Norcantharidin Enhances the Antitumor Effect of 5-Fluorouracil by Inducing Apoptosis of Cervical Cancer Cells: Network Pharmacology, Molecular Docking, and Experimental Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Lines and Cell Culture
2.3. Cell Viability Assay
2.4. Combination Index (CI)
2.5. Colony Formation Assay
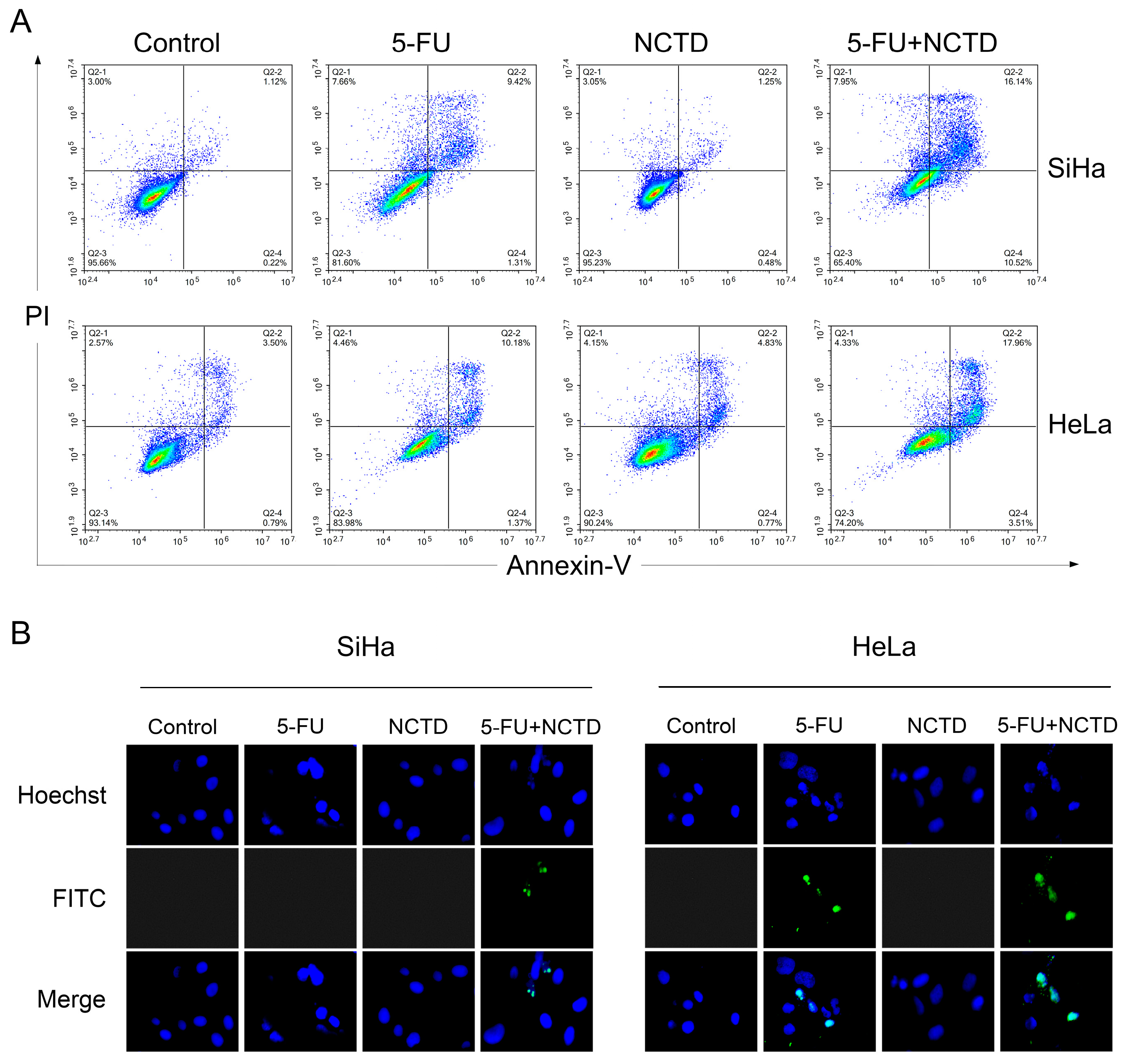
2.6. Annexin V-FITC/PI Staining Assay
2.7. Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) Assay
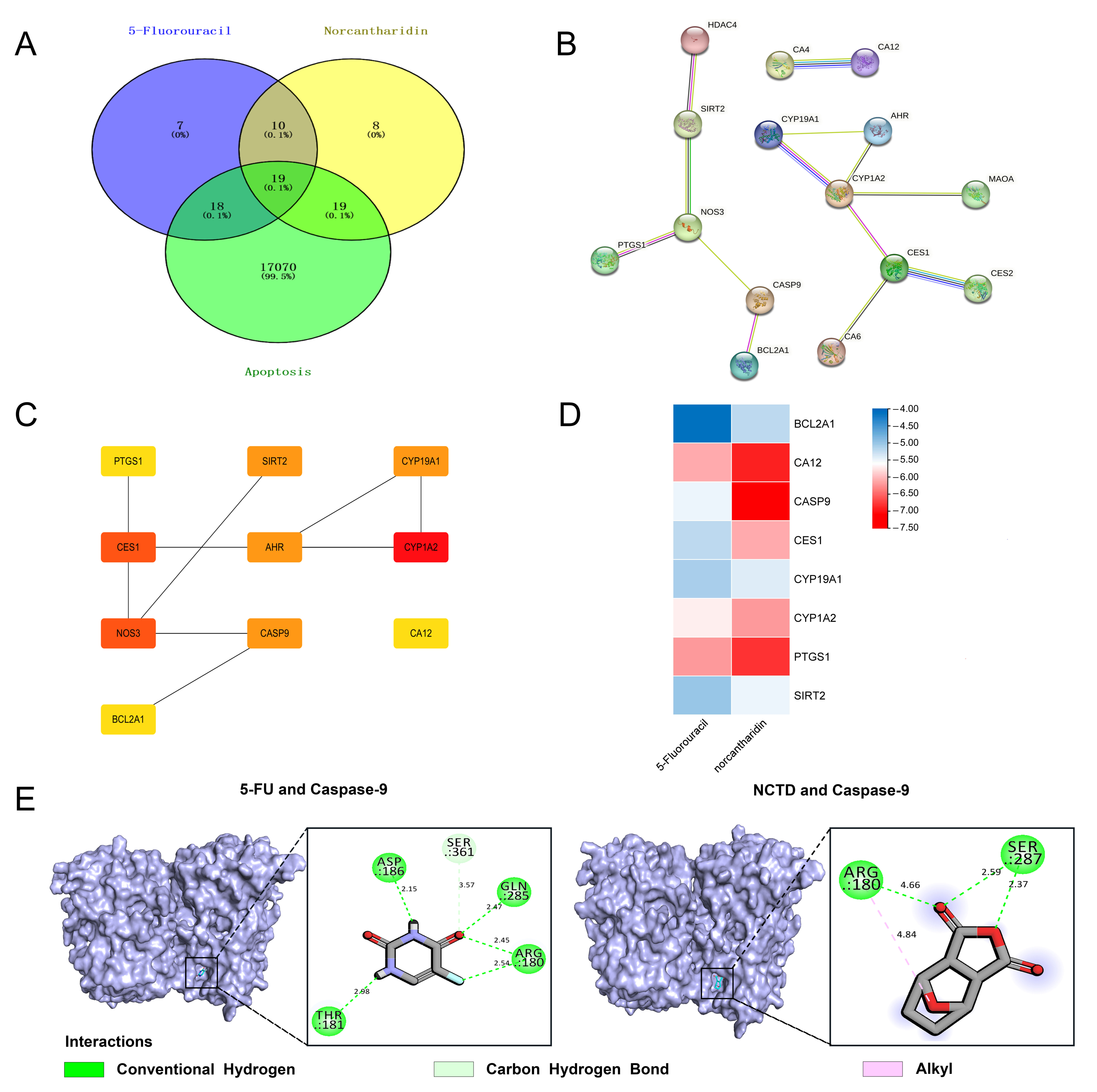
2.8. Network Pharmacology Analysis
2.9. Molecular Docking
2.10. Molecular Dynamics Simulations
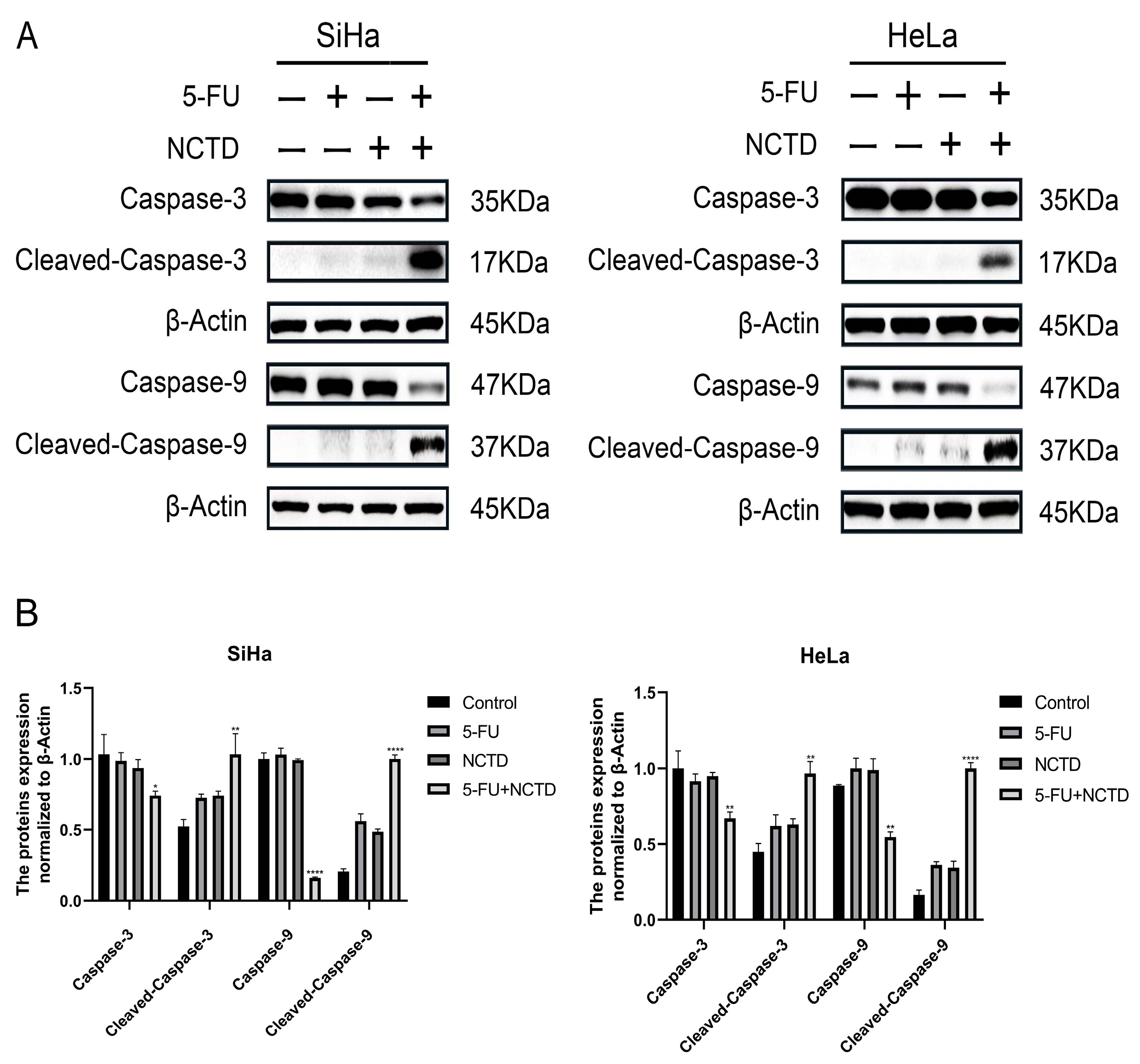
2.11. Immunoblotting Analysis
2.12. Statistical Analysis
3. Results
3.1. The Single-Drug Treatment of NCTD or 5-FU Increases the Cytotoxic of Cervical Cancer Cell Lines
3.2. The Combination of NCTD and 5-FU Is Synergistic in Cervical Cancer Cell Lines
3.3. NCTD Increased 5-FU-Mediated Cytotoxicity through the Induction of Apoptosis
3.4. Network Pharmacological Analysis Showed That the Combination of NCTD and 5-FU May Induce Apoptosis of Cervical Cancer Cells by Regulating the Caspase-Dependent Pathway
3.5. NCTD Enhanced 5-FU-Induced Apoptosis by the Caspase-Dependent Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferenczy, A.; Franco, E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002, 3, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Shai, A.; Pitot, H.; Lambert, P. E6-associated