Assessment of the Concentration of Transforming Growth Factor Beta 1–3 in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Group
2.3. Control Group
2.4. Securing the Extracted Material for Molecular Examination
2.5. Extraction of Whole Ribonucleic Acid (RNA)
2.6. Determination of Changes in the Expression Profile of TGF-β-1-3 mRNA in Degenerated and Control IVDs Using RT-qPCR
2.7. Determination of the Profile of TGF-β-1-3 Proteins Through ELISA and Western Blot Separation Procedures in Degenerated and Healthy IVDs
2.8. IHC Analysis
2.9. Statistical Analysis
3. Results
3.1. Changes in the Expression Profile of TGF-β-1-3 mRNA in Degenerated and Control IVDs
3.2. TGF-β-1-3 Protein Expression Profile Determined Through the Enzyme-Linked Immunosorbent Assay (ELISA) Technique
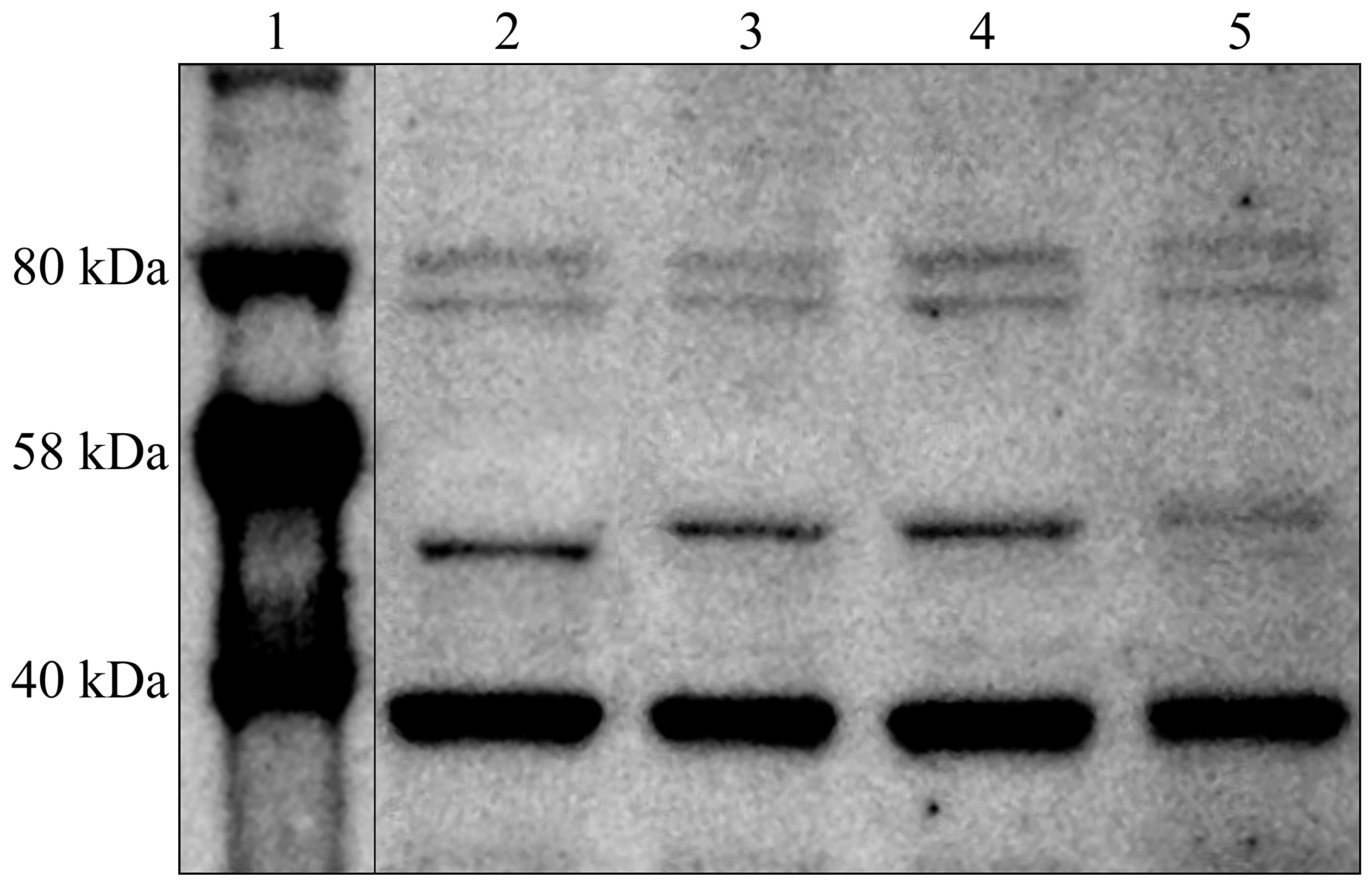
3.3. TGF-β-1-3 Concentration Profile in Degenerated and Control IVDs Determined Using the Western Blot Technique
3.4. TGF-β-1-3 Expression in IVDs Collected from Patients in the Study Group and Control Group Participants, Determined Through the IHC Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of Intervertebral Disc Degeneration. JOR SPINE 2020, 3, e1076. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.-J.; Cui, H.; Pan, H.; MC Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful Intervertebral Disc Degeneration and Inflammation: From Laboratory Evidence to Clinical Interventions. Bone Res. 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath Jr, L.B.; Härtl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar] [CrossRef]
- Ciapetti, G.; Granchi, D.; Devescovi, V.; Leonardi, E.; Greggi, T.; Di Silvestre, M.; Baldini, N. Ex Vivo Observation of Human Intervertebral Disc Tissue and Cells Isolated from Degenerated Intervertebral Discs. Eur. Spine. J. 2012, 21, 10–19. [Google Scholar] [CrossRef][Green Version]
- Yamabe, D.; Murakami, H.; Chokan, K.; Endo, H.; Oikawa, R.; Sawamura, S.; Doita, M. Evaluation of Water Content in Lumbar Intervertebral Discs and Facet Joints Before and After Physiological Loading Using T2 Mapping MRI. Spine 2017, 42, E1423–E1428. [Google Scholar] [CrossRef]
- Abu-Awwad, A.; Folescu, R.; Pop, D.L.; Motoc, A.G.M.; Oprea, D.M.; Tudoran, M.; Zamfir, C.L.; Faur, C.I.; Vermesan, D.; Deleanu, B.N. Morphometric Characteristics of Fibrocartilaginous Tissue in the Herniated Intervertebral Disc. Rom. J. Morphol. Embryol 2019, 60, 629–634. [Google Scholar]
- Adams, M.A.; Roughley, P.J. What Is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Groen, G.J.; Baljet, B.; Drukker, J. Nerves and Nerve Plexuses of the Human Vertebral Column. Am. J. Anat. 1990, 188, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.B.; Groenen, P.S.; Vissers, K.C.; van Helmond, N.; Stanton-Hicks, M.D. The Pathways and Processes Underlying Spinal Transmission of Low Back Pain: Observations From Dorsal Root Ganglion Stimulation Treatment. Neuromodulation 2021, 24, 610–621. [Google Scholar] [CrossRef]
