Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’ from Different Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. ‘Shatian Yu’ Fruit Harvesting
2.2. Sample Pretreatment
2.3. Metabolite Analysis Using UPLC–MS/MS
2.4. Multivariate Pattern Analysis and Differential Metabolites Selected
2.5. Metabolic Pathway Analysis
2.6. Correlation Analysis
3. Results
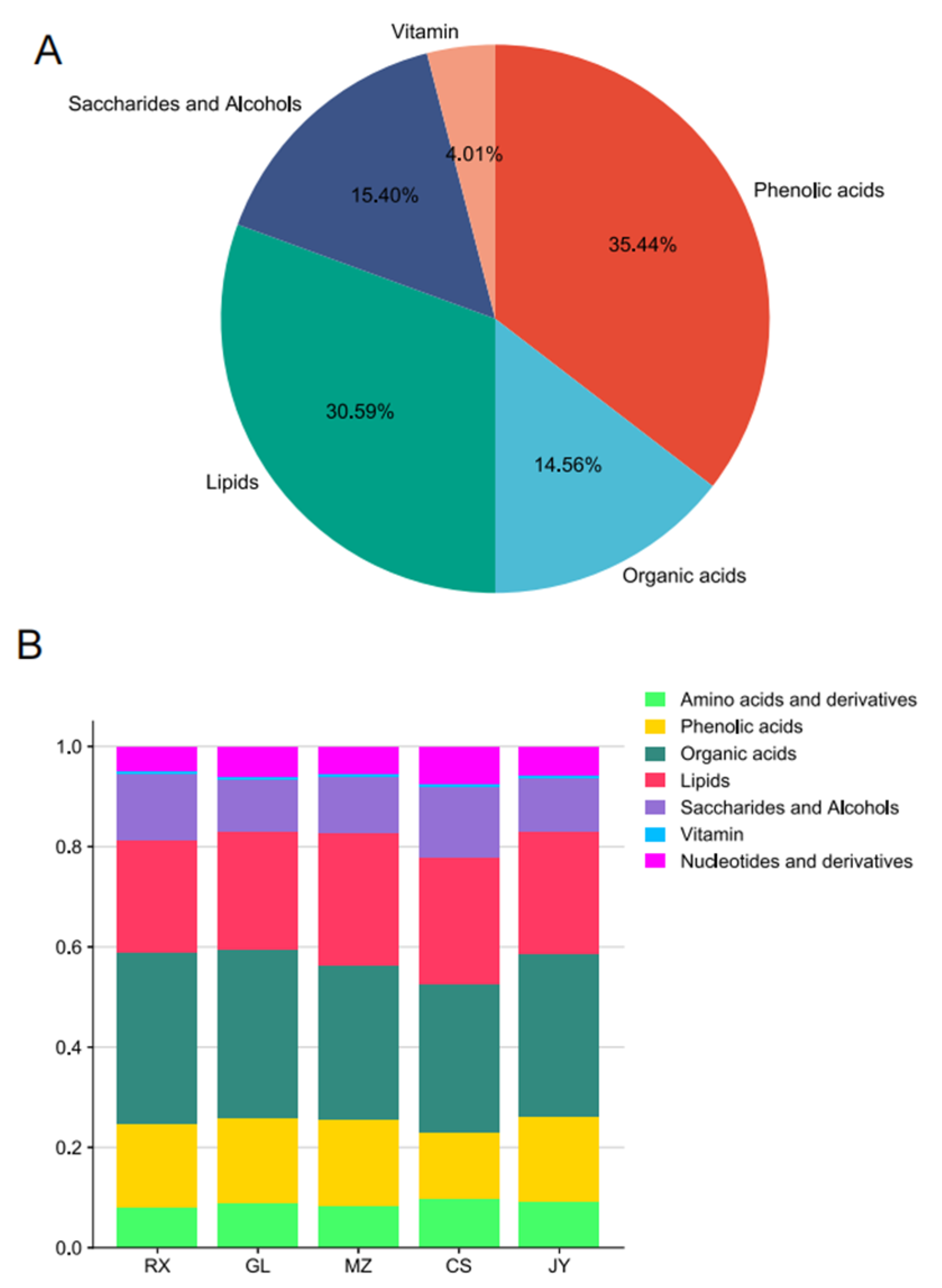
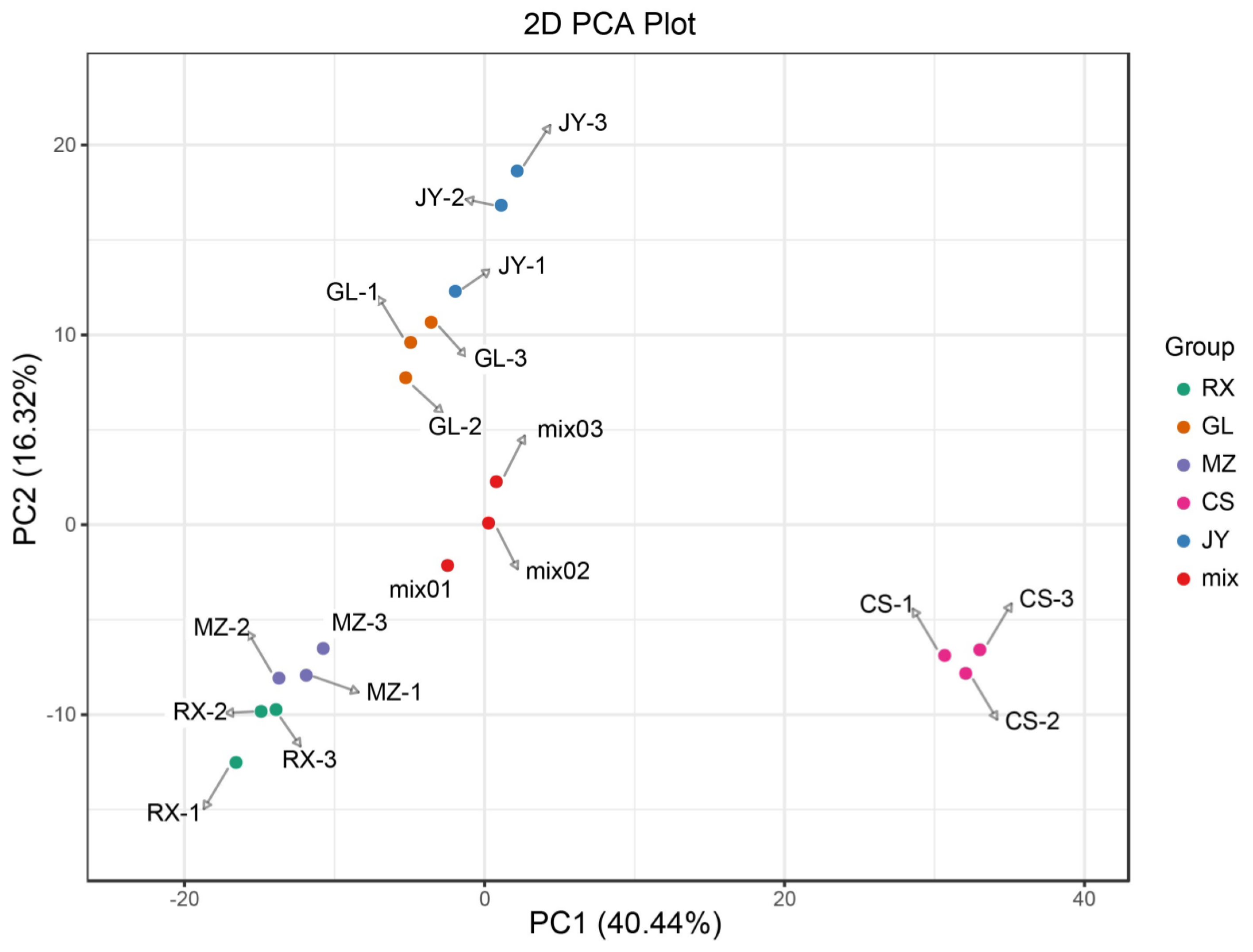
3.1. Comprehensive Metabolite Profiling of ‘Shatian Yu’ Across Different Production Areas
3.2. Differential Metabolite Analysis in ‘Shatian Yu’ from Various Production Areas
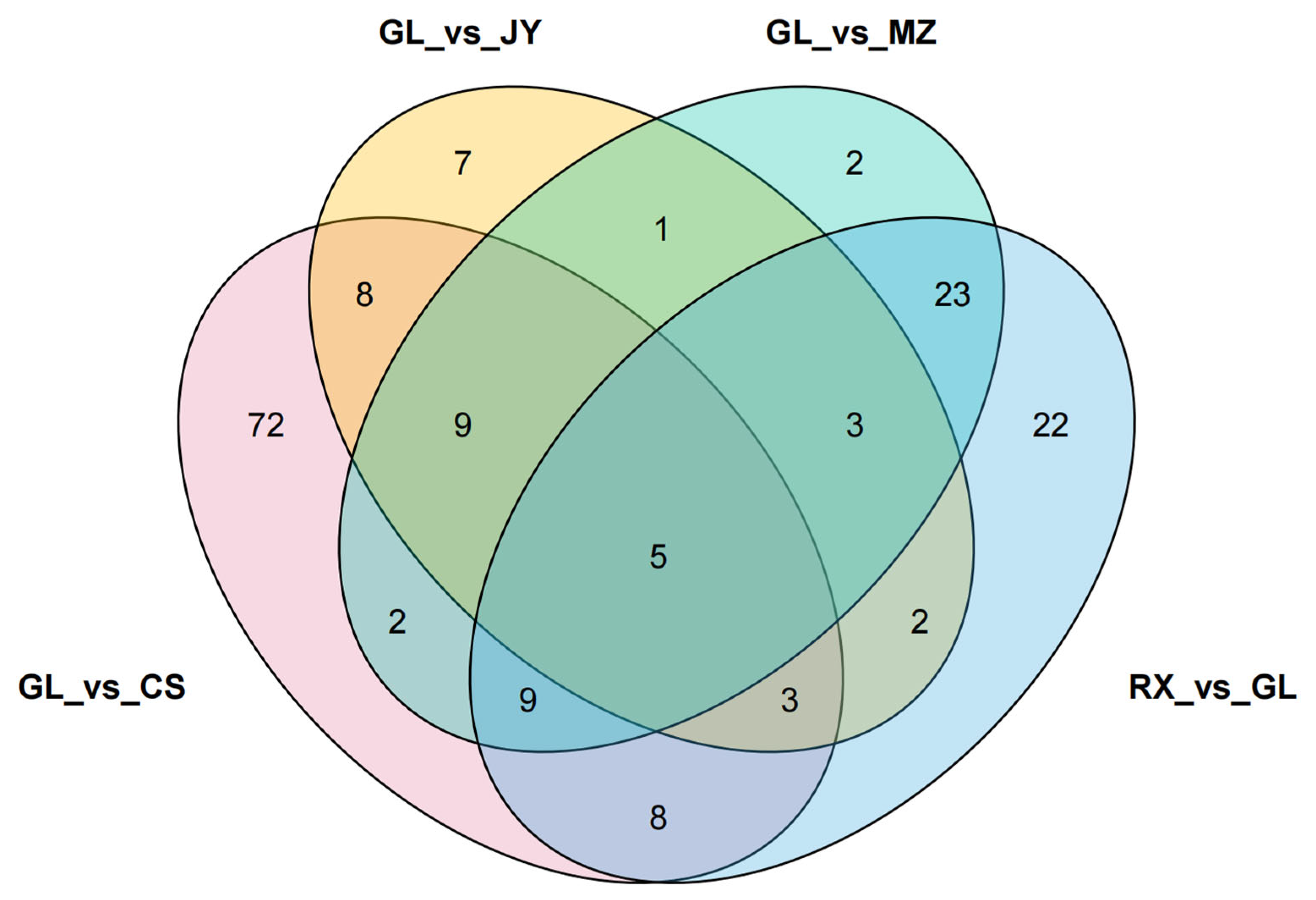
3.3. Comparative Analysis of Differential Metabolites in ‘Shatian Yu’ from Different Origins
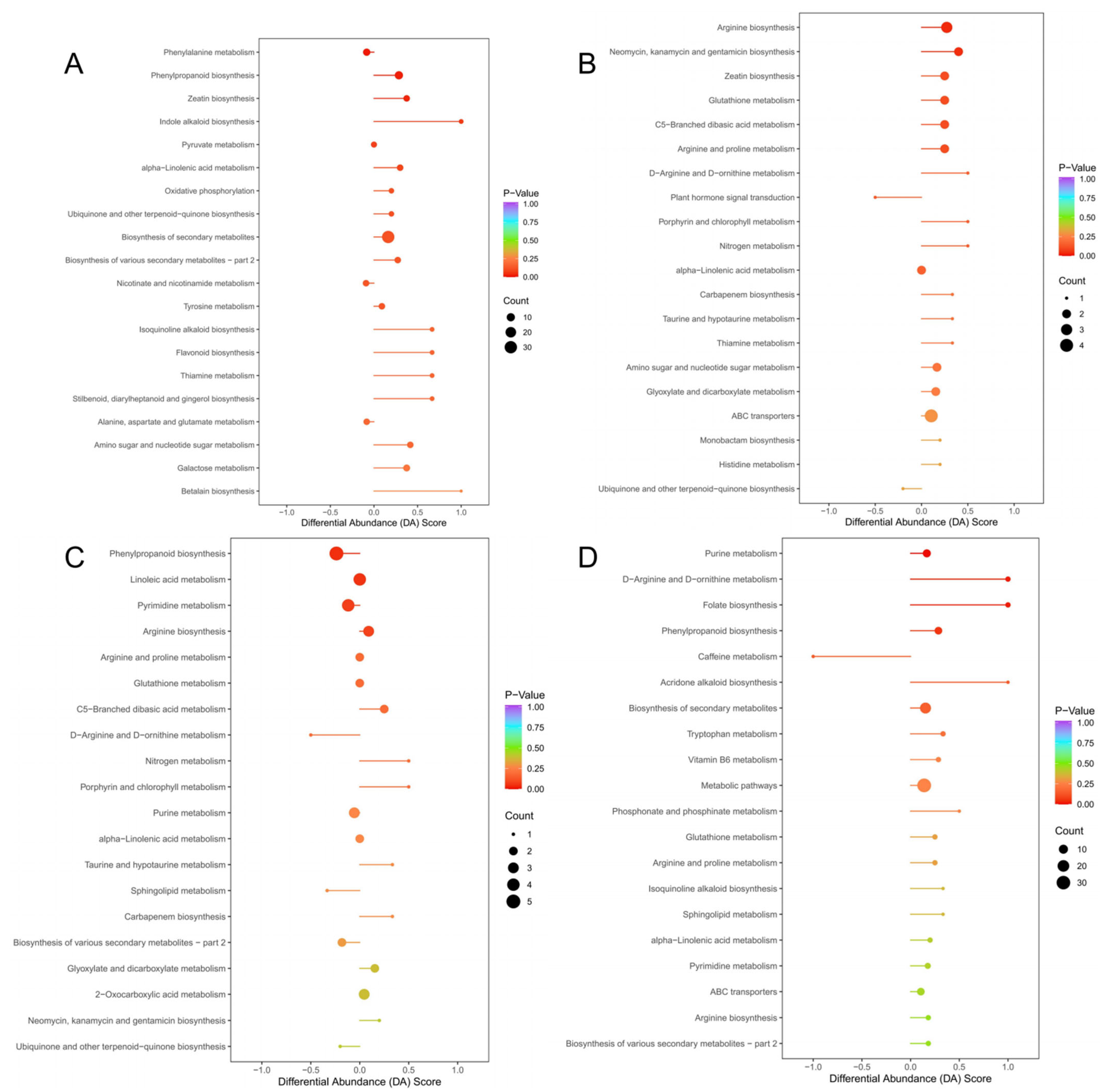
3.4. KEGG Pathway Analysis of ‘Shatian Yu’ Metabolites
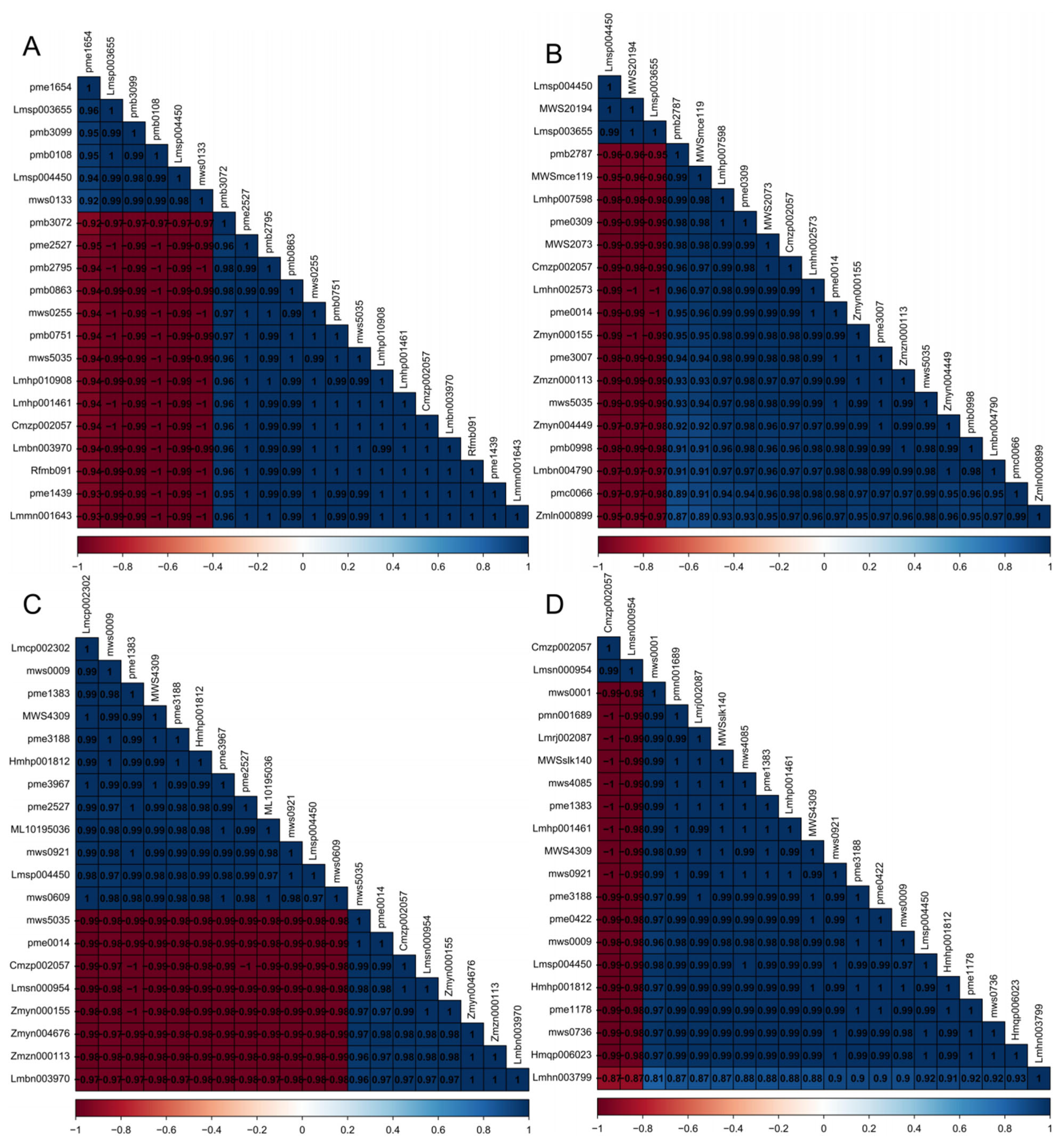
3.5. Correlation Analysis of Differential Substances
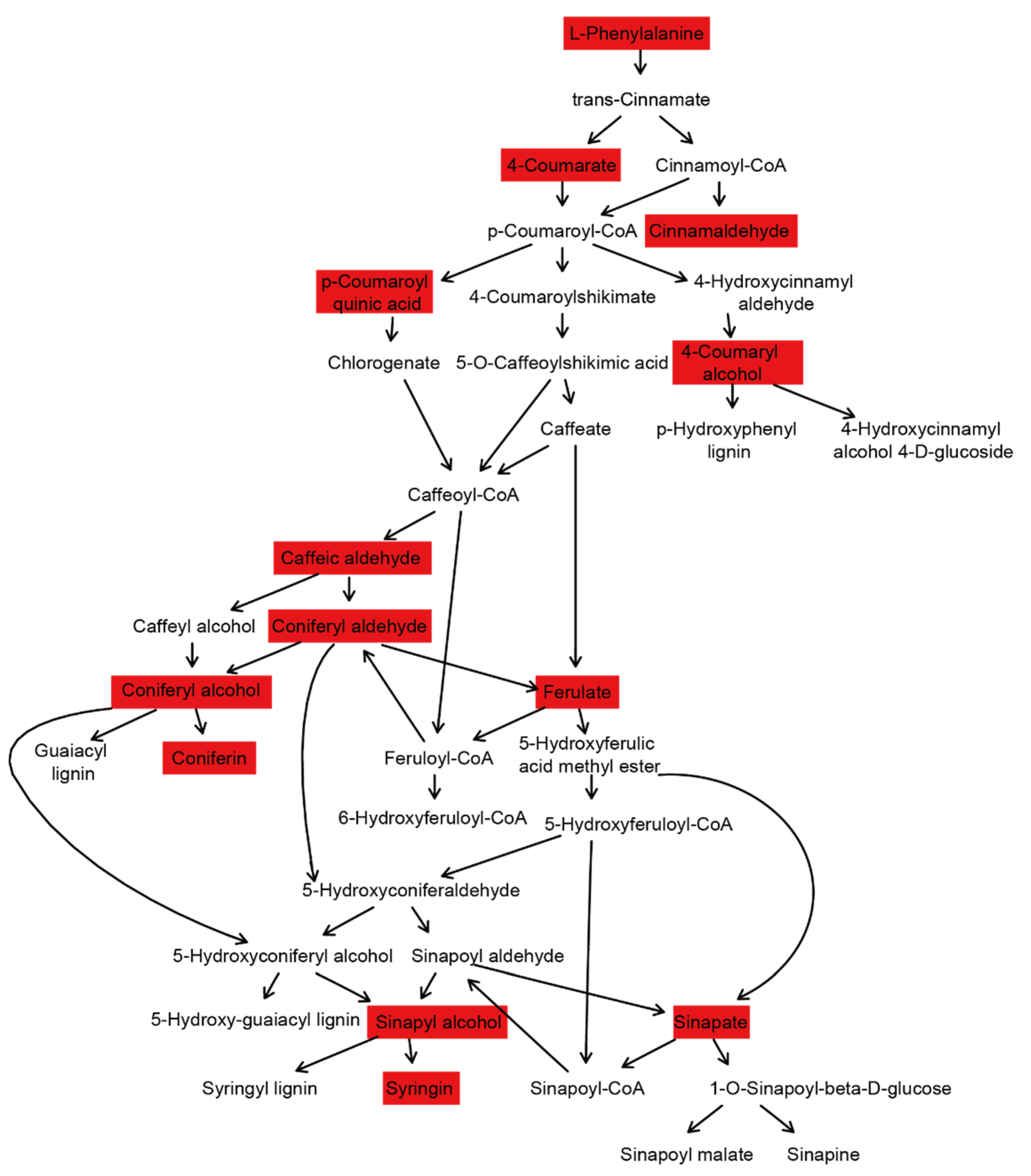
3.6. Phenylpropanoid Pathway Analysis in ‘Shatian Yu’ from Different Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, B.; Ye, S.; Qiu, X.; Liao, L.; Sun, G.; Luo, J.; Dai, L.; Rong, Y.; Wang, Z. Transcriptome analyses of two citrus cultivars (Shiranuhi and Huangguogan) in seedling etiolation. Sci. Rep. 2017, 7, 46245. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef]
- Sapkota, B.; Devkota, H.P.; Poudel, P. Citrus maxima (Brum.) Merr.(Rutaceae): Bioactive chemical constituents and pharmacological activities. Evid.-Based Complement. Altern. Med. 2022, 2022, 8741669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Miller, E.G.; Porter, J.L.; Binnie, W.H.; Guo, I.Y.; Hasegawa, S. Further studies on the anticancer activity of citrus limonoids. J. Agric. Food Chem. 2004, 52, 4908–4912. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, G. An insight into the role of citrus bioactives in modulation of colon cancer. J. Funct. Foods 2015, 13, 239–261. [Google Scholar] [CrossRef]
- Yang, H.; Li, M.; Kui, X.; Sun, Y.; Li, J.-A.; Qiu, D. Comparative studies on the quality and lycopene content of pomelo (Citrus grandis Osbeck) cultivars. Nat. Prod. Commun. 2020, 15, 1934578X20953290. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Rudaz, S.; Hae Choi, Y.; Kyong Kim, H. Plant metabolomics: From holistic data to relevant biomarkers. Curr. Med. Chem. 2013, 20, 1056–1090. [Google Scholar]
- Rolin, D.; Baldet, P.; Just, D.; Chevalier, C.; Biran, M.; Raymond, P. NMR study of low subcellular pH during the development of cherry tomato fruit. Funct. Plant Biol. 2000, 27, 61–69. [Google Scholar] [CrossRef]
- Goh, R.M.V.; Pua, A.; Liu, S.Q.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Lau, H.; Tan, L.P.; Zhang, W.L.; Yu, B. Characterisation of volatile and non-volatile compounds in pomelo by gas chromatography-olfactometry, gas chromatography and liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Essent. Oil Res. 2020, 32, 132–143. [Google Scholar] [CrossRef]
- Xiao, L.; Shibuya, T.; Watanabe, T.; Kato, K.; Kanayama, Y. Effect of Light Quality on Metabolomic, Ionomic, and Transcriptomic Profiles in Tomato Fruit. Int. J. Mol. Sci. 2022, 23, 13288. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Bianconi, M.; Poletti, S.; Negri, S.; Munari, F.; Ceoldo, S.; Guzzo, F. Metabolomic profiling and antioxidant activity of fruits representing diverse apple and pear cultivars. Biology 2021, 10, 380. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an optimized workflow for global metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Chilakala, S.; Mehtab, V.; Tallapally, M.; Vemula, M.; Shaikh, A.S.; Chenna, S.; Upadhyayula, V. SEC-MS/MS determination of amino acids from mango fruits and application of the method for studying amino acid perturbations due to post harvest ripening. LWT 2021, 138, 110680. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Recent trends in the analysis of amino acids in fruits and derived foodstuffs. Anal. Bioanal. Chem. 2013, 405, 7941–7956. [Google Scholar] [CrossRef]
- Das, A.; Fröhlich, D.; Achanta, L.B.; Rowlands, B.D.; Housley, G.D.; Klugmann, M.; Rae, C.D. L-aspartate, L-ornithine and L-ornithine-L-aspartate (LOLA) and their impact on brain energy metabolism. Neurochem. Res. 2020, 45, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, H.; Wang, T.; Xu, Q.; Zhang, C.; Fan, X.; Ma, Q.; Chen, N.; Xie, X. Current status on metabolic engineering for the production of l-aspartate family amino acids and derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Canbay, A. Hepatoprotection by L-ornithine L-aspartate in non-alcoholic fatty liver disease. Dig. Dis. 2018, 37, 63–68. [Google Scholar] [CrossRef]
- Chen, T.-J.; Kukley, M. Glutamate receptors and glutamatergic signalling in the peripheral nerves. Neural Regen. Res. 2020, 15, 438–447. [Google Scholar]
- He, S.; Zhang, X.; Qu, S. Glutamate, glutamate transporters, and circadian rhythm sleep disorders in neurodegenerative diseases. ACS Chem. Neurosci. 2018, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-S.; Chung, Y.-H.; Hsieh, M.-H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Feron, O. Nitric oxide synthases: Which, where, how, and why? J. Clin. Investig. 1997, 100, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K. I. arginine. Biomed. Pharmacother. 2002, 56, 439–445. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J. Nitric oxide regulation of plant metabolism. Mol. Plant 2022, 15, 228–242. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Schroeder, R.A.; Kuo, P.C. Nitric oxide: Physiology and pharmacology. Anesth. Analg. 1995, 81, 1052–1059. [Google Scholar] [PubMed]
