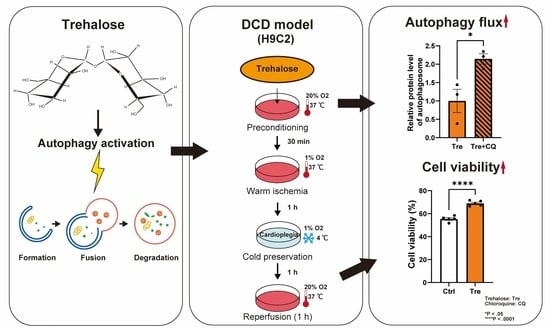
Effects of Trehalose Preconditioning on H9C2 Cell Viability and Autophagy Activation in a Model of Donation after Circulatory Death for Heart Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
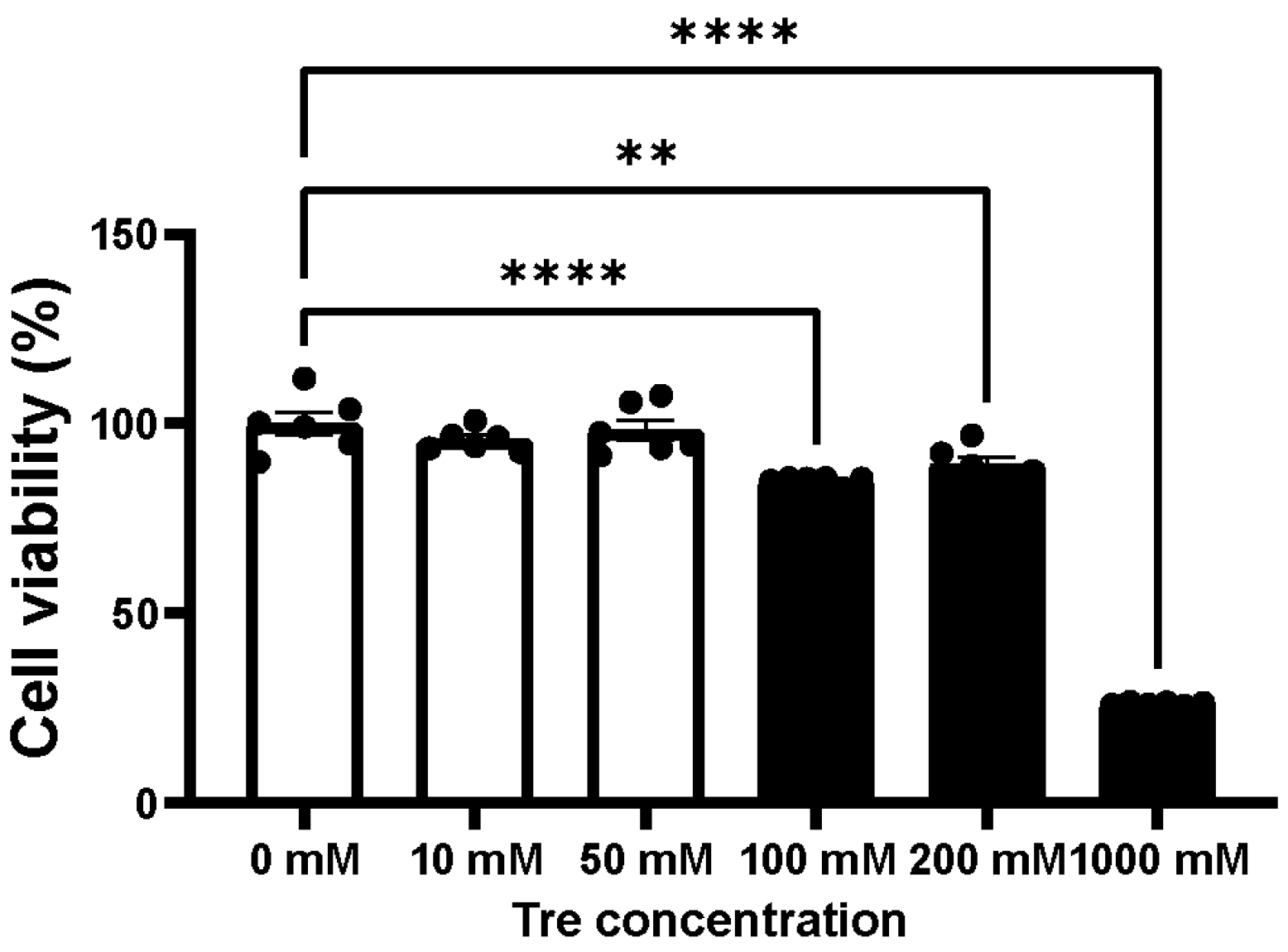
2.2. Assessment of Cellular Toxicity for Trehalose and Chloroquine
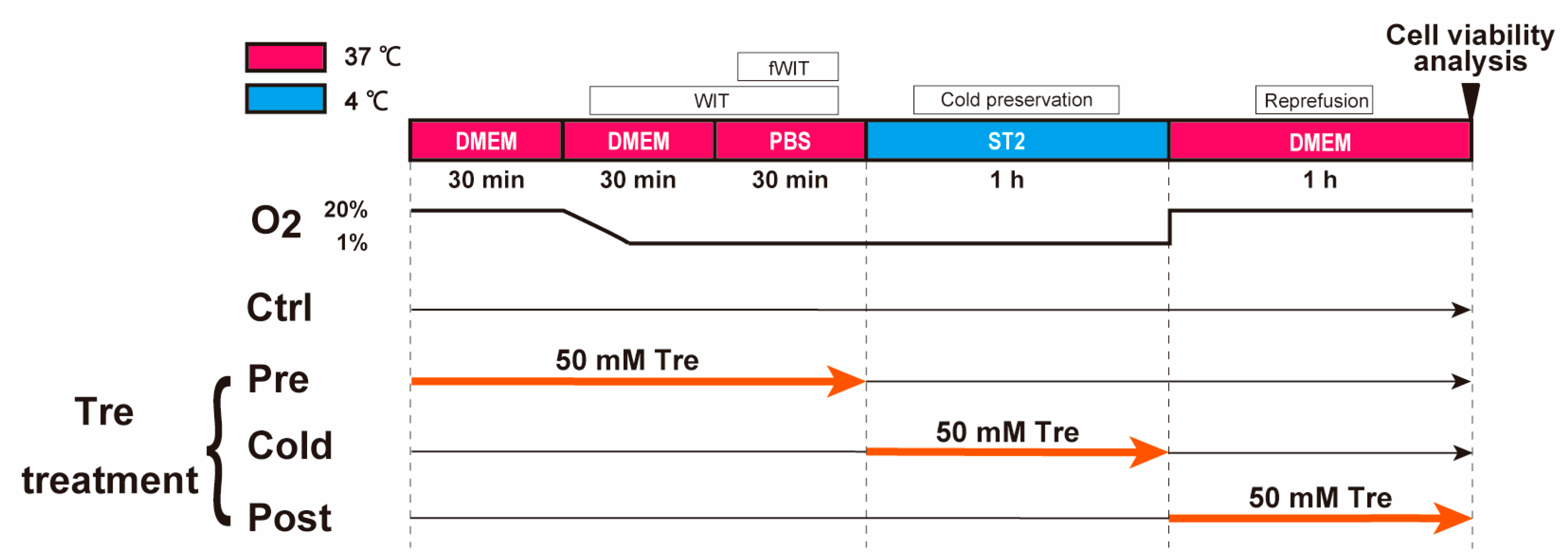
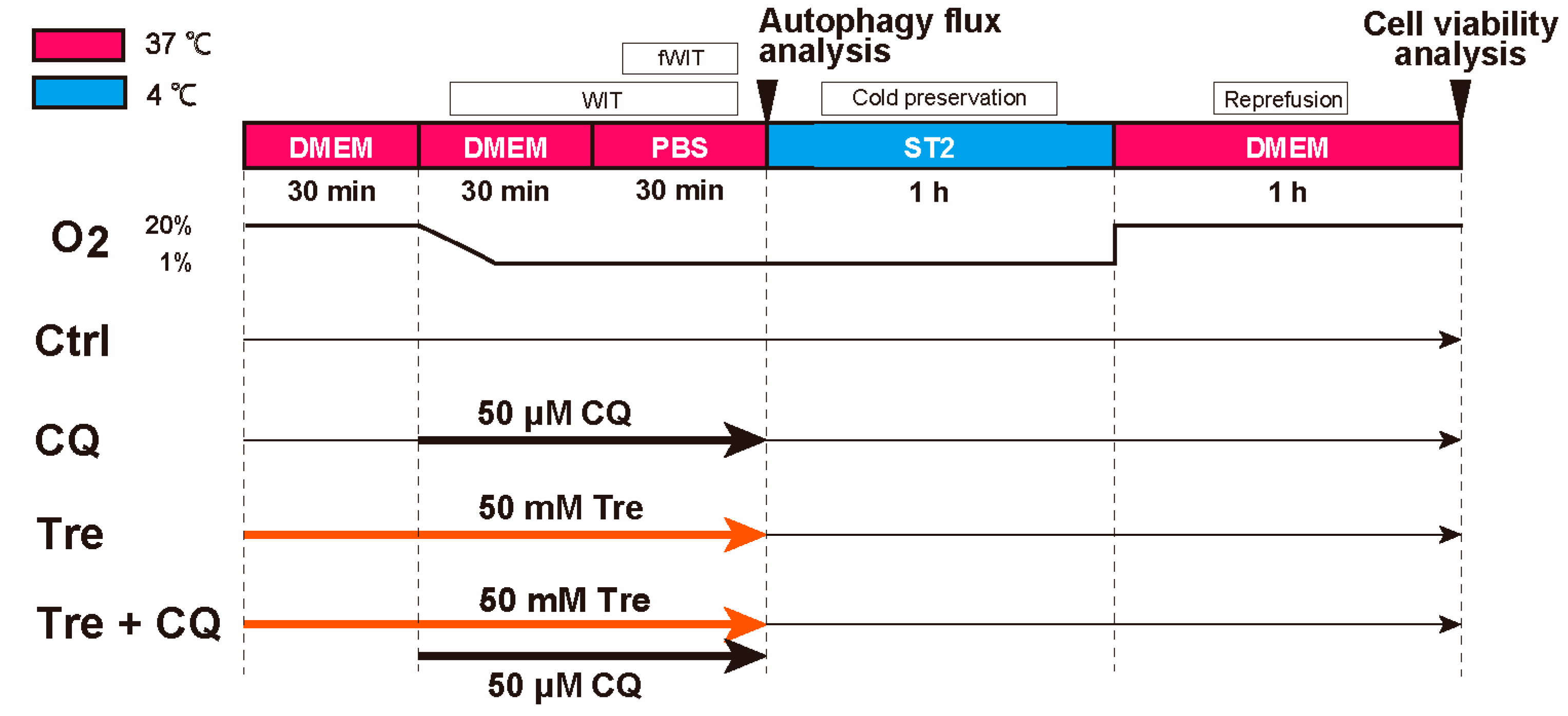
2.3. Protocol of a Cellular DCD Model and Trehalose Treatments
2.4. Validation of Oxygen Concentrations and pH in Different Solutions
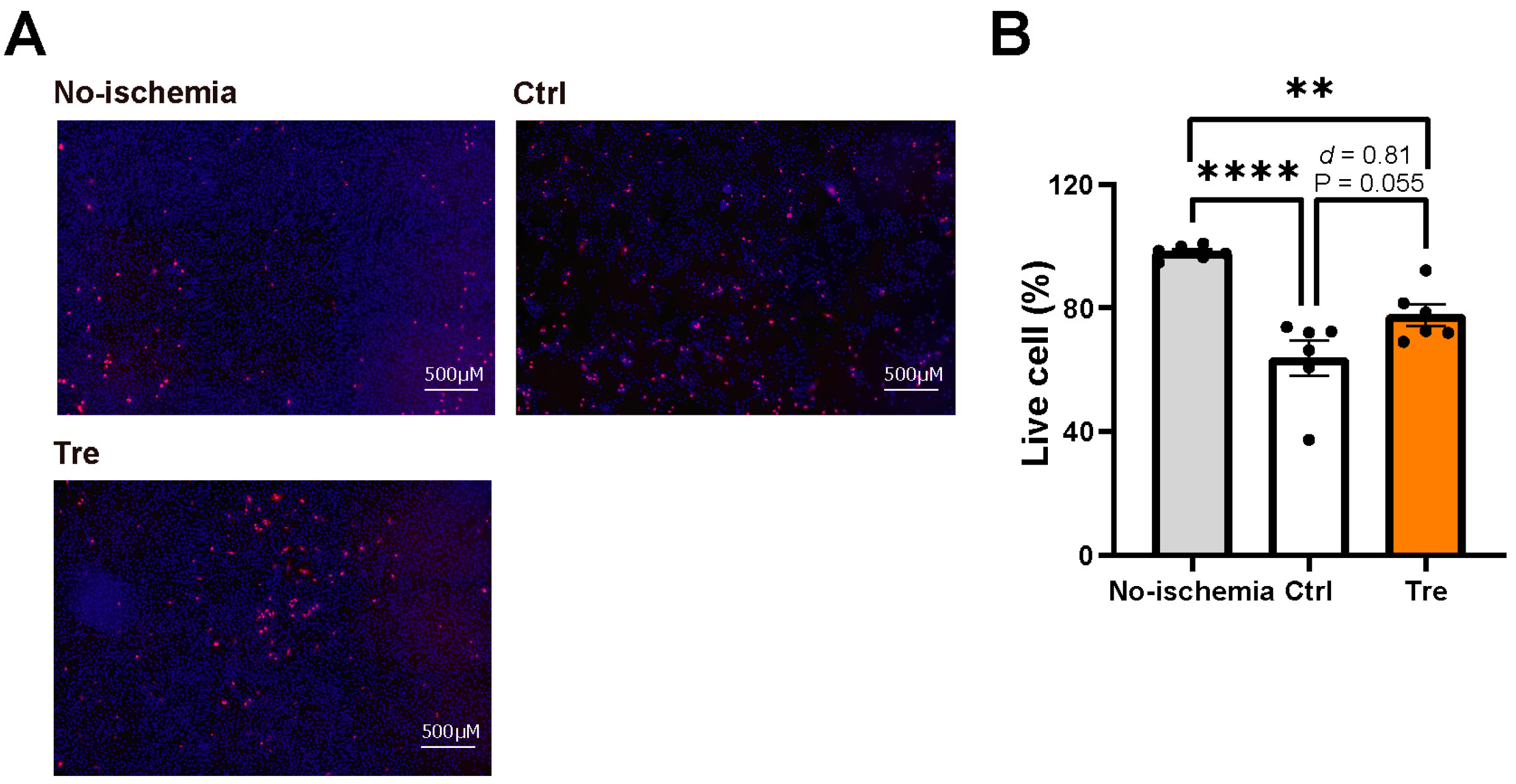
2.5. Assessment of Cell Viability in the DCD Model
2.6. Assessment of Cell Viability after Using Chloroquine, an Autophagy Inhibitor
2.7. Analysis of Autophagy Flux
2.8. Statistical Analysis
3. Results
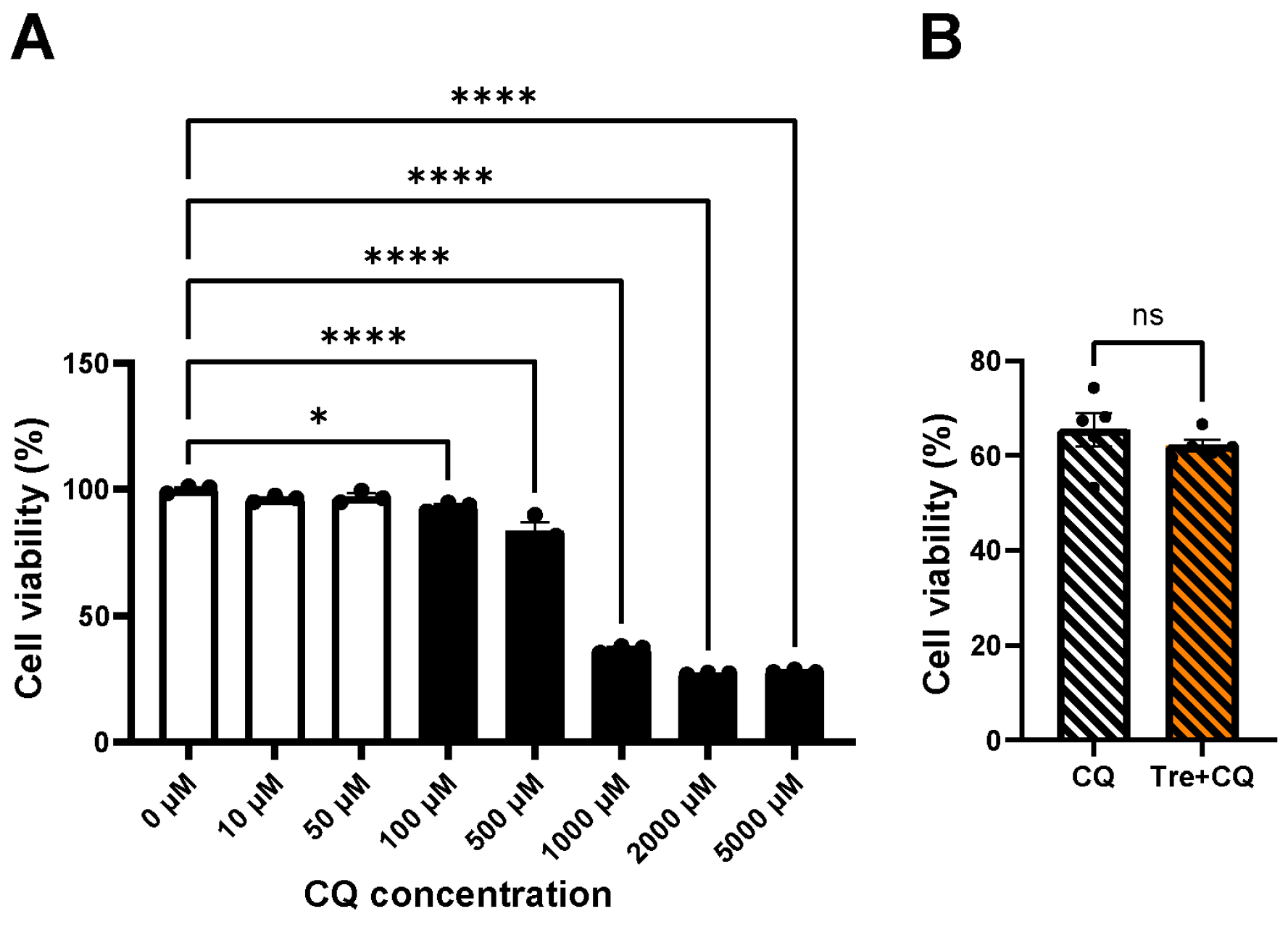
3.1. Cellular Toxicity of Trehalose
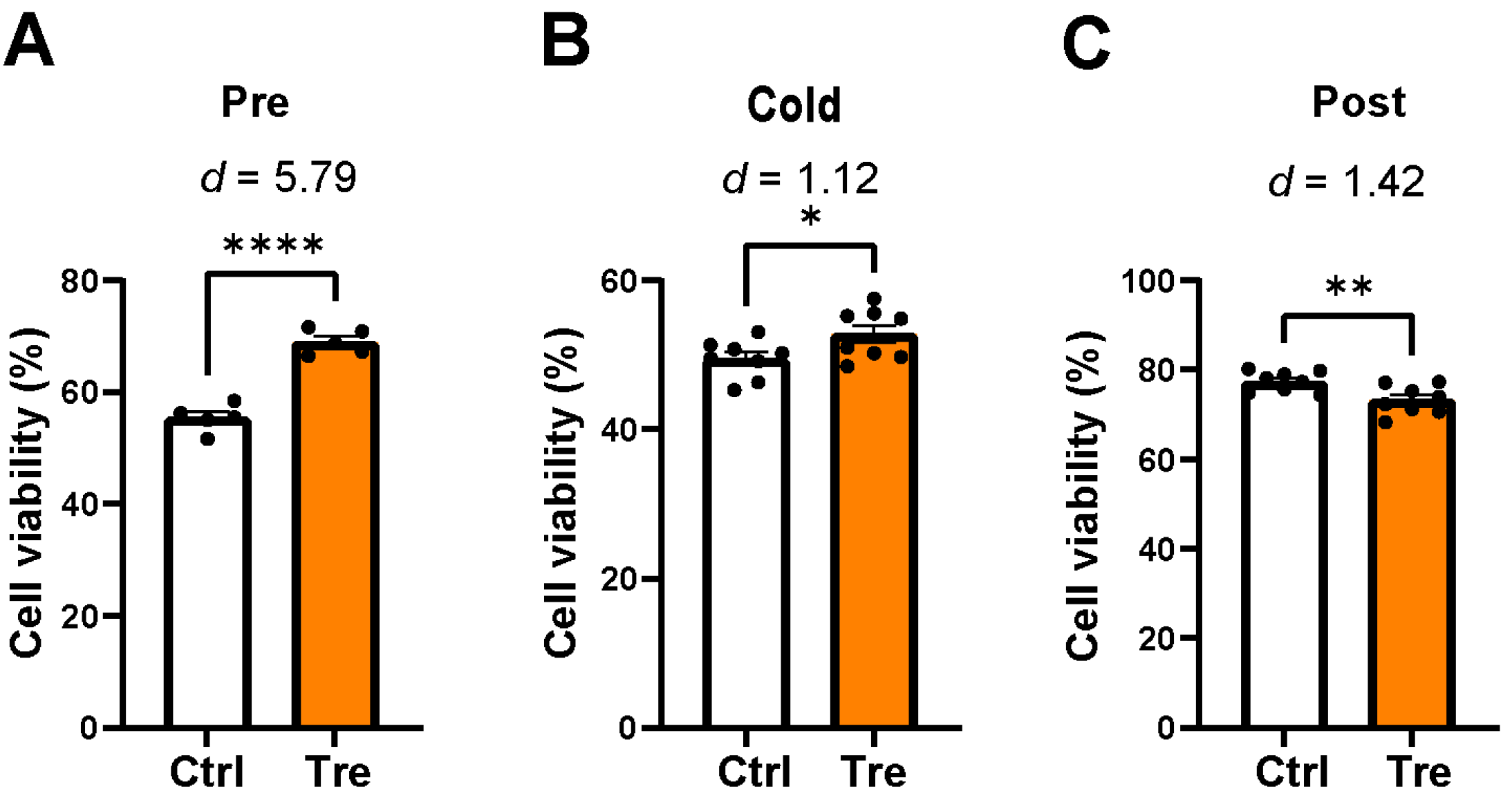