- Yoshimura, N.; Dennison, E.; Wilman, C.; Hashimoto, T.; Cooper, C. Epidemiology of Chronic Disc Degeneration and Osteoarthritis of the Lumbar Spine in Britain and Japan: A Comparative Study. J. Rheumatol. 2000, 27, 429–433. [Google Scholar]
- Kettler, A.; Wilke, H.-J. Review of Existing Grading Systems for Cervical or Lumbar Disc and Facet Joint Degeneration. Eur. Spine J. 2006, 15, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Brazill, J.M.; Beeve, A.T.; Craft, C.S.; Ivanusic, J.J.; Scheller, E.L. Nerves in Bone: Evolving Concepts in Pain and Anabolism. J. Bone Miner. Res. 2019, 34, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Kennon, J.C.; Awad, M.E.; Chutkan, N.; DeVine, J.; Fulzele, S. Current Insights on Use of Growth Factors as Therapy for Intervertebral Disc Degeneration. Biomol. Concepts 2018, 9, 43–52. [Google Scholar] [CrossRef]
- Mahyudin, F.; Prakoeswa, C.R.S.; Notobroto, H.B.; Tinduh, D.; Ausrin, R.; Rantam, F.A.; Suroto, H.; Utomo, D.N.; Rhatomy, S. An Update of Current Therapeutic Approach for Intervertebral Disc Degeneration: A Review Article. Ann. Med. Surg. 2022, 77, 103619. [Google Scholar]
- Skaper, S.D. Neurotrophic Factors: An Overview. In Neurotrophic Factors: Methods and Protocols; Skaper, S.D., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 1–17. ISBN 978-1-4939-7571-6. [Google Scholar]
- Sahay, A.S.; Jadhav, A.T.; Sundrani, D.P.; Wagh, G.N.; Joshi, S.R. Differential Expression of Nerve Growth Factor (NGF) and Brain Derived Neurotrophic Factor (BDNF) in Different Regions of Normal and Preeclampsia Placentae. Clin. Exp. Hypertens 2020, 42, 360–364. [Google Scholar] [CrossRef]
- Hsiao, S.J.; Zehir, A.; Sireci, A.N.; Aisner, D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J. Mol. Diagn. 2019, 21, 553–571. [Google Scholar] [CrossRef]
- Kirkeby, A.; Barker, R.A. Parkinson Disease and Growth Factors - Is GDNF Good Enough? Nat. Rev. Neurol. 2019, 15, 312–314. [Google Scholar] [CrossRef]
- Cintrón-Colón, A.F.; Almeida-Alves, G.; Boynton, A.M.; Spitsbergen, J.M. GDNF Synthesis, Signaling, and Retrograde Transport in Motor Neurons. Cell Tissue Res. 2020, 382, 47–56. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Ma, K.; Zhao, L.; Lin, H.; Shao, Z. TGF-β Signaling in Intervertebral Disc Health and Disease. Osteoarthr. Cartil. 2019, 27, 1109–1117. [Google Scholar] [CrossRef]
- Cui, L.; Wei, H.; Li, Z.-M.; Dong, X.-B.; Wang, P.-Y. TGF-Β1 Aggravates Degenerative Nucleus Pulposus Cells Inflammation and Fibrosis through the Upregulation of Angiopoietin-like Protein 2 Expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12025–12033. [Google Scholar]
- Lee, Y.-J.; Kong, M.-H.; Song, K.-Y.; Lee, K.-H.; Heo, S.-H. The Relation Between Sox9, TGF-Β1, and Proteoglycan in Human Intervertebral Disc Cells. J. Korean Neurosurg. Soc. 2008, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.; Bachmeier, B.; Boos, N. Expression of Fibronectin and TGF-SS1 mRNA and Protein Suggest Altered Regulation of Extracellular Matrix in Degenerated Disc Tissue. Eur. Spine J. 2005, 14, 17–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolonen, J.; Grönblad, M.; Virri, J.; Seitsalo, S.; Rytömaa, T.; Karaharju, E. Transforming