Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3
Abstract
1. Introduction
2. Materials and Methods
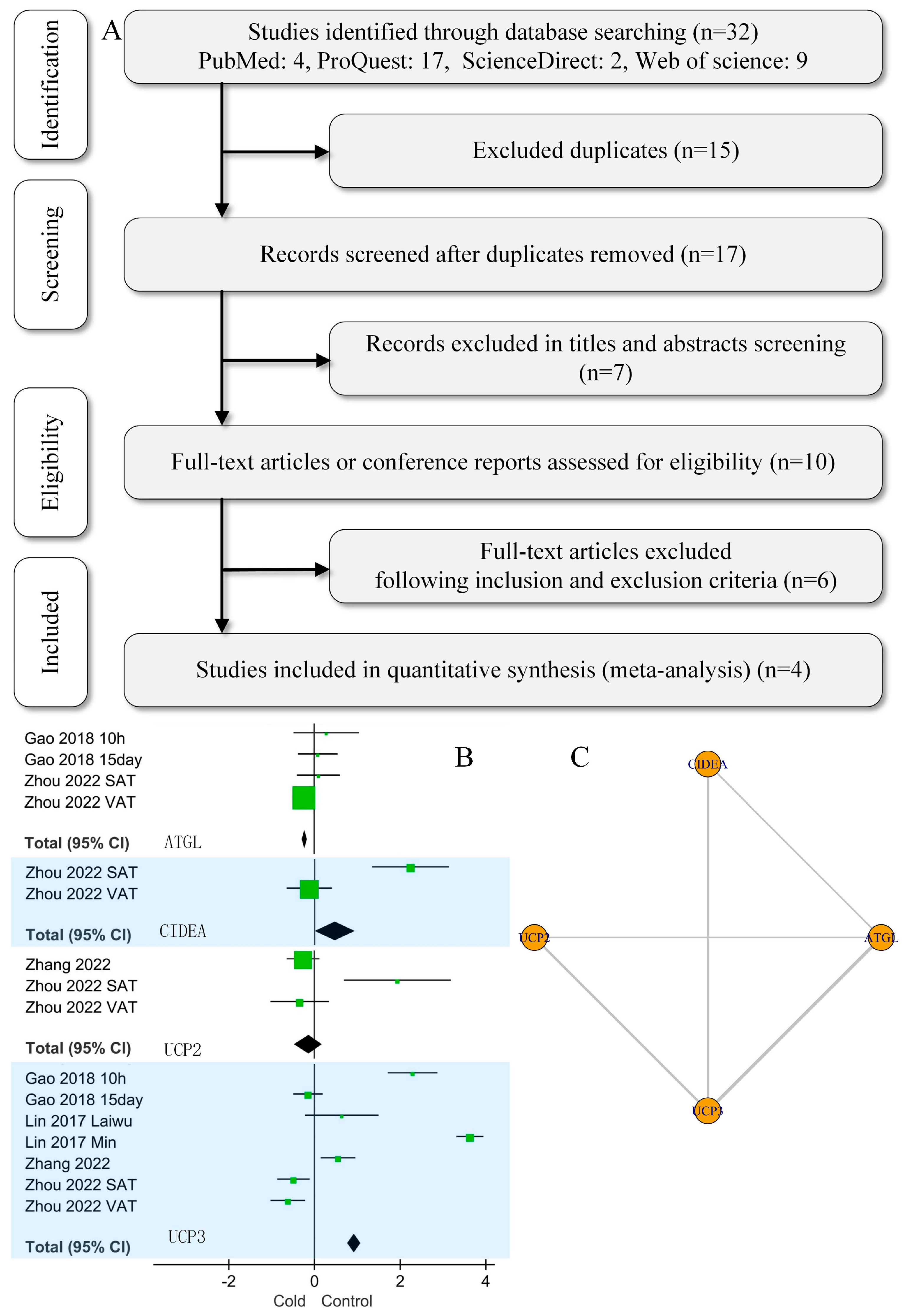
2.1. Database Search Strategy and Study Inclusion
2.2. Data Extraction
2.3. Traditional and Network Meta-Analyses
2.4. Molecular Docking of UCP1, UCP2, and UCP3
3. Results
3.1. Traditional Meta-Analysis
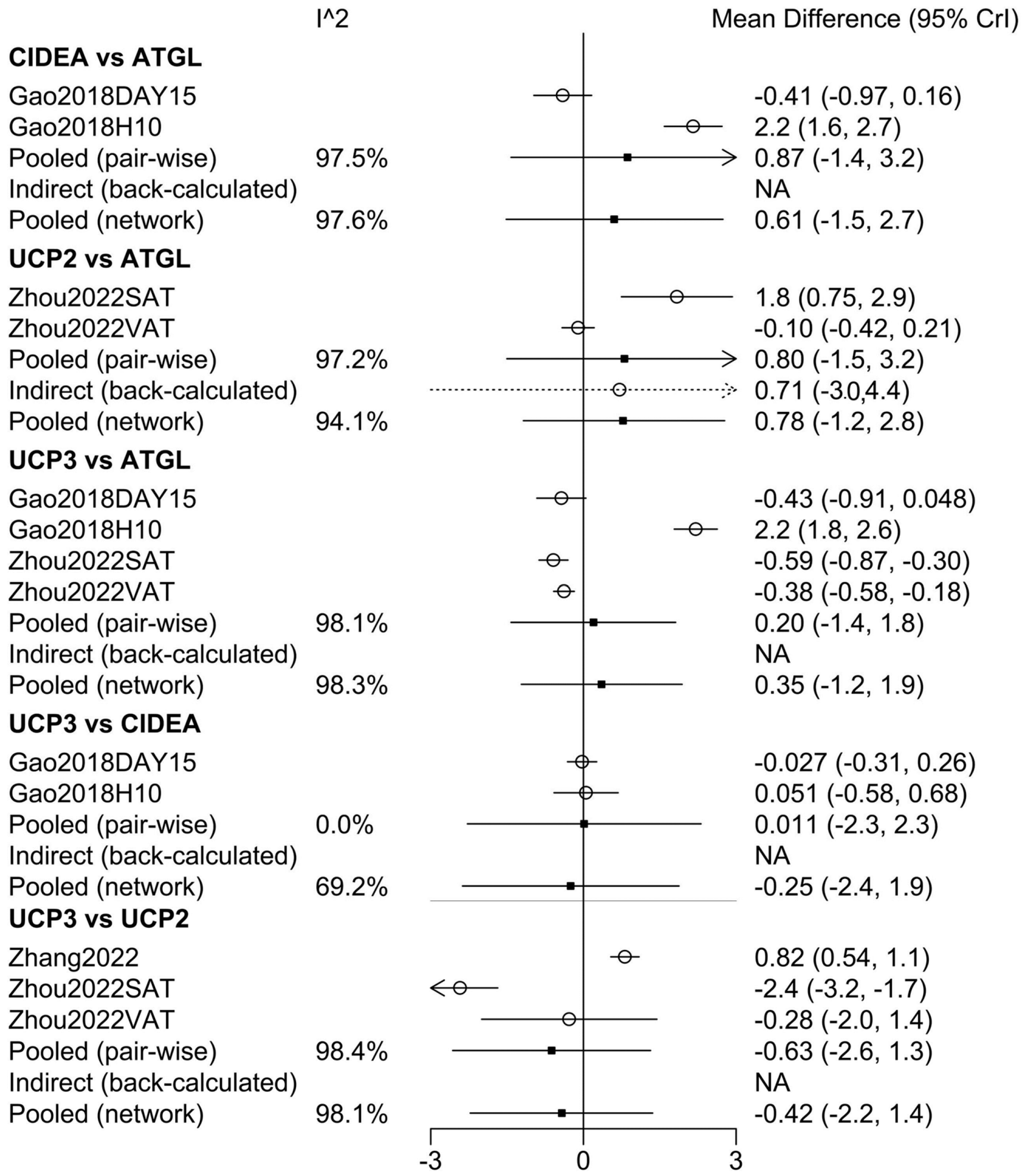
3.2. Network Meta-Analysis
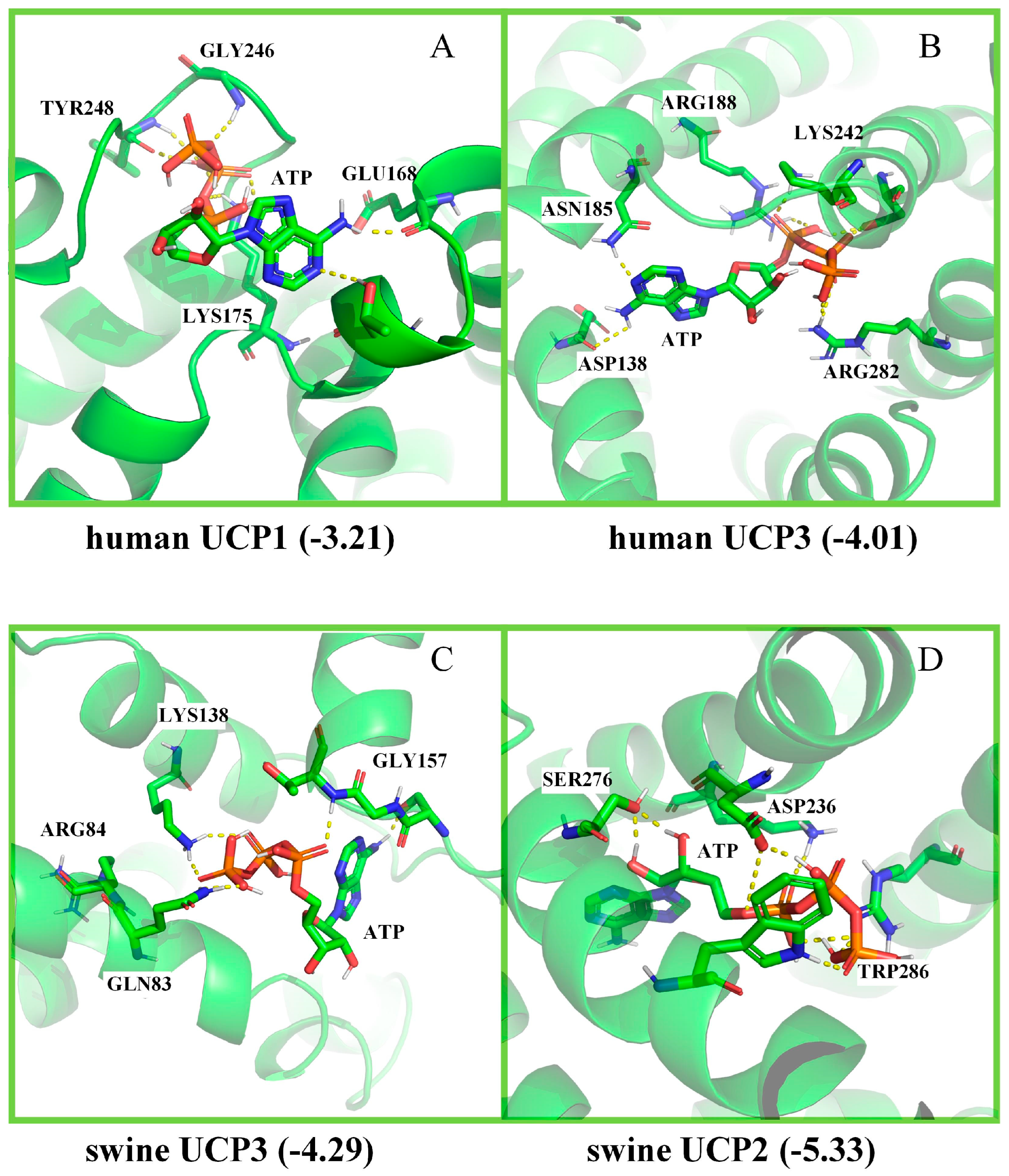
3.3. Molecular Docking of UCP1, UCP2, and UCP3
4. Discussion
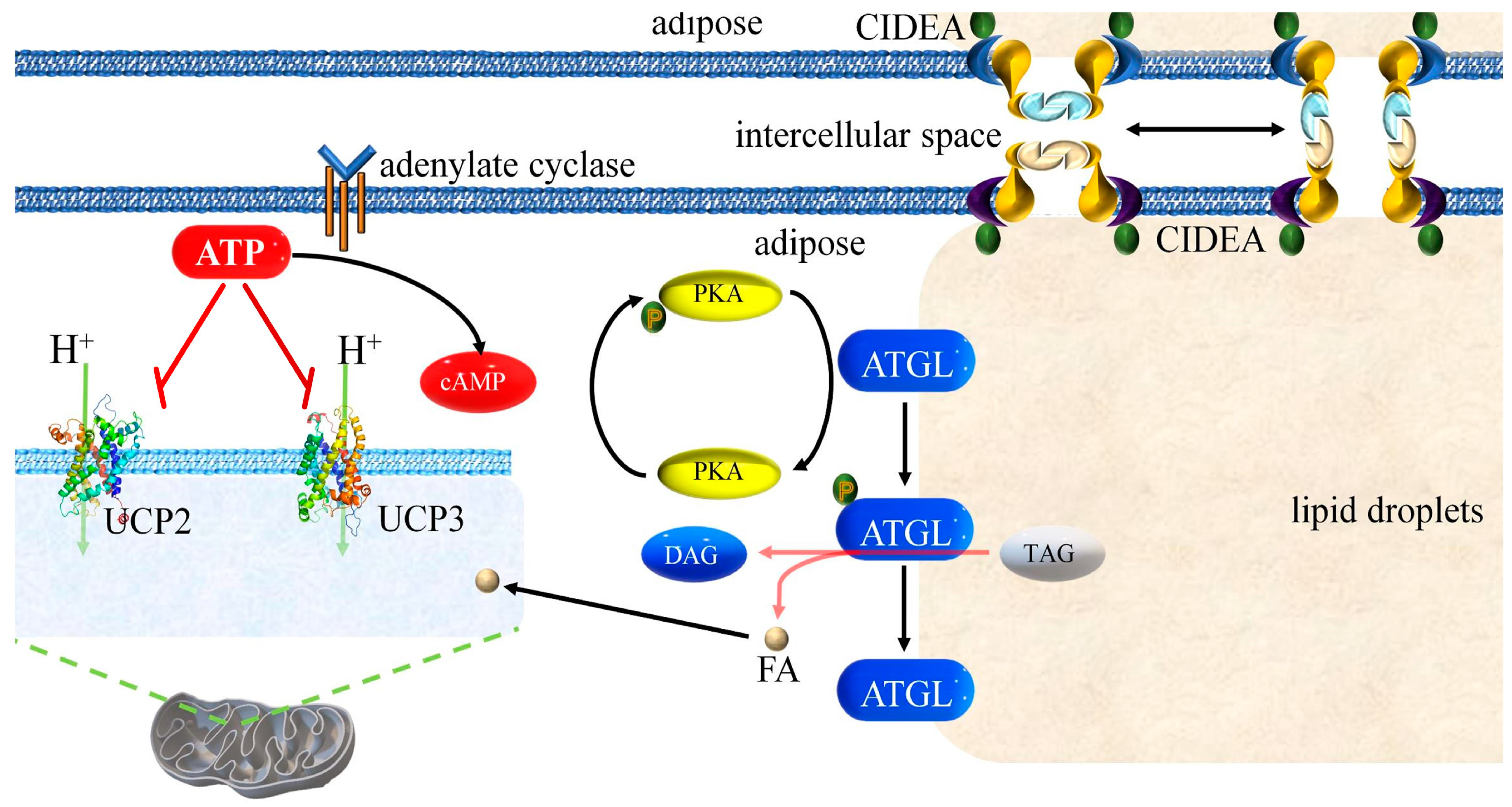
4.1. Effect of Cold Stress on Adipocyte Gene Expression
4.2. Transformation of Pig Adipocytes and Induction Factors
4.3. Mutual Regulation of ATGL, CIDEA, UCP2, and UCP3
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohseni-Takalloo, S.; Mozaffari-Khosravi, H.; Mohseni, H.; Mirzaei, M.; Hosseinzadeh, M. Metabolic syndrome prediction using non-invasive and dietary parameters based on a support vector machine. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, A.J.; Veliova, M.; Acin-Perez, R.; Villalobos, F.; Petcherski, A.; Tombolato, A.; Liesa, M.; Shirihai, O.S. Mitochondria isolated from lipid droplets of white adipose tissue reveal functional differences based on lipid droplet size. Life Sci. Alliance 2024, 7, e202301934. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Kubota, T.; Ishikura, T.; Nakayama, M.; Kobayashi, A.; Yazaki, J.; Uchida, K.; Matsuda, M.; Kondo, T.; Ohara, O.; et al. Characterization of metabolic phenotypes and distinctive genes in mice with low-weight gain. FASEB J. 2024, 38, e23339. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.P.; Layer, J.V.; Heja, D.; Hirose, T.; Lassiter, G.; Firl, D.J.; Paragas, V.B.; Akkad, A.; Chhangawala, S.; Colvin, R.B.; et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature 2023, 622, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Long, Q.; Zhao, J.; Wu, W.; Lin, Z.; Sun, W.; Gu, P.; Deng, T.; Loomes, K.M.; Wu, D.; et al. Cold-Induced Reprogramming of Subcutaneous White Adipose Tissue Assessed by Single-Cell and Single-Nucleus RNA Sequencing. Research 2023, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ikeda, K.; Yoneshiro, T.; Scaramozza, A.; Tajima, K.; Wang, Q.; Kim, K.; Shinoda, K.; Sponton, C.H.; Brown, Z.; et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 2019, 565, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tao, C.; Cao, C.; Zheng, Q.; Lam, S.M.; Shui, G.; Liu, X.; Li, K.; Zhao, J.; Wang, Y. Adipose lipidomics and RNA-Seq analysis revealed the enhanced mitochondrial function in UCP1 knock-in pigs. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1375–1383. [Google Scholar] [CrossRef]
- Huesca-Gomez, C.; Torres-Paz, Y.E.; Fuentevilla-Alvarez, G.; Gonzalez-Moyotl, N.J.; Ramirez-Marroquin, E.S.; Vasquez-Jimenez, X.; Sainz-Escarrega, V.; Soto, M.E.; Samano, R.; Gamboa, R. Expressions of mRNA and encoded proteins of mitochondrial uncoupling protein genes (UCP1, UCP2, and UCP3) in epicardial and mediastinal adipose tissue and associations with coronary artery disease. Arch. Endocrinol. Metab. 2023, 67, 214–223. [Google Scholar] [CrossRef]