3.2. Effects of Trehalose Treatments on Cell Viability in the DCD Model
3.3. Cell Viability after Trehalose Using Autophagy Inhibitor, Chloroquine
3.4. Autophagy Flux in Trehalose Preconditioning
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsich, E.; Singh, T.P.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Perch, M.; Potena, L.; Sadavarte, A.; Lindblad, K.; Zuckermann, A.; et al. The International thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-ninth adult heart transplantation report-2022; focus on transplant for restrictive heart disease. J. Heart Lung Transplant. 2022, 41, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Bryner, B.S.; Casalinova, S.; Shah, A.; Smith, J.W.; Fiedler, A.G.; Daneshmand, M.; Silvestry, S.; et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N. Engl. J. Med. 2023, 388, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, S.E.; Jansz, P.C.; Macdonald, P.S. Heart transplantation following donation after circulatory death: Expanding the donor pool. J. Heart Lung Transplant. 2021, 40, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Moeslund, N.; Ertugrul, I.A.; Hu, M.A.; Dalsgaard, F.F.; Ilkjaer, L.B.; Ryhammer, P.; Pedersen, M.; Erasmus, M.E.; Eiskjaer, H. Ex-situ oxygenated hypothermic machine perfusion in donation after circulatory death heart transplantation following either direct procurement or in-situ normothermic regional perfusion. J. Heart Lung Transplant. 2023, 42, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Gao, H.; Zhou, N.; Wang, P.; Zhang, Y.; Zhang, A.; Jia, Z.; Huang, S. Trehalose attenuates renal ischemia-reperfusion injury by enhancing autophagy and inhibiting oxidative stress and inflammation. Am. J. Physiol.-Ren. Physiol. 2020, 318, F994–F1005. [Google Scholar] [CrossRef] [PubMed]