protein is required for human papillomavirus type 16 E6 to cause cervical cancer in mice. Cancer Res. 2010, 70, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xie, X.; Liu, J.; Qiao, Y.; Zhao, F.; Wu, T.; Zhang, J.; Ma, D.; Kong, B.; Chen, W.; et al. Elimination of Cervical Cancer: Challenges Promoting the HPV Vaccine in China. Indian J. Gynecol. Oncol. 2021, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.; Laimins, L. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luke, C.J.; Pak, S.C.; Shi, V.; Chen, L.; Moore, J.; Andress, A.P.; Jayachandran, K.; Zhang, J.; Huang, Y.; et al. SERPINB3 (SCCA1) inhibits cathepsin L and lysoptosis, protecting cervical cancer cells from chemoradiation. Commun. Biol. 2022, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Cassidy, J.; Saltz, L.; Twelves, C.; Van Cutsem, E.; Hoff, P.; Kang, Y.; Saini, J.P.; Gilberg, F.; Cunningham, D. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: A meta-analysis of individual data from 6171 patients. Ann. Oncol. 2011, 22, 2604–2609. [Google Scholar] [CrossRef]
- Lyu, N.; Wang, X.; Li, J.; Lai, J.; Chen, Q.; Li, S.; Deng, H.; He, M.; Mu, L.; Zhao, M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 468–480. [Google Scholar] [CrossRef]