- Davis, M.R.; Ortegon, D.P.; Kerby, J.D.; Ignarro, L.J.; Kashyap, V.S. Endothelial dysfunction after arterial thrombosis is ameliorated by L-arginine in combination with thrombolysis. J. Vasc. Interv. Radiol. 2003, 14, 233–239. [Google Scholar] [CrossRef] [PubMed]
- de Francisco, L.; Pinto, D.; Rosseto, H.; Toledo, L.; Santos, R.; Tobaldini-Valério, F.; Svidzinski, T.; Bruschi, M.; Sarmento, B.; Oliveira, M.B.P. Evaluation of radical scavenging activity, intestinal cell viability and antifungal activity of Brazilian propolis by-product. Food Res. Int. 2018, 105, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S. Bioavailable Citrus sinensis extract: Polyphenolic composition and biological activity. Molecules 2017, 22, 623. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chuang, Y.-C.; Hsu, H.-W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Fraga, L.N.; Coutinho, C.P.; Rozenbaum, A.C.; de Castro Tobaruela, E.; Lajolo, F.M.; Hassimotto, N.M.A. Blood pressure and body fat% reduction is mainly related to flavanone phase II conjugates and minor extension by phenolic acid after long-term intake of orange juice. Food Funct. 2021, 12, 11278–11289. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S. Dietary consumption of phenolic acids and prostate cancer: A case-control study in sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.; Wang, K. Methyl jasmonate promotes phospholipid remodeling and jasmonic acid signaling to alleviate chilling injury in peach fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K. Jasmonic acid: An essential plant hormone. Int. J. Mol. Sci. 2020, 21, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Zhang, Z.; Chen, Y.; Tian, S. Mushroom alcohol controls gray mold caused by Botrytis cinerea in harvested fruit via activating the genes involved in jasmonic acid signaling pathway. Postharvest Biol. Technol. 2022, 186, 111843. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Tang, S.; Jin, P. Control of anthracnose rot and quality deterioration in loquat fruit with methyl jasmonate. J. Sci. Food Agric. 2008, 88, 1598–1602. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.; Chen, Q.; Chen, J.; Zhang, X.; Wei, L.; Deng, L.; Yao, S.; Zeng, K. Transcription factor CsERF1B regulates postharvest citrus fruit resistance to Penicillium digitatum. Postharvest Biol. Technol. 2023, 198, 112260. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Palmieri, B.; Iannitti, T.; Capone, S.; Flescher, E. A preliminary study of the local treatment of preneoplastic and malignant skin lesions using methyl jasmonate. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 333–336. [Google Scholar]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.d.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl jasmonate: Putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef]