Growth Factor Beta Receptor Induction in Herniated Intervertebral Disc Tissue: An Immunohistochemical Study. Eur. Spine J. 2001, 10, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, J.; Skubutyte, R.; Kepler, C.K.; Huang, Z.; Anderson, D.G.; Shapiro, I.M.; Risbud, M.V. Smad3 Controls β-1,3-Glucuronosyltransferase 1 Expression in Rat Nucleus Pulposus Cells: Implications of Dysregulated Expression in Disc Disease. Arthritis Rheum. 2012, 64, 3324–3333. [Google Scholar] [CrossRef]
- Schroeder, M.; Viezens, L.; Schaefer, C.; Friedrichs, B.; Algenstaedt, P.; Rüther, W.; Wiesner, L.; Hansen-Algenstaedt, N. Chemokine Profile of Disc Degeneration with Acute or Chronic Pain: Laboratory Investigation. J. Neurosurg. Spine 2013, 18, 496–503. [Google Scholar] [CrossRef]
- Abbott, R.D.; Purmessur, D.; Monsey, R.D.; Brigstock, D.R.; Laudier, D.M.; Iatridis, J.C. Degenerative Grade Affects the Responses of Human Nucleus Pulposus Cells to Link-N, CTGF, and TGFβ3. J. Spinal Disord. Tech. 2013, 26, E86–E94. [Google Scholar] [CrossRef]
- Tsarouhas, A.; Soufla, G.; Tsarouhas, K.; Katonis, P.; Pasku, D.; Vakis, A.; Tsatsakis, A.M.; Spandidos, D.A. Molecular Profile of Major Growth Factors in Lumbar Intervertebral Disc Herniation: Correlation with Patient Clinical and Epidemiological Characteristics. Mol. Med. Rep. 2017, 15, 2195–2203. [Google Scholar] [CrossRef][Green Version]
- Wan, Z.Y.; Shan, H.; Liu, T.F.; Song, F.; Zhang, J.; Liu, Z.H.; Ma, K.L.; Wang, H.Q. Emerging Issues Questioning the Current Treatment Strategies for Lumbar Disc Herniation. Front. Surg. 2022, 9, 814531. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Li, Y.; Feng, J.; Pang, H.; Wang, J.; Liu, Q. Association of transforming growth factor-β1 with pathological grading of intervertebral disc degeneration. Nan Fang Yi Ke Da Xue Xue Bao 2012, 32, 897–900. [Google Scholar]
- Koroth, J.; Buko, E.O.; Abbott, R.; Johnson, C.P.; Ogle, B.M.; Stone, L.S.; Ellingson, A.M.; Bradley, E.W. Macrophages and Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 1367. [Google Scholar] [CrossRef]
- Yang, H.; Cao, C.; Wu, C.; Yuan, C.; Gu, Q.; Shi, Q.; Zou, J. TGF-Βl Suppresses Inflammation in Cell Therapy for Intervertebral Disc Degeneration. Sci. Rep. 2015, 5, 13254. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuan, C.; Wu, C.; Qian, J.; Shi, Q.; Li, X.; Zhu, X.; Zou, J. The Role of TGF-β1/Smad2/3 Pathway in Platelet-rich Plasma in Retarding Intervertebral Disc Degeneration. J. Cell Mol. Med. 2016, 20, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Stich, S.; Jagielski, M.; Fleischmann, A.; Meier, C.; Bussmann, P.; Kohl, B.; Schmidt, J.; Krüger, J.-P.; Endres, M.; Cabraja, M. Degeneration of Lumbar Intervertebral Discs: Characterization of Anulus Fibrosus Tissue and Cells of Different Degeneration Grades. Int. J. Mol. Sci. 2020, 21, 2165. [Google Scholar] [CrossRef]
- An, H.S.; Masuda, K.; Inoue, N. Intervertebral Disc Degeneration: Biological Biomechanical Factors. J. Orthop. Sci. 2006, 11, 541–552. [Google Scholar] [CrossRef]