- Christie, D.; Bull, C.; Gebski, V.; Langlands, A. Concurrent 5-fluorouracil, mitomycin C and irradiation in locally advanced cervix cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1995, 37, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Wang, G.-S. Medical uses of mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.-T.; Sun, J.; Shi, Y.-J.; Zhang, X.-F.; Zou, J.-B.; Cheng, J.-X.; Fan, Y.; Guo, D.-Y.; Tian, H. Review targeted drug delivery systems for norcantharidin in cancer therapy. J. Nanobiotechnol. 2022, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: Research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef]
- Guo, G.T.; Jian, F.Z.; Qiao, L.L.; Jia, Q.W.; Bin, H.K.; Shao, H.L. Synthesis and antiproliferative assay of norcantharidin derivatives in cancer cells. Med. Chem. 2013, 10, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Cao, J.; Fan, Y.-Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhu, Y.Q.; Mei, J.J.; Liu, S.Q.; Luo, J. Involvement of mitochondrial pathway in NCTD-induced cytotoxicity in human hepG2 cells. J. Exp. Clin. Cancer Res. 2010, 29, 145. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.; Ahmad, M.; Alnemri, E.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Oltvai, Z.; Milliman, C.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhao, C.; Zhang, C.; Xiao, Y.; Yan, G.; Liu, L.; Pan, H. Elucidation of the anti-inflammatory mechanism of Er Miao San by integrative approach of network pharmacology and experimental verification. Pharmacol. Res. 2022, 175, 106000. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wei, H.; Li, K.; Zhu, S.; Li, J.; Chen, C.; Zhang, D.; Li, Y.; Zhu, L.; Yi, X.; et al. The traditional chinese medicine monomer Ailanthone improves the therapeutic efficacy of anti-PD-L1 in melanoma cells by targeting c-Jun. J. Exp. Clin. Cancer Res. 2022, 41, 346. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Liu, L.; Che, C.; Li, W. Exploring the Antiovarian Cancer Mechanisms of Salvia Miltiorrhiza Bunge by Network Pharmacological Analysis and Molecular Docking. Comput. Math. Methods Med. 2022, 2022, 7895246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Zheng, J.; Shao, C.; Zhang, D. Identification of predictors based on drug targets highlights accurate treatment of goserelin in breast and prostate cancer. Cell Biosci. 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. TIG 1997, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sussman, J.L.; Lin, D.; Jiang, J.; Manning, N.O.; Prilusky, J.; Ritter, O.; Abola, E.E. Biological crystallography. Protein Data Bank (PDB): Database of three-dimensional structural information of biological macromolecules. Acta Crystallogr. Sect. D Struct. Biol. 1998, 54, 1078–1084. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.; Frank, M.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.W.S.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Cohen, G. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef]
- Amărandi, R.-M.; Al-Matarneh, M.-C.; Popovici, L.; Ciobanu, C.I.; Neamțu, A.; Mangalagiu, I.I.; Danac, