Telomerase Reverse Transcriptase-Promoter Mutation in Young Patients with Bladder Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Selection
2.2. Mutation Analysis
2.3. Evaluations
2.4. Statistical Analysis
3. Results
3.1. Epidemiological and Clinical Parameters
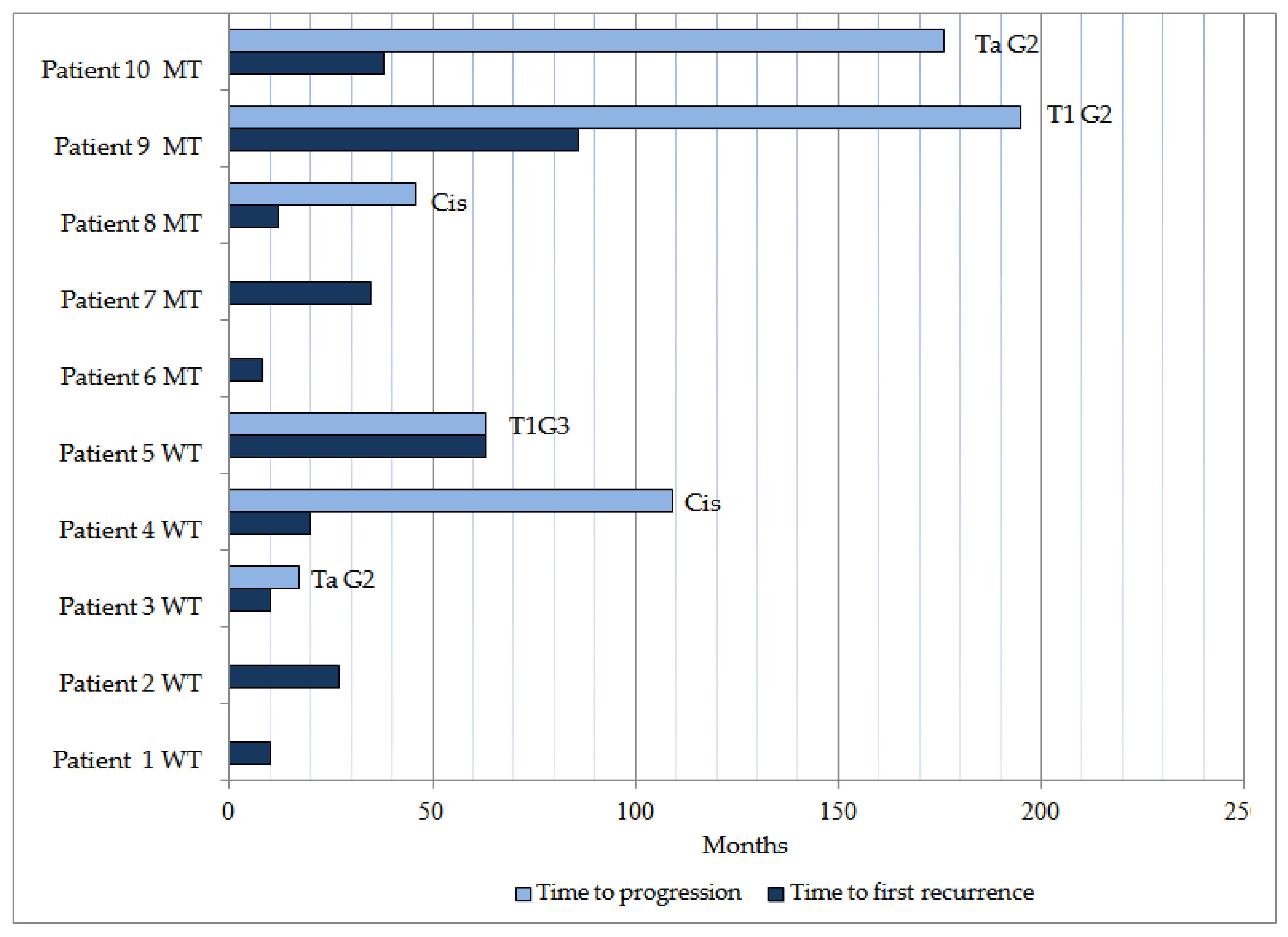
3.2. Disease Evolution
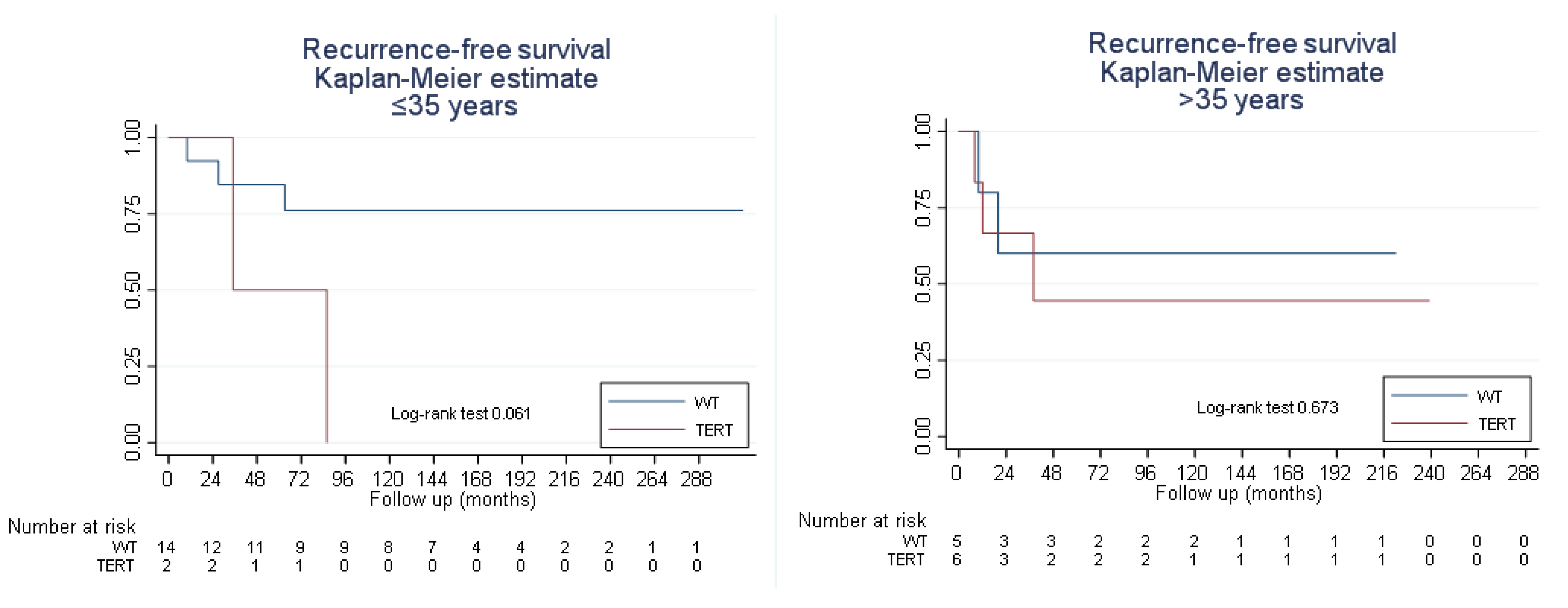
3.3. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell 1985, 43 Pt 1, 405–413. [Google Scholar] [CrossRef]
- Hosseini-Asl, S.; Atri, M.; Modarressi, M.H.; Salhab, M.; Mokbel, K.; Mehdipour, P. The expression of hTR and hTERT in human breast cancer: Correlation with clinico-pathological parameters. Int. Semin. Surg. Oncol. 2006, 3, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cong, Y.-S.; Wright, W.E.; Shay, J.W. Human Telomerase and Its Regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of telomerase in cancer. Cell. Mol. Life Sci. 2016, 73, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere maintenance mechanisms in cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flández, M.; Marqués, M.; Márquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Wang, M.; Lopez-Beltran, A. Biological and clinical perspectives of TERT promoter mutation detection on bladder cancer diagnosis and management. Hum. Pathol. 2023, 33, 56–75. [Google Scholar] [CrossRef]
- Günes, C.; Wezel, F.; Southgate, J.; Bolenz, C. Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat. Rev. Urol. 2018, 15, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Fujita, K.; Netto, G.J.; Nonomura, N. Clinical Application of TERT Promoter Mutations in Urothelial Carcinoma. Front. Oncol. 2021, 11, 705440. [Google Scholar] [CrossRef]
- Isharwal, S.; Audenet, F.; Drill, E.; Pietzak, E.J.; Iyer, G.; Ostrovnaya, I.; Cha, E.; Donahue, T.; Arcila, M.; Jayakumaran, G.; et al. Prognostic Value of TERT Alterations, Mutational and Copy Number Alterations Burden in Urothelial Carcinoma. Eur. Urol. Focus 2019, 5, 201–204. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; De Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Cao, J.L.; Abuduwufuer, A.; Wang, L.M.; Yuan, X.S.; Lv, W.; Hu, J. Clinical characteristics and prognostic significance of TERT promoter mutations in cancer: A cohort study and a meta-analysis. PLoS ONE 2016, 11, e0146803. [Google Scholar] [CrossRef]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Platt, F.M.; Knowles, M.A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 2014, 65, 367–369. [Google Scholar] [CrossRef]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A., Jr.; et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef]
- Kurtis, B.; Zhuge, J.; Ojaimi, C.; Ye, F.; Cai, D.; Zhang, D.; Fallon, J.T.; Zhong, M. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann. Diagn. Pathol. 2016, 21, 7–11. [Google Scholar] [CrossRef]
- Siraj, A.K.; Bu, R.; Iqbal, K.; Parvathareddy, S.K.; Siraj, N.; Siraj, S.; Diaz, M.R.F.; Rala, D.R.; Benito, A.D.; Sabido, M.A.; et al. Telomerase reverse transcriptase promoter mutations in cancers derived from multiple organ sites among middle eastern population. Genomics 2020, 112, 1746–1753. [Google Scholar] [CrossRef]
- Wan, S.; Liu, X.; Hua, W.; Xi, M.; Zhou, Y.; Wan, Y. The role of telomerase reverse transcriptase (TERT) promoter mutations in prognosis in bladder cancer. Bioengineered 2021, 12, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, P.; Li, C.; Huang, Y.; Li, X.; Wang, Y.; Chen, C.; Lv, Z.; Tang, A.; Sun, X.; et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: A genomic and molecular study. Eur. Urol. 2014, 65, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Pietzak, E.J.; Bagrodia, A.; Cha, E.K.; Drill, E.N.; Iyer, G.; Isharwal, S.; Ostrovnaya, I.; Baez, P.; Li, Q.; Berger, M.F.; et al. Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur. Urol. 2017, 72, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Novakova, Z.V.; Bevizova, K.; Danisovic, L.; Ziaran, S. Biomarkers of Bladder Cancer: Cell-Free DNA, Epigenetic Modifications and Non-Coding RNAs. Int. J. Mol. Sci. 2022, 23, 13206. [Google Scholar] [CrossRef] [PubMed]
