Blocking Microglial Proliferation by CSF-1R Inhibitor Does Not Alter the Neuroprotective Effects of Adoptive Regulatory T Cells in 3xTg Alzheimer’s Disease Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
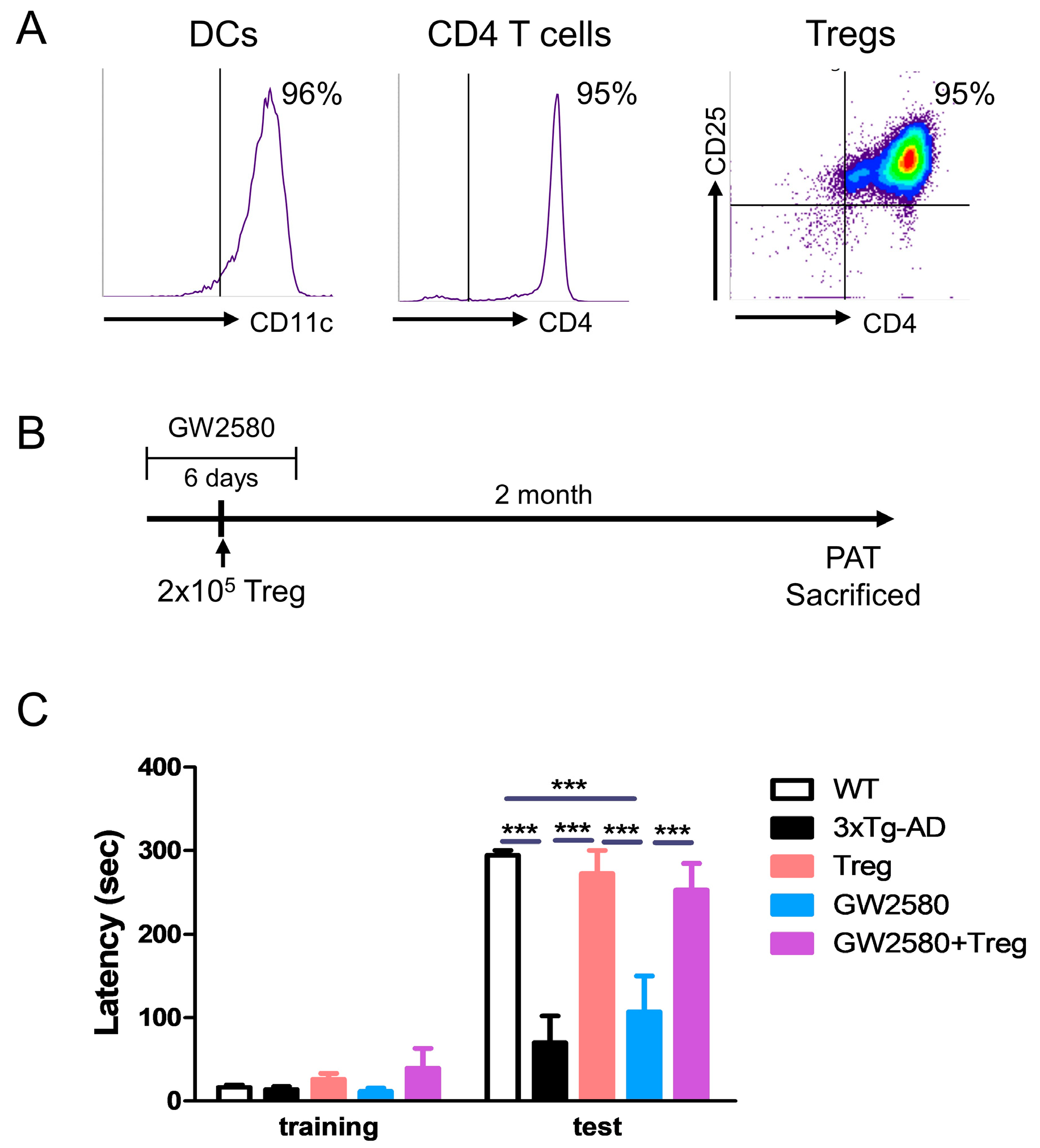
2.2. Manufacturing and Adoptive Transfer of Tregs
2.3. Drug Treatment and Adoptive Transfer
2.4. Flow Cytometry
2.5. Passive Avoidance Test (PAT)
2.6. Immunohistochemistry Analysis
2.7. RNA Extraction and RT-PCR Assays
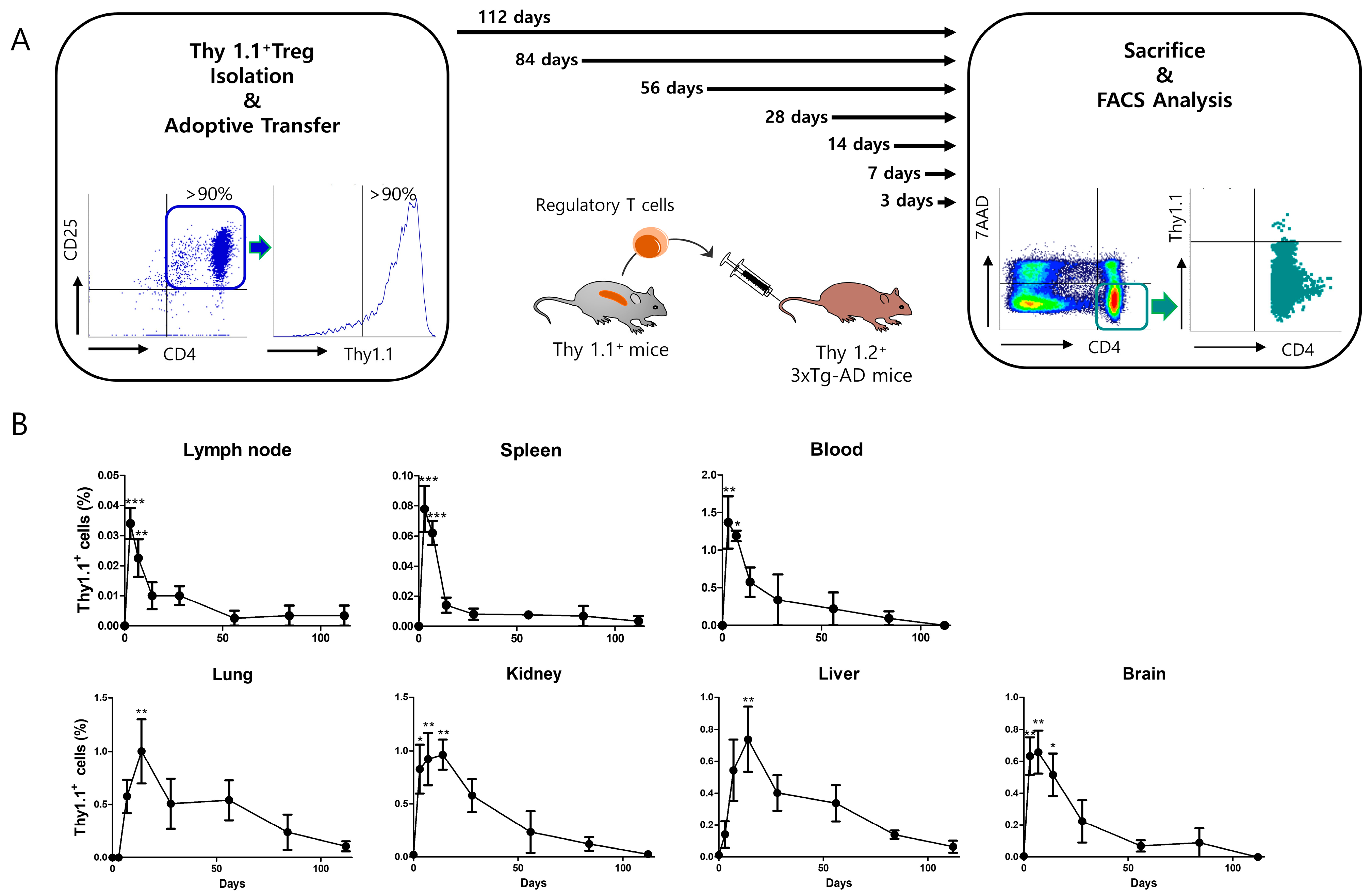
2.8. Thy 1.1 Treg Trafficking
2.9. Statistical Analysis
3. Results
3.1. CSF-1R Inhibition Reduced Microglia and Macrophage Population in 4-Month 3xTg-AD Mice
3.2. Adoptive Transfer of Tregs Attenuates Learning and Memory Impairment in GW2580-Treated 3xTg-AD Mice
3.3. Adoptive Transfer of Tregs Attenuates AD Pathology in GW2580-Treated 3xTg-AD Mice
3.4. Adoptive Transfer of Tregs Modulates Microglial Polarization in GW2580-Treated 3xTg-AD Mice
3.5. Adoptively Transferred Tregs Survive up to 112 Days in Several Tissues of 3xTg-AD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cummings, J.L.; Cole, G. Alzheimer disease. JAMA 2002, 287, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.D.; Zimmerman, S.; Suchindran, C.; Reed, P.; Wang, L.; Boustani, M.; Sudha, S. The public health impact of Alzheimer’s disease, 2000-2050: Potential implication of treatment advances. Annu. Rev. Public Health 2002, 23, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: A field in its infancy. J. Alzheimer’s Dis. JAD 2010, 19, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Morales, I.; Jimenez, J.M.; Mancilla, M.; Maccioni, R.B. Tau oligomers and fibrils induce activation of microglial cells. J. Alzheimer’s Dis. JAD 2013, 37, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Liesz, A.; Kleinschnitz, C. Regulatory T Cells in Post-stroke Immune Homeostasis. Transl. Stroke Res. 2016, 7, 313–321. [Google Scholar] [CrossRef]
- Bailey-Bucktrout, S.L.; Bluestone, J.A. Regulatory T cells: Stability revisited. Trends Immunol. 2011, 32, 301–306. [Google Scholar] [CrossRef]
- Baek, H.; Ye, M.; Kang, G.H.; Lee, C.; Lee, G.; Choi, D.B.; Jung, J.; Kim, H.; Lee, S.; Kim, J.S.; et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 2016, 7, 69347–69357. [Google Scholar] [CrossRef]