- Ustawa z Dnia 1 Lipca 2005 r. o Pobieraniu, Przechowywaniu i Przeszczepianiu Komórek, Tkanek i Narządów. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20051691411 (accessed on 13 February 2024).
- Staszkiewicz, R.; Gralewski, M.; Gładysz, D.; Bryś, K.; Garczarek, M.; Gadzieliński, M.; Marcol, W.; Sobański, D.; Grabarek, B.O. Evaluation of the Concentration of Growth Associated Protein-43 and Glial Cell-Derived Neurotrophic Factor in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine. Mol. Pain 2023, 19, 17448069231158287. [Google Scholar] [CrossRef] [PubMed]
- Staszkiewicz, R.; Gładysz, D.; Bryś, K.; Garczarek, M.; Gadzieliński, M.; Marcol, W.; Sobański, D.; Grabarek, B.O. Usefulness of Detecting Brain-Derived Neurotrophic Factor in Intervertebral Disc Degeneration of the Lumbosacral Spine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e938663. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Yu, L.-P.; Qian, W.-W.; Yin, G.-Y.; Ren, Y.-X.; Hu, Z.-Y. MRI Assessment of Lumbar Intervertebral Disc Degeneration with Lumbar Degenerative Disease Using the Pfirrmann Grading Systems. PLoS ONE 2012, 7, e48074. [Google Scholar] [CrossRef]
- Hasanović-Vučković, S.; Jusufbegović, M.; Vegar-Zubović, S.; Milišić, L.; Šehić, A.; Hasanbegović, I.; Beganović, A. Assessment of Lumbar Spine Disc Degeneration in Coherence to Pfirrman Grades and Oswestry Disability Index. J. Health Sci. 2020, 10, 191–195. [Google Scholar] [CrossRef]
- Pagani, S.; Maglio, M.; Sicuro, L.; Fini, M.; Giavaresi, G.; Brogini, S. RNA Extraction from Cartilage: Issues, Methods, Tips. Int. J. Mol. Sci. 2023, 24, 2120. [Google Scholar] [CrossRef]
- Peirson, S.N.; Butler, J.N. RNA Extraction From Mammalian Tissues. In Circadian Rhythms: Methods and Protocols; Rosato, E., Ed.; Humana Press: Totowa, NJ, USA, 2007; ISBN 978-1-59745-257-1. [Google Scholar]
- Leonova, O.N.; Elgaeva, E.E.; Golubeva, T.S.; Peleganchuk, A.V.; Krutko, A.V.; Aulchenko, Y.S.; Tsepilov, Y.A. A Protocol for Recruiting and Analyzing the Disease-Oriented Russian Disc Degeneration Study (RuDDS) Biobank for Functional Omics Studies of Lumbar Disc Degeneration. PLoS ONE 2022, 17, e0267384. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0267384 (accessed on 22 August 2024). [CrossRef]
- Ruettger, A.; Neumann, S.; Wiederanders, B.; Huber, R. Comparison of Different Methods for Preparation and Characterization of Total RNA from Cartilage Samples to Uncover Osteoarthritis in Vivo. BMC Res. Notes 2010, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ Ecosystem: Open-source Software for Image Visualization, Processing, and Analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; van Kruining Kodele, S.; Creemers, L.; Joosten, B. Biomolecular therapies for chronic dis-cogenic low back pain: A narrative review. JOR Spine 2024, 7, e1345. [Google Scholar] [CrossRef]
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421. [Google Scholar] [CrossRef]
- Voisin, A.; Damon-Soubeyrand, C.; Bravard, S.; Saez, F.; Drevet, J.R.; Guiton, R. Differential Expression and Localisation of TGF-β Isoforms and Receptors in the Murine Epididymis. Sci. Rep. 2020, 10, 995. [Google Scholar] [CrossRef]
- Bian, Q.; Ma, L.; Jain, A.; Crane, J.L.; Kebaish, K.; Wan, M.; Zhang, Z.; Edward Guo, X.; Sponseller, P.D.; Séguin, C.A. Mechanosignaling Activation of TGFβ Maintains Intervertebral Disc Homeostasis. Bone Res. 2017, 5, 1–14. [Google Scholar] [CrossRef]
- Nakawaki, M.; Uchida, K.; Miyagi, M.; Inoue, G.; Kawakubo, A.; Satoh, M.; Takaso, M. Changes in Nerve Growth Factor Expression and Macrophage Phenotype Following Intervertebral Disc Injury in Mice. J. Orthop. Res. 2019, 37, 1798–1804. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Liu, M.-Q.; Chen, H.-W.; Wu, Z.-L.; Gao, Y.-C.; Ma, Z.-J.; He, X.-G.; Kang, X.-W. NF-κB Signalling Pathways in Nucleus Pulposus Cell Function and Intervertebral Disc Degeneration. Cell Prolif. 2021, 54, e13057. [Google Scholar] [CrossRef]
- Van Der Kraan, P.M. The Changing Role of TGFβ in Healthy, Ageing and Osteoarthritic Joints. Nat. Rev. Rheumatol. 2017, 13, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Jain, A.; Xu, X.; Kebaish, K.; Crane, J.L.; Zhang, Z.; Wan, M.; Ma, L.; Riley, L.H.; Sponseller, P.D. Excessive Activation of TGFβ by Spinal Instability Causes Vertebral Endplate Sclerosis. Sci. Rep. 2016, 6, 27093. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Lee, J.-W.; Moon, E.-J.; Chung, Y.G.; Kim, O.-S.; Kim, H.-J. Anabolic Effects of Peniel 2000, a Peptide That Regulates TGF-Β1 Signaling on Intervertebral Disc Degeneration. Spine 2013, 38, E49–E58. [Google Scholar] [CrossRef]
- Ni, L.; Zheng, Y.; Gong, T.; Xiu, C.; Li, K.; Saijilafu; Li, B.; Yang, H.; Chen, J. Proinflammatory Macrophages Promote Degenerative Phenotypes in Rat Nucleus Pulpous Cells Partly through ERK and JNK Signaling. J. Cell. Physiol. 2019, 234, 5362–5371. [Google Scholar] [CrossRef]
- Li, W.; Liu, T.; Wu, L.; Chen, C.; Jia, Z.; Bai, X.; Ruan, D. Blocking the Function of Inflammatory Cytokines and Mediators by Using IL-10 and TGF-β: A Potential Biological Immunotherapy for Intervertebral Disc Degeneration in a Beagle Model. Int. J. Mol. Sci. 2014, 15, 17270–17283. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Guo, Z.; Su, W.; Zhou, R.; Zhang, G.; Yang, S.; Wu, X.; Qiu, C.; Cong, W.; Shen, N.; Guo, J. Exosomal MATN3 of Urine-Derived Stem Cells Ameliorates Intervertebral Disc Degeneration by Antisenescence Effects and Promotes NPC Proliferation and ECM Synthesis by Activating TGF-β. Oxidative Med. Cell. Longev. 2021, 2021, 5542241. [Google Scholar] [CrossRef]
- Stich, S.; Möller, A.; Cabraja, M.; Krüger, J.P.; Hondke, S.; Endres, M.; Ringe, J.; Sittinger, M. Chemokine CCL25 Induces Migration and Extracellular Matrix Production of Anulus Fibrosus-Derived Cells. Int. J. Mol. Sci. 2018, 19, 2207. [Google Scholar] [CrossRef] [PubMed]
- Hondke, S.; Cabraja, M.; Krüger, J.P.; Stich, S.; Hartwig, T.; Sittinger, M.; Endres, M. Proliferation, Migration, and ECM Formation Potential of Human Annulus Fibrosus Cells Is Independent of Degeneration Status. CARTILAGE 2020, 11, 192–202. [Google Scholar] [CrossRef]
- Hu, B.; Shi, C.; Tian, Y.; Zhang, Y.; Xu, C.; Chen, H.; Cao, P.; Yuan, W. TGF-β Induces up-Regulation of Chondroitin Sulfate Synthase 1 (CHSY1) in Nucleus Pulposus Cells through MAPK Signaling. Cell Physiol. Biochem. 2015, 37, 793–804. [Google Scholar] [CrossRef]
- Hu, B.; Xu, C.; Cao, P.; Tian, Y.; Zhang, Y.; Shi, C.; Xu, J.; Yuan, W.; Chen, H. TGF-β Stimulates Expression of Chondroitin Polymerizing Factor in Nucleus Pulposus Cells through the Smad3, RhoA/ROCK1, and MAPK Signaling Pathways. J. Cell Biochem. 2018, 119, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Setton, L.A. Biomaterials for Intervertebral Disc Regeneration and Repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, L.; Deng, X.; Shi, D.; Wu, F.; Liang, H.; Huang, D.; Shao, Z. Mesenchymal Stem Cells Protect Nucleus Pulposus Cells from Compression-Induced Apoptosis by Inhibiting the Mitochondrial Pathway. Stem Cells Int. 2017, 2017, 984312. [Google Scholar] [CrossRef]
- Fontana, G.; See, E.; Pandit, A. Current Trends in Biologics Delivery to Restore Intervertebral Disc Anabolism. Adv. Drug Deliv. Rev. 2015, 84, 146–158. [Google Scholar] [CrossRef]
- Nagano, S.; Matsunaga, S.; Takae, R.; Morimoto, N.; Suzuki, S.; Yoshida, H. Immunolocalization of Transforming Growth Factor-Betas and Their Receptors in the Intervertebral Disk of Senescence-Accelerated Mouse. Int. J. Oncol. 2000, 17, 461–467. [Google Scholar] [CrossRef]
- Matsunaga, S.; Nagano, S.; Onishi, T.; Motimoto, N.; Suzuki, S. kOMIYA, s Age-Related Changes in Expression of Transforming Growth Factor-β and Receptors in Cells of Intervertebral Discs. J. Neurosurg. Spine 2003, 98, 63–67. [Google Scholar]
- Zheng, L.; Cao, Y.; Ni, S.; Qi, H.; Ling, Z.; Xu, X.; Zou, X.; Wu, T.; Deng, R.; Hu, B.; et al. Ciliary Parathyroid Hormone Signaling Activates Transforming Growth Factor-β to Maintain Intervertebral Disc Homeostasis during Aging. Bone Res. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Yoon, S.; Attallah-Wasif, E.; Tsai, K.; Fei, Q.; Hutton, W. The Expression of Anabolic Cytokines in Intervertebral Discs. Spine 2006, 31, 1770–1774. [Google Scholar] [CrossRef]
- Hiyama, A.; Mochida, J.; Omi, H.; Serigano, K.; Sakai, D. Cross Talk between Smad Transcription Factors and TNF-α in Intervertebral Disc Degeneration. Biochem. Biophys. Res. Commun. 2008, 369, 679–685. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Guo, M.-K.; Wan, Z.-Y.; Song, F.; Wang, H.-Q. Emerging Evidence on Noncoding-RNA Regulatory Machinery in Intervertebral Disc Degeneration: A Narrative Review. Arthritis Res. Ther. 2020, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.-L.; Gao, Y.-S.; Wang, X.-M.; Yu, X. Histological Changes of Cervical Disc Tissue in Patients with Degenerative Ossification. J. Korean Neurosurg. Soc. 2022, 65, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Deng, R.; Yan, H.; Liu, X.; Kang, R. Intervertebral Disc Degeneration and Inflammatory Microenvironment: Expression, Pathology, and Therapeutic Strategies. Inflamm. Res. 2023, 72, 1811–1828. [Google Scholar] [CrossRef] [PubMed]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PMR 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Nishida, K.; Kang, J.D.; Gilbertson, L.G.; Moon, S.H.; Suh, J.K.; Vogt, M.T.; Robbins, P.D.; Evans, C.H. Modulation of the Biologic Activity of the Rabbit Intervertebral Disc by Gene Therapy: An in Vivo Study of Adenovirus-Mediated Transfer of the Human Transforming Growth Factor Beta 1 Encoding Gene. Spine 1999, 24, 2419–2425. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 2017, 7, 45623. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Shen, B.; Diwan, A.; Hoyland, J.A.; Richardson, S.M. Therapeutic Potential of Growth Differentiation Factors in the Treatment of Degenerative Disc Diseases. JOR Spine 2019, 2, e1045. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Gerami, H.; Jun, P.; Funabashi, M.; Kawchuk, G.; Goldstein, A.; Foltz, W.; Sussman, M.; Eek, B.C.; et al. NTG-101: A Novel Molecular Therapy That Halts the Progression of Degenerative Disc Disease. Sci. Rep. 2018, 8, 16809. [Google Scholar] [CrossRef]
- Risbud, M.V.; Di Martino, A.; Guttapalli, A.; Seghatoleslami, R.; Denaro, V.; Vaccaro, A.R.; Albert, T.J.; Shapiro, I.M. Toward an Optimum System for Intervertebral Disc Organ Culture: TGF-Beta 3 Enhances Nucleus Pulposus and Anulus Fibrosus Survival and Function through Modulation of TGF-Beta-R Expression and ERK Signaling. Spine 2006, 31, 884–890. [Google Scholar] [CrossRef]
- Hegewald, A.A.; Zouhair, S.; Endres, M.; Cabraja, M.; Woiciechowsky, C.; Thomé, C.; Kaps, C. Towards Biological Anulus Repair: TGF-Β3, FGF-2 and Human Serum Support Matrix Formation by Human Anulus Fibrosus Cells. Tissue Cell 2013, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Lufkin, T.; Kraus, P. Development and Degeneration of the Intervertebral Disc—Insights from Across Species. Vet. Sci. 2023, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lyu, M.; Lu, Q.; Cheung, K.; Leung, V. Current Perspectives on Nucleus Pulposus Fibrosis in Disc Degeneration and Repair. Int. J. Mol. Sci. 2022, 23, 6612. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Yurube, T.; Nishida, K. Gene Therapy Approach for Intervertebral Disc Degeneration: An Update. Neurospine 2020, 17, 3–14. [Google Scholar] [CrossRef]
- Hou, Z.; Tan, R.; Zhang, Y. Snapshots of a Tiny Ancestral Nuclease of Cas9. Trends Biochem. Sci 2023, 48, 9–10. [Google Scholar] [CrossRef]


| mRNA | Oligonucleotide Sequence | Tm (°C) |
|---|---|---|
| TGF-β1 | Forward: 5’-GGCCAGATCCTGTCCAAGC-3’ Reverse: 5’-GTGGGTTTCCACCATTAGCAC-3’ | 85.4 |
| TGF-β2 | Forward: 5’-CAGCACACTCGATATGGACCA-3’ Reverse: 5’-CCTCGGGCTCAGGATAGTCT-3’ | 88.7 |
| TGF-β3 | Forward: 5’-CTGGATTGTGGTTCCATGCA-3’ Reverse: 5’-TCCCCGAATGCCTCACAT-3’ | 86.6 |
| GAPDH | Forward: 5’-GGTGAAGGTCGGAGTCAACGGA-3’ Reverse: 5’-GAGGGATCTCGCTCCTGGAAGA-3’ | 86.4 |
| Isoform of TGF-β | Group | Concentration (pg/mL) | 95% Cl |
|---|---|---|---|
| TGF-β1 a,b,d,e | Control | 276 ± 19 | 210–345 |
| Study | 2797 ± 132 | 2541–3098 | |
| Pfirrmann 2 | 2876 ± 123 | 2456–3087 | |