- Yossepowitch, O.; Dalbagni, G. Transitional Cell Carcinoma of the Bladder in Young Adults: Presentation, Natural History and Outcome. J. Urol. 2002, 168, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. The TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Roggisch, J.; Ecke, T.; Koch, S. Molecular identification of telomerase reverse transcriptase (TERT) promotor mutations in primary and recurrent tumors of invasive and noninvasive urothelial bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 77.e17–77.e25. [Google Scholar] [CrossRef]
- Jahnson, S.; Söderkvist, P.; Aljabery, F.; Olsson, H. Telomerase reverse transcriptase mutation and the p53 pathway in T1 urinary bladder cancer. BJU Int. 2022, 129, 601–609. [Google Scholar] [CrossRef]
- Giedl, J.; Rogler, A.; Wild, A.; Riener, M.O.; Filbeck, T.; Burger, M.; Rümmele, P.; Hurst, C.; Knowles, M.; Hartmann, A.; et al. TERT core promotor mutations in early-onset bladder cancer. J. Cancer 2016, 7, 915–920. [Google Scholar] [CrossRef]
- Nardelli, C.; Aveta, A.; Pandolfo, S.D.; Tripodi, L.; Russo, F.; Imbimbo, C.; Castaldo, G.; Pastore, L. Microbiome Profiling in Bladder Cancer Patients Using the First-morning Urine Sample. Eur. Urol. Open Sci. 2023, 59, 18–26. [Google Scholar] [CrossRef]
- Leão, R.; Lee, D.; Figueiredo, A.; Hermanns, T.; Wild, P.; Komosa, M.; Lau, I.; Mistry, M.; Nunes, N.M.; Price, A.J.; et al. Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int. J. Cancer 2019, 144, 1676–1684. [Google Scholar] [CrossRef]
- Eich, M.L.; Rodriguez Pena, M.D.C.; Springer, S.U.; Taheri, D.; Tregnago, A.C.; Salles, D.C.; Bezerra, S.M.; Cunha, I.W.; Fujita, K.; Ertoy, D.; et al. Incidence and distribution of UroSEEK gene panel in a multi-institutional cohort of bladder urothelial carcinoma. Mod. Pathol. 2019, 32, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Huang, C.Y.; Jhuang, Y.L.; Chen, C.C.; Jeng, Y.M. Biological Significance of TERT Promoter Mutation in Papillary Urothelial Neoplasm of Low Malignant Potential. Histopathology 2018, 72, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.J.; Ju, Y.; Gordon, N.S.; Zeegers, M.P.; Cheng, K.K.; James, N.D.; Bryan, R.T.; Ward, D.G. Toward Personalised Liquid Biopsies for Urothelial Carcinoma: Characterisation of ddPCR and Urinary cfDNA for the Detection of the TERT 228 G>A/T Mutation. Bladder Cancer 2018, 4, 41–48. [Google Scholar] [CrossRef]