- Giedt, M.S.; Thomalla, J.M.; White, R.P.; Johnson, M.R.; Lai, Z.W.; Tootle, T.L.; Welte, M.A. Adipose triglyceride lipase promotes prostaglandin-dependent actin remodeling by regulating substrate release from lipid droplets. Development 2023, 150, dev201516. [Google Scholar] [CrossRef]
- Moro, C.; Lafontan, M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H358–H368. [Google Scholar] [CrossRef] [PubMed]
- Vegliante, R.; Di Leo, L.; Ciccarone, F.; Ciriolo, M.R. Hints on ATGL implications in cancer: Beyond bioenergetic clues. Cell Death Dis. 2018, 9, 316. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, L. Structural basis for the binding of DNP and purine nucleotides onto UCP1. Nature 2023, 620, 226–231. [Google Scholar] [CrossRef]
- Gou, W.; Wei, H.; Swaby, L.; Green, E.; Wang, H. Deletion of Spinophilin Promotes White Adipocyte Browning. Pharmaceuticals 2023, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Barneda, D.; Planas-Iglesias, J.; Gaspar, M.L.; Mohammadyani, D.; Prasannan, S.; Dormann, D.; Han, G.S.; Jesch, S.A.; Carman, G.M.; Kagan, V.; et al. The brown adipocyte protein CIDEA promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. eLife 2015, 4, e07485. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhu, R.; Zhang, S.; Liu, S.; Wang, Y.; Wu, Y.; Xing, S.; Liao, X.; Mi, J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. NPJ Biofilms Microbiomes 2022, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Wang, L.; Ling, D.; Nong, Q.; Xie, J.; Zhu, X.; Shan, T. Cold Exposure Induces Depot-Specific Alterations in Fatty Acid Composition and Transcriptional Profile in Adipose Tissues of Pigs. Front. Endocrinol. 2022, 13, 827523. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qimuge, N.R.; Qin, J.; Cai, R.; Li, X.; Chu, G.Y.; Pang, W.J.; Yang, G.S. Acute and chronic cold exposure differentially affects the browning of porcine white adipose tissue. Animal 2018, 12, 1435–1441. [Google Scholar] [CrossRef]
- Lin, J.; Cao, C.; Tao, C.; Ye, R.; Dong, M.; Zheng, Q.; Wang, C.; Jiang, X.; Qin, G.; Yan, C.; et al. Cold adaptation in pigs depends on UCP3 in beige adipocytes. J. Mol. Cell Biol. 2017, 9, 364–375. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, L.; Liu, D.; Ma, H.; Radovic, C. A meta-analysis: Effect of androgens on reproduction in sows. Front. Endocrinol. 2023, 14, 1094466. [Google Scholar] [CrossRef]
- Kucukmetin, A.; Biliatis, I.; Naik, R.; Bryant, A. Laparoscopically assisted radical vaginal hysterectomy versus radical abdominal hysterectomy for the treatment of early cervical cancer. Cochrane Database Syst. Rev. 2013, 2013, CD006651. [Google Scholar] [CrossRef] [PubMed]
- U-Din, M.; de Mello, V.D.; Tuomainen, M.; Raiko, J.; Niemi, T.; Fromme, T.; Klavus, A.; Gautier, N.; Haimilahti, K.; Lehtonen, M.; et al. Cold-stimulated brown adipose tissue activation is related to changes in serum metabolites relevant to NAD(+) metabolism in humans. Cell Rep. 2023, 42, 113131. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Mottis, A.; Zietak, M.; De Franco, F.; van der Velpen, V.; Gariani, K.; Ryu, D.; Cialabrini, L.; Matilainen, O.; Liscio, P.; et al. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 2018, 563, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Sim, S.M.; Park, J.S.; Hong, J.S.; Suh, H. Modulation of corticosterone and changes of signal molecules in the HPA axis after cold water swimming stress. Anim. Cells Syst. 2021, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Santander, Y.A.; Gerez, E.M.; Hocht, C.; Polizio, A.H.; Mayer, M.A.; Taira, C.A.; Fraga, C.G.; Galleano, M.; Carranza, A. Fructose increases corticosterone production in association with NADPH metabolism alterations in rat epididymal white adipose tissue. J. Nutr. Biochem. 2017, 46, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Kurachi, K.; Takagi, S.; Saneyasu, T.; Kamisoyama, H. Role of Corticosterone in Lipid Metabolism in Broiler Chick White Adipose Tissue. J. Poult. Sci. 2022, 59, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.H.; Hu, Y.T.; Zhou, X.; Huang, Z.S.; Ye, J.M.; Rao, Y. Matrine counteracts obesity in mice via inducing adipose thermogenesis by activating HSF1/PGC-1alpha axis. Pharmacol. Res. 2022, 177, 106136. [Google Scholar] [CrossRef] [PubMed]
- Crowder, M.K.; Shrestha, S.; Cartailler, J.P.; Collins, S. Protein kinase D1 (Prkd1) deletion in brown adipose tissue leads to altered myogenic gene expression after cold exposure, while thermogenesis remains intact. Physiol. Rep. 2023, 11, e15576. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zheng, M.; Zhang, J.; Ye, Y.; Duan, M.; Chamba, Y.; Wang, Z.; Shang, P. Transcriptomics-Based Study of Differentially Expressed Genes Related to Fat Deposition in Tibetan and Yorkshire Pigs. Front. Vet. Sci. 2022, 9, 919904. [Google Scholar] [CrossRef] [PubMed]
- Feyera, T.; Lashkari, S.; Johannsen, J.C.; Llaurado-Calero, E.; Zhe, L.; Theil, P.K.; Jensen, S.K. Supplementation of palmitoleic acid improved piglet growth and reduced body temperature drop upon cold exposure. J. Anim. Sci. 2023, 101, skad372. [Google Scholar] [CrossRef]
- He, Y.; Liang, Z.; Wang, J.; Tang, H.; Li, J.; Cai, J.; Liao, Y. Ceiling culture of human mature white adipocytes with a browning agent: A novel approach to induce transdifferentiation into beige adipocytes. Front. Bioeng. Biotechnol. 2022, 10, 905194. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.; Guerra, B.A.; Boucher, J.; Mori, M.A. A Method to Induce Brown/Beige Adipocyte Differentiation from Murine Preadipocytes. Bio-Protocol 2021, 11, e4265. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, C.; Liu, J.; Zhao, Y.; Pan, J.; Tao, C.; Wang, Y. Distinct Transcriptional Responses of Skeletal Muscle to Short-Term Cold Exposure in Tibetan Pigs and Bama Pigs. Int. J. Mol. Sci. 2023, 24, 7431. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
| Species evaluated included, but were not limited to, pigs | Pigs not used |
| English literature | Non-English |
| Analysis of ATGL, CIDEA, UCP2, and UCP3 alone or with other genes in pigs | No melatonin treatment for pigs |
| Adipose tissue-data included | No adipose-tissue data |
| Study Year | Breed (Number) | Tissue | Temperature, Treatment Time | Genes | Age or Weight | Measurement | |
|---|---|---|---|---|---|---|---|
| 1 | Gao 2018 [18] | NM (9) | Adipocytes | 4 °C, 10 h; 8 °C, 15 days | UCP3, CIDEA, ATGL | 5 days | Relative mRNA expression |
| 2 | Lin 2017 [19] | Wuzhishan (4), Min (4) | Adipocytes | 4 °C, 4 h | UCP3 | 5 weeks | Relative mRNA expression |
| 3 | Zhang 2022 [16] | NM (5) | Fat tissue | 18 °C, 48 h | UCP2, UCP3 | NM | Transcript expression levels |
| 4 | Zhou 2022 [17] | Duroc × Landrace × Yorkshire (6) | SAT, VAT | 5–7 °C, 14 h | UCP2, UCP3, ATGL | 120–125 kg | tpm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lv, L.; Liu, D.; Ma, H.; Wang, L.; Fu, B.; Wang, F. Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3. Curr. Issues Mol. Biol. 2024, 46, 3866-3876. https://doi.org/10.3390/cimb46050240
Guo Z, Lv L, Liu D, Ma H, Wang L, Fu B, Wang F. Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3. Current Issues in Molecular Biology. 2024; 46(5):3866-3876. https://doi.org/10.3390/cimb46050240
Chicago/Turabian StyleGuo, Zhenhua, Lei Lv, Di Liu, Hong Ma, Liang Wang, Bo Fu, and Fang Wang. 2024. "Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3" Current Issues in Molecular Biology 46, no. 5: 3866-3876. https://doi.org/10.3390/cimb46050240
APA StyleGuo, Z., Lv, L., Liu, D., Ma, H., Wang, L., Fu, B., & Wang, F. (2024). Network Meta-Analysis: Effect of Cold Stress on the Gene Expression of Swine Adipocytes ATGL, CIDEA, UCP2, and UCP3. Current Issues in Molecular Biology, 46(5), 3866-3876. https://doi.org/10.3390/cimb46050240