- Longo, N.; Holt, R.J. Glycerol phenylbutyrate for the maintenance treatment of patients with deficiencies in enzymes of the urea cycle. Expert Opin. Orphan Drugs 2017, 5, 999–1010. [Google Scholar] [CrossRef]
- Challoumas, D.; Kirwan, P.D.; Borysov, D.; Clifford, C.; McLean, M.; Millar, N.L. Topical glyceryl trinitrate for the treatment of tendinopathies: A systematic review. Br. J. Sports Med. 2019, 53, 251–262. [Google Scholar] [CrossRef]
- Appleton, J.P.; Sprigg, N.; Bath, P.M. Therapeutic potential of transdermal glyceryl trinitrate in the management of acute stroke. CNS Drugs 2017, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, J.; Dai, Z.; Xu, L.; Fan, T.; Jing, T.; Chen, M.; Gou, M. Commonly and specifically activated defense responses in maize disease lesion mimic mutants revealed by integrated transcriptomics and metabolomics analysis. Front. Plant Sci. 2021, 12, 638792. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, J.; Wang, Z.; Cheng, K.; Zhang, P.; Tian, G.; Liu, X.; Guo, E.; Du, Y.; Wang, Y. Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress. BMC Plant Biol. 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Galleano, M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: From experimental models to humans. Redox Biol. 2021, 42, 101914. [Google Scholar] [CrossRef]
- Zhou, D.; Bai, Z.; Guo, T.; Li, J.; Li, Y.; Hou, Y.; Chen, G.; Li, N. Dietary flavonoids and human top-ranked diseases: The perspective of in vivo bioactivity and bioavailability. Trends Food Sci. Technol. 2022, 120, 374–386. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Li, M.; Song, F.; Liu, S.; Qin, Y.; Hu, B.; Cui, X. Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’ from Different Origins. Curr. Issues Mol. Biol. 2024, 46, 12830-12846. https://doi.org/10.3390/cimb46110764
Peng Y, Li M, Song F, Liu S, Qin Y, Hu B, Cui X. Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’ from Different Origins. Current Issues in Molecular Biology. 2024; 46(11):12830-12846. https://doi.org/10.3390/cimb46110764
Chicago/Turabian StylePeng, Yujiao, Meixin Li, Fangfei Song, Shuilan Liu, Yuxiang Qin, Baoqing Hu, and Xueyu Cui. 2024. "Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’ from Different Origins" Current Issues in Molecular Biology 46, no. 11: 12830-12846. https://doi.org/10.3390/cimb46110764
APA StylePeng, Y., Li, M., Song, F., Liu, S., Qin, Y., Hu, B., & Cui, X. (2024). Identification of Primary Metabolite Profiles Reveals Quality Characteristics of Citrus maxima ‘Shatian Yu’ from Different Origins. Current Issues in Molecular Biology, 46(11), 12830-12846. https://doi.org/10.3390/cimb46110764