- Descotes, F.; Kara, N.; Decaussin-Petrucci, M.; Piaton, E.; Geiguer, F.; Rodriguez-Lafrasse, C.; Terrier, J.E.; Lopez, J.; Ruffion, A. Non-invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine. Br. J. Cancer 2017, 117, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Dahmcke, C.M.; Steven, K.E.; Larsen, L.K.; Poulsen, A.L.; Abdul-Al, A.; Dahl, C.; Guldberg, P. A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria. Eur. Urol. 2016, 70, 916–919. [Google Scholar] [CrossRef]
- Shuai, H.; Duan, X.; Zhou, J.J.; Liu, Y.; Wu, T. Effect of the TERT Mutation on the Prognosis of Patients with Urothelial Carcinoma: A Systematic Review and Meta-Analysis. BMC Urol. 2023, 23, 177. [Google Scholar] [CrossRef]
- Mollica, V.; Tassinari, E.; Santoni, M.; Marchese, P.V.; Giunchi, F.; Maloberti, T.; Tateo, V.; Ricci, C.; Rosellini, M.; Marchetti, A.; et al. TERT Promoter Mutations and the Outcome of Patients with Advanced Urothelial Carcinoma Treated by Platinum-Based Chemotherapy or Pembrolizumab. Pathol. Res. Pract. 2024, 253, 155008. [Google Scholar] [CrossRef]

| Clinical and Pathological Characteristics | Total | WT pTERT | Mutated pTERT | p-Value |
|---|---|---|---|---|
| No. of patients | 29 | 20 (69%) | 9 (31%) | |
| Sex | ||||
| Male | 21 (72.4%) | 15 (75%) | 6 (66.7%) | 0.675 |
| Female | 8 (27.6%) | 5 (25%) | 3 (33.3%) | |
| Age, years; mean (SD) | 34.1 (4.7) | 33.3 (4.2) | 35.8 (5.2) | 0.107 |
| Personal Background | ||||
| Smoker | 19 (65.5%) | 14 (70%) | 5 (55.6%) | 0.675 |
| HBP | 1 (3.4%) | 1 (5%) | 0 | 1 |
| DL | 1 (3.4%) | 1 (5%) | 0 | 1 |
| DM | 1 (3.4%) | 1 (5%) | 0 | 1 |
| Other | 10 (34.5%) | 6 (30%) | 4 (44.4%) | 0.68 |
| ASA | ||||
| 1 | 22 (75.9%) | 17 (85%) | 5 (55.6%) | 0.212 |
| 2 | 5 (17.2%) | 2 (10%) | 3 (33.3%) | |
| 3 | 2 (6.9%) | 1 (5%) | 1 (11.1%) | |
| Cytology | ||||
| Positive | 7 (24.1%) | 3 (15%) | 4 (44.4%) | 0.005 |
| Suspicious | 2 (6.9%) | 0 | 2 (22.2%) | |
| Negative | 20 (69%) | 17 (85%) | 3 (33.3%) | |
| Clinical diagnosis | ||||
| Macroscopic hematuria | 26 (89.7%) | 18 (90%) | 8 (88.9%) | 1 |
| Urinary syndrome | 3 (10.3%) | 2 (10%) | 1 (11.1%) | 1 |
| Location | ||||
| Trigone | 4 (13.8%) | 2 (10%) | 2 (22.2%) | 0.568 |
| Posterior wall | 5 (17.2%) | 1 (5%) | 4 (44.4%) | 0.022 |
| Right wall | 6 (20.7%) | 5 (25%) | 1 (11.1%) | 0.633 |
| Left wall | 14 (48.3%) | 12 (60%) | 2 (22.2%) | 0.109 |
| Anterior wall | 2 (6.9%) | 0 | 2 (22.2%) | 0.089 |
| Neck—posterior urethra | 3 (10.3%) | 2 (10%) | 1 (11.1%) | 1 |
| Stage at diagnosis (T) | ||||
| Ta | 22 (75.9%) | 16 (80%) | 6 (66.7%) | 0.744 |
| T1 | 1 (3.4%) | 1 (5%) | 0 | |
| T2 | 4 (13.8%) | 2 (10%) | 2 (22.2%) | |
| T3 | 2 (6.9%) | 1 (5%) | 1 (11.1%) | |
| Grade at diagnosis (G) | ||||
| PUNLMP | 3 (10.3%) | 3 (15%) | 0 | 0.478 |
| Carcinoma G1 | 20 (69%) | 14 (70%) | 6 (66.7%) | |
| Carcinoma G3 | 6 (20.7%) | 3 (15%) | 3 (33.3%) | |
| Tumor size, cm; median (IQR) | 2 (1–3) | 2 (1–2) | 2 (1–4) | 0.358 |
| Recurrence | 10 (34.5%) | 5 (25%) | 5 (55.6%) | 0.12 |
| Progression | 6 (20.7%) | 3 (15%) | 3 (33.3%) | 0.339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez González, S.; Heredia-Soto, V.; Girón de Francisco, M.; Pérez-Fernández, E.; Casans-Francés, R.; Mendiola Sabio, M.; González-Peramato, P. Telomerase Reverse Transcriptase-Promoter Mutation in Young Patients with Bladder Tumors. Curr. Issues Mol. Biol. 2024, 46, 2845-2855. https://doi.org/10.3390/cimb46040178
Pérez González S, Heredia-Soto V, Girón de Francisco M, Pérez-Fernández E, Casans-Francés R, Mendiola Sabio M, González-Peramato P. Telomerase Reverse Transcriptase-Promoter Mutation in Young Patients with Bladder Tumors. Current Issues in Molecular Biology. 2024; 46(4):2845-2855. https://doi.org/10.3390/cimb46040178
Chicago/Turabian StylePérez González, Sonia, Victoria Heredia-Soto, Manuel Girón de Francisco, Elia Pérez-Fernández, Rubén Casans-Francés, Marta Mendiola Sabio, and Pilar González-Peramato. 2024. "Telomerase Reverse Transcriptase-Promoter Mutation in Young Patients with Bladder Tumors" Current Issues in Molecular Biology 46, no. 4: 2845-2855. https://doi.org/10.3390/cimb46040178
APA StylePérez González, S., Heredia-Soto, V., Girón de Francisco, M., Pérez-Fernández, E., Casans-Francés, R., Mendiola Sabio, M., & González-Peramato, P. (2024). Telomerase Reverse Transcriptase-Promoter Mutation in Young Patients with Bladder Tumors. Current Issues in Molecular Biology, 46(4), 2845-2855. https://doi.org/10.3390/cimb46040178






