species with contrasting habitat specialization. Environ. Exp. Bot. 2021, 182, 104336. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef] [PubMed]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Lwalaba, J.L.W.; Zvobgo, G.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Kirika, B.A.; Tshibangu, A.K.; Adil, M.F.; Sehar, S.; Mukobo, R.P.; et al. High accumulation of phenolics and amino acids confers tolerance to the combined stress of cobalt and copper in barley (Hordeum vulagare). Plant Physiol. Biochem. 2020, 155, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Samota, M.K.; Tanti, A.J.; Babu, A. Trichoderma reesei induces defense-related biochemical markers associated with resistance to Fusarium dieback in tea crop. Biol. Control 2023, 180, 105200. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Chakraborty, N.; Chandra, S.; Sarkar, A.; Ghosh, S.; Dasgupta, A.; Acharya, K. An in planta approach for understanding defense responses in tomato plants against Fusarium oxysporum Schltdl. J. Plant Pathol. 2023, 105, 129–136. [Google Scholar] [CrossRef]
- Gershenzon, J.; Ullah, C. Plants protect themselves from herbivores by optimizing the distribution of chemical defenses. Proc. Natl. Acad. Sci. USA. 2022, 119, e2120277119. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; João, M.; Queiroz, R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, L.; Su, J.; Tian, B.; Fang, A.; Yu, Y.; Bi, C.; Yang, Y. Integrated Metabolo-transcriptomics Reveals the Defense Response of Homogentisic Acid in Wheat against Puccinia striiformis f. sp. tritici. J. Agric. Food Chem. 2022, 70, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Campos, L.; Lisón, P.; Pilar López-Gresa, M.; Rodrigo, I.; Zacarés, L.; Conejero, V.; Bellés, J.M. Transgenic Tomato Plants Overexpressing Tyramine N-Hydroxycinnamoyltransferase Exhibit Elevated Hydroxycinnamic Acid Amide Levels and Enhanced Resistance to Pseudomonas syringae. Mol. Plant-Microbe Interact. 2014, 27, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef]
- Ghorbi, M.; Momeni, H.; Rashidi, V.; Ahmadzadeh, A.; Yarnia, M. Changes in Phenolic Acid Levels in Wheat Cultivars Inoculated with Pyrenophora tritici-repentis race 1. J. Agric. Sci. Technol. 2023, 25, 185–198. [Google Scholar] [CrossRef]
- Atak, A.; Göksel, Z.; Yılmaz, Y. Changes in Major Phenolic Compounds of Seeds, Skins, and Pulps from Various Vitis spp. and the Effect of Powdery and Downy Mildew Diseases on Their Levels in Grape Leaves. Plants 2021, 10, 2554. [Google Scholar] [CrossRef]
- Whitney, K.; Gracia-Gomez, G.; Anderson, J.A.; Simsek, S. Time Course Metabolite Profiling of Fusarium Head Blight-Infected Hard Red Spring Wheat Using Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole Time of Flight/MS. J. Agric. Food Chem. 2022, 70, 4152–4163. [Google Scholar] [CrossRef] [PubMed]
- Macoy, D.M.; Kim, W.Y.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Kim, S.B.; Van den Broeck, L.; Karre, S.; Choi, H.; Christensen, S.A.; Wang, G.F.; Jo, Y.; Cho, W.K.; Balint-Kurti, P. Analysis of the transcriptomic, metabolomic, and gene regulatory responses to Puccinia sorghi in maize. Mol. Plant Pathol. 2021, 22, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Carucci, A.; Rodrigues-Pousada, R.A.; Tisi, A.; Franchi, S.; Tavladoraki, P.; Angelini, R.; Cona, A. The Apoplastic Copper AMINE OXIDASE1 Mediates Jasmonic Acid-Induced Protoxylem Differentiation in Arabidopsis Roots. Plant Physiol. 2015, 168, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Møller, I.M. Plant Physiology and Development; Ian, M., Murphy, A.S., Zeiger, E., Eds.; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Moreira, X.; Abdala-Roberts, L.; Hidalgo-Galvez, M.D.; Vázquez-González, C.; Pérez-Ramos, I.M. Micro-climatic effects on plant phenolics at the community level in a Mediterranean savanna. Sci. Rep. 2020, 10, 14757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, J.; Ye, F.; Zhao, G. Non-covalent interaction between ferulic acid and arabinan-rich pectic polysaccharide from rapeseed meal. Int. J. Biol. Macromol. 2017, 103, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Singh, N.K.; Prasad, M. Multi-omics approaches for strategic improvement of stress tolerance in underutilized crop species: A climate change perspective. Adv. Genet. 2019, 103, 1–38. [Google Scholar] [CrossRef]
- Caldana, C.; Degenkolbe, T.; Cuadros-Inostroza, A.; Klie, S.; Sulpice, R.; Leisse, A.; Steinhauser, D.; Fernie, A.R.; Willmitzer, L.; Hannah, M.A. High-density kinetic analysis of the metabolomic and transcriptomic response of Arabidopsis to eight environmental conditions. Plant J. 2011, 67, 869–884. [Google Scholar] [CrossRef]
- Wu, X.; Gong, F.; Cao, D.; Hu, X.; Wang, W. Advances in crop proteomics: PTMs of proteins under abiotic stress. Proteomics 2016, 16, 847–865. [Google Scholar] [CrossRef]
- Neilson, K.A.; Gammulla, C.G.; Mirzaei, M.; Imin, N.; Haynes, P.A. Proteomic analysis of temperature stress in plants. Proteomics 2010, 10, 828–845. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Keyster, M.; Klein, A. No Exogenous Caffeic Acid Alters Physiological and Molecular Responses in Chia (Salvia hispanica L.) Title. S. Afr. J. Bot. 2017, 100, 339–340. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, R.D.; Grewal, S.K. Mitigation of salinity-induced oxidative damage in wheat (Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol. Plant 2017, 39, 221. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Plant proteomic research for improvement of food crops under stresses: A review. Mol. Omi. 2021, 17, 860–880. [Google Scholar] [CrossRef]
- Nkomo, M.A. The role of p-coumaric acid on physiological and biochemical response of chia seedling under salt stress. Ph.D. Thesis, University of the Western cape, Cape Town, South Africa, 2020. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khawula, S.; Gokul, A.; Niekerk, L.-A.; Basson, G.; Keyster, M.; Badiwe, M.; Klein, A.; Nkomo, M. Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses. Curr. Issues Mol. Biol. 2024, 46, 81-95. https://doi.org/10.3390/cimb46010007
Khawula S, Gokul A, Niekerk L-A, Basson G, Keyster M, Badiwe M, Klein A, Nkomo M. Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses. Current Issues in Molecular Biology. 2024; 46(1):81-95. https://doi.org/10.3390/cimb46010007
Chicago/Turabian StyleKhawula, Sindiswa, Arun Gokul, Lee-Ann Niekerk, Gerhard Basson, Marshall Keyster, Mihlali Badiwe, Ashwil Klein, and Mbukeni Nkomo. 2024. "Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses" Current Issues in Molecular Biology 46, no. 1: 81-95. https://doi.org/10.3390/cimb46010007