| Pfirrmann 3 | 3127 ± 165 | 2987–3456 | |
| Pfirrmann 4 | 3198 ± 176 | 2898–3298 | |
| Pfirrmann 5 | 1987 ± 156 | 1765–2321 | |
| TGF-β2 a,b,e | Control | 159 ± 17 | 140–198 |
| Study | 1918 ± 176 | 1561–2198 | |
| Pfirrmann 2 | 1834 ± 201 | 1656–2098 | |
| Pfirrmann 3 | 2034 ± 165 | 1871–2198 | |
| Pfirrmann 4 | 2043 ± 156 | 1876–2198 | |
| Pfirrmann 5 | 1761 ± 198 | 1456–2001 | |
| TGF-β3 a,b,c,d,e | Control | 152 ± 11 | 134–189 |
| Study | 2573 ± 102 | 2345–2761 | |
| Pfirrmann 2 | 2545 ± 165 | 2456–2671 | |
| Pfirrmann 3 | 2767 ± 187 | 2571–2981 | |
| Pfirrmann 4 | 2761 ± 209 | 2671–2891 | |
| Pfirrmann 5 | 2219 ± 187 | 2091–2348 |
| Isoform of TGF-β | Group | Optical Density | 95% Cl |
|---|---|---|---|
| TGF-β1 a,b,c,e,f | Control | 1.11 ± 0.13 | 1.04–1.18 |
| Study | 4.78 ± 0.65 | 4.45–5.11 | |
| Pfirrmann 2 | 3.29 ± 0.91 | 2.83–3.75 | |
| Pfirrmann 3 | 6.42 ± 1.23 | 5.79–7.04 | |
| Pfirrmann 4 | 7.29 ± 1.87 | 6.34–8.24 | |
| Pfirrmann 5 | 2.09 ± 0.54 | 1.82–2.36 | |
| TGF-β2 a,c,f | Control | 2.10 ± 0.32 | 1.93–2.26 |
| Study | 8.78 ± 0.18 | 8.69–8.87 | |
| Pfirrmann 2 | 5.44 ± 0.23 | 5.33–5.56 | |
| Pfirrmann 3 | 7.01 ± 0.55 | 6.73–7.28 | |
| Pfirrmann 4 | 12.11 ± 2.32 | 10.94–13.28 | |
| Pfirrmann 5 | 10.57 ± 1.98 | 9.57–11.57 | |
| TGF-β3 a,c,d,e,f | Control | 1.09 ± 0.18 | 0.99–1.18 |
| Study | 4.31 ± 0.98 | 3.81–4.81 | |
| Pfirrmann 2 | 2.00 ± 0.14 | 1.93–2.07 | |
| Pfirrmann 3 | 4.34 ± 0.71 | 3.98–4.70 | |
| Pfirrmann 4 | 5.21 ± 0.56 | 4.93–5.49 | |
| Pfirrmann 5 | 5.68 ± 0.34 | 5.51–5.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszkiewicz, R.; Gładysz, D.; Sobański, D.; Bolechała, F.; Golec, E.; Sobańska, M.; Strojny, D.; Turek, A.; Grabarek, B.O. Assessment of the Concentration of Transforming Growth Factor Beta 1–3 in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine. Curr. Issues Mol. Biol. 2024, 46, 12813-12829. https://doi.org/10.3390/cimb46110763
Staszkiewicz R, Gładysz D, Sobański D, Bolechała F, Golec E, Sobańska M, Strojny D, Turek A, Grabarek BO. Assessment of the Concentration of Transforming Growth Factor Beta 1–3 in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine. Current Issues in Molecular Biology. 2024; 46(11):12813-12829. https://doi.org/10.3390/cimb46110763
Chicago/Turabian StyleStaszkiewicz, Rafał, Dorian Gładysz, Dawid Sobański, Filip Bolechała, Edward Golec, Małgorzata Sobańska, Damian Strojny, Artur Turek, and Beniamin Oskar Grabarek. 2024. "Assessment of the Concentration of Transforming Growth Factor Beta 1–3 in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine" Current Issues in Molecular Biology 46, no. 11: 12813-12829. https://doi.org/10.3390/cimb46110763
APA StyleStaszkiewicz, R., Gładysz, D., Sobański, D., Bolechała, F., Golec, E., Sobańska, M., Strojny, D., Turek, A., & Grabarek, B. O. (2024). Assessment of the Concentration of Transforming Growth Factor Beta 1–3 in Degenerated Intervertebral Discs of the Lumbosacral Region of the Spine. Current Issues in Molecular Biology, 46(11), 12813-12829. https://doi.org/10.3390/cimb46110763