- Reynolds, A.D.; Banerjee, R.; Liu, J.; Gendelman, H.E.; Mosley, R.L. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J. Leukoc. Biol. 2007, 82, 1083–1094. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Huang, A.; Wen, S.; Liao, B.; Appel, S.H. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain J. Neurol. 2011, 134, 1293–1314. [Google Scholar] [CrossRef]
- Park, S.Y.; Yang, H.; Ye, M.; Liu, X.; Shim, I.; Chang, Y.T.; Bae, H. Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice. J. Neuroinflamm. 2022, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Park, S.Y.; Baek, H.; Lee, C.; Chung, G.; Liu, X.; Lee, J.H.; Kim, B.; Kwon, M.; Choi, H.; et al. Adoptive therapy with amyloid-beta specific regulatory T cells alleviates Alzheimer’s disease. Theranostics 2022, 12, 7668–7680. [Google Scholar] [CrossRef]
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Soto-Diaz, K.; Vailati-Riboni, M.; Louie, A.Y.; McKim, D.B.; Gaskins, H.R.; Johnson, R.W.; Steelman, A.J. Treatment with the CSF1R Antagonist GW2580, Sensitizes Microglia to Reactive Oxygen Species. Front. Immunol. 2021, 12, 734349. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Kevadiya, B.D.; Muhammad, I.K.; Herskovitz, J.; Olson, K.E.; Mosley, R.L.; Gendelman, H.E. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol. Neurodegener. 2020, 15, 32. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Edinger, M.; Hoffmann, P.; Ermann, J.; Drago, K.; Fathman, C.G.; Strober, S.; Negrin, R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003, 9, 1144–1150. [Google Scholar] [CrossRef]
- Mottet, C.; Uhlig, H.H.; Powrie, F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003, 170, 3939–3943. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; Carpentier, P.A.; Anger, H.A.; Miller, S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002, 169, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Yeapuri, P.; Machhi, J.; Lu, Y.; Abdelmoaty, M.M.; Kadry, R.; Patel, M.; Bhattarai, S.; Lu, E.; Namminga, K.L.; Olson, K.E.; et al. Amyloid-beta specific regulatory T cells attenuate Alzheimer’s disease pathobiology in APP/PS1 mice. Mol. Neurodegener. 2023, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Li, P.; Zhu, W.; Cai, W.; Liu, Z.; Wang, Y.; Luo, W.; Stetler, R.A.; Leak, R.K.; Yu, W.; et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain J. Neurol. 2017, 140, 1914–1931. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, Y.R.; Tung, S.L.; Dudreuilh, C.; Lechler, R.I.; Fruhwirth, G.O.; Lombardi, G. The Future of Regulatory T Cell Therapy: Promises and Challenges of Implementing CAR Technology. Front. Immunol. 2020, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Baeten, P.; Van Zeebroeck, L.; Kleinewietfeld, M.; Hellings, N.; Broux, B. Improving the Efficacy of Regulatory T Cell Therapy. Clin. Rev. Allergy Immunol. 2022, 62, 363–381. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.C.; Anderton, S.M. Adaptive immune responses in CNS autoimmune disease: Mechanisms and therapeutic opportunities. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 774–790. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Perera, C.J.; Moalem-Taylor, G. The role of regulatory T cells in nervous system pathologies. J. Neurosci. Res. 2018, 96, 951–968. [Google Scholar] [CrossRef]
- Olmos-Alonso, A.; Schetters, S.T.; Sri, S.; Askew, K.; Mancuso, R.; Vargas-Caballero, M.; Holscher, C.; Perry, V.H.; Gomez-Nicola, D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain J. Neurol. 2016, 139, 891–907. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Perry, V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2015, 21, 169–184. [Google Scholar] [CrossRef]
- Fagan, S.G.; Bechet, S.; Dev, K.K. Fingolimod Rescues Memory and Improves Pathological Hallmarks in the 3xTg-AD Model of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.; Blurton-Jones, M.; West, B.L.; Green, K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain J. Neurol. 2016, 139, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.P.; Hohsfield, L.A.; Kramar, E.A.; Soreq, L.; Lee, R.J.; Pham, S.T.; Najafi, A.R.; Spangenberg, E.E.; Wood, M.A.; West, B.L.; et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 2018, 17, e12832. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qin, C.; Chen, M.; Yu, H.H.; Chu, Y.H.; Chen, T.J.; Bosco, D.B.; Wu, L.J.; Bu, B.T.; Wang, W.; et al. Regulatory T cells protect against brain damage by alleviating inflammatory response in neuromyelitis optica spectrum disorder. J. Neuroinflamm. 2021, 18, 201. [Google Scholar] [CrossRef]
- Zhou, K.; Zhong, Q.; Wang, Y.C.; Xiong, X.Y.; Meng, Z.Y.; Zhao, T.; Zhu, W.Y.; Liao, M.F.; Wu, L.R.; Yang, Y.R.; et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 967–979. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Tan, N.; Nova-Lamperti, E.; McGregor, R.; Lechler, R.I.; Lombardi, G.; Scotta, C. Expanded Regulatory T Cells Induce Alternatively Activated Monocytes with a Reduced Capacity to Expand T Helper-17 Cells. Front. Immunol. 2018, 9, 1625. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| β-actin | GTG CTA TGT TGC TCT AGA CTT CG | ATG CCA CAG GAT TCC ATA CC |
| NOS2 | AGG ACA TCC TGC GGC AGC | GCT TTA ACC CCT CCT GTA |
| TNF-α | GGC AGG TTC TGT CCC TTT CAC | TTC TGT GCT CAT GGT GTC TTT TCT |
| IL-1β | AAG CCT CGT GCT GTC GGA CC | TGA GGC CCA AGG CCA CAG G |
| IL-6 | TTC CAT CCA GTT GCC TTC TTG | GGG AGT GGT ATC CTC TGT GAA GTC |
| Ym1 | TGG AGG ATG GAA GTT TGG AC | GAG TAG CAG CCT TGG AAT GT |
| IL-10 | CAG CCG GGA AGA CAA TAA CTG | CCG CAG CTC TAG GAG CAT GT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Cha, N.; Kim, S.; Chae, S.; Lee, W.-j.; Jung, H.; Bae, H. Blocking Microglial Proliferation by CSF-1R Inhibitor Does Not Alter the Neuroprotective Effects of Adoptive Regulatory T Cells in 3xTg Alzheimer’s Disease Mice. Curr. Issues Mol. Biol. 2024, 46, 2871-2883. https://doi.org/10.3390/cimb46040180
Park S-Y, Cha N, Kim S, Chae S, Lee W-j, Jung H, Bae H. Blocking Microglial Proliferation by CSF-1R Inhibitor Does Not Alter the Neuroprotective Effects of Adoptive Regulatory T Cells in 3xTg Alzheimer’s Disease Mice. Current Issues in Molecular Biology. 2024; 46(4):2871-2883. https://doi.org/10.3390/cimb46040180
Chicago/Turabian StylePark, Seon-Young, Nari Cha, Soyoung Kim, Songah Chae, Won-jun Lee, Hyunjae Jung, and Hyunsu Bae. 2024. "Blocking Microglial Proliferation by CSF-1R Inhibitor Does Not Alter the Neuroprotective Effects of Adoptive Regulatory T Cells in 3xTg Alzheimer’s Disease Mice" Current Issues in Molecular Biology 46, no. 4: 2871-2883. https://doi.org/10.3390/cimb46040180