- Suno, K.; Shingu, Y.; Wakasa, S. Protective effects of trehalose preconditioning on cardiac and coronary endothelial function through eNOS signaling pathway in a rat model of ischemia-reperfusion injury. Mol. Cell. Biochem. 2022, 477, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Burda, R.; Burda, J.; Morochovič, R. Ischemic Tolerance-A Way to Reduce the Extent of Ischemia-Reperfusion Damage. Cells 2023, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Dieu, A.; Foguenne, M.; Bonaccorsi-Riani, E. Experimental and Clinical Aspects of Sevoflurane Preconditioning and Postconditioning to Alleviate Hepatic Ischemia-Reperfusion Injury: A Scoping Review. Int. J. Mol. Sci. 2023, 24, 2340. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229. [Google Scholar] [CrossRef]
- Hong, L.; Sun, Y.; An, J.-Z.; Wang, C.; Qiao, S.-G. Sevoflurane Preconditioning Confers Delayed Cardioprotection by Upregulating AMP-Activated Protein Kinase Levels to Restore Autophagic Flux in Ischemia-Reperfusion Rat Hearts. Med. Sci. Monit. 2020, 26, e922176. [Google Scholar] [CrossRef]
- Sciarretta, S.; Yee, D.; Nagarajan, N.; Bianchi, F.; Saito, T.; Valenti, V.; Tong, M.; Del Re, D.P.; Vecchione, C.; Schirone, L.; et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 1999–2010. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, J. mTOR-Independent autophagy inducer trehalose rescues against insulin resistance-induced myocardial contractile anomalies: Role of p38 MAPK and Foxo1. Pharmacol. Res. 2016, 111, 357–373. [Google Scholar] [CrossRef]
- Zahedi, N.; Pourajam, S.; Zaker, E.; Kouhpayeh, S.; Mirbod, S.M.; Tavangar, M.; Boshtam, M.; Hatami Kahkesh, K.; Qian, Q.; Zhang, F.; et al. The potential therapeutic impacts of trehalose on cardiovascular diseases as the environmental-influenced disorders: An overview of contemporary findings. Environ. Res. 2023, 226, 115674. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-Q.; Li, A.; Yang, Y.; Li, X.-X.; Zhang, L.-N.; Guo, H.-C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018, 193, 124–131. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, Y.; Qi, Y.; Chang, C.; Jiao, Y.; Bai, Y.; Guo, Z. Metformin ameliorates ferroptosis in cardiac ischemia and reperfusion by reducing NOX4 expression via promoting AMPKα. Pharm. Biol. 2023, 61, 886–896. [Google Scholar] [CrossRef]
- Gong, F.; Ge, T.; Liu, J.; Xiao, J.; Wu, X.; Wang, H.; Zhu, Y.; Xia, D.; Hu, B. Trehalose inhibits ferroptosis via NRF2/HO-1 pathway and promotes functional recovery in mice with spinal cord injury. Aging 2022, 14, 3216–3232. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Omasa, M.; Hasegawa, S.; Bando, T.; Hanaoka, N.; Yoshimura, T.; Nakamura, T.; Wada, H. Application of ET-Kyoto solution in clinical lung transplantation. Ann. Thorac. Surg. 2004, 77, 338–339. [Google Scholar] [CrossRef] [PubMed]

| Solutions | Oxygen Concentrations | Decrease Rate (%) | |
|---|---|---|---|
| Before Hypoxia | After Hypoxia | ||
| DMEM (37 °C) | 1.61 μL/mL | 0.68 μL/mL | 58 |
| PBS (37 °C) | 6.20 mL/L | 2.30 mL/L | 63 |
| PBS (4 °C) | 14.60 mg/L | 7.18 mg/L | 51 |
| Solutions | pH | Decrease | |
|---|---|---|---|
| Before | After | ||
| DMEM (37 °C, 30 min hypoxia) | 8.18 | 7.80 | 0.38 |
| PBS (37 °C, 30 min hypoxia) | 7.41 | 6.74 | 0.67 |
| ST2 (4 °C, 1 h hypoxia) | 7.89 | 6.92 | 0.97 |
| DMEM (37 °C, 30 min normoxia) | 8.20 | 8.03 | 0.17 |
| DMEM (37 °C, 1 h normoxia) | 8.35 | 8.01 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Shingu, Y.; Wakasa, S. Effects of Trehalose Preconditioning on H9C2 Cell Viability and Autophagy Activation in a Model of Donation after Circulatory Death for Heart Transplantation. Curr. Issues Mol. Biol. 2024, 46, 3353-3363. https://doi.org/10.3390/cimb46040210
Gao J, Shingu Y, Wakasa S. Effects of Trehalose Preconditioning on H9C2 Cell Viability and Autophagy Activation in a Model of Donation after Circulatory Death for Heart Transplantation. Current Issues in Molecular Biology. 2024; 46(4):3353-3363. https://doi.org/10.3390/cimb46040210
Chicago/Turabian StyleGao, Jingwen, Yasushige Shingu, and Satoru Wakasa. 2024. "Effects of Trehalose Preconditioning on H9C2 Cell Viability and Autophagy Activation in a Model of Donation after Circulatory Death for Heart Transplantation" Current Issues in Molecular Biology 46, no. 4: 3353-3363. https://doi.org/10.3390/cimb46040210
APA StyleGao, J., Shingu, Y., & Wakasa, S. (2024). Effects of Trehalose Preconditioning on H9C2 Cell Viability and Autophagy Activation in a Model of Donation after Circulatory Death for Heart Transplantation. Current Issues in Molecular Biology, 46(4), 3353-3363. https://doi.org/10.3390/cimb46040210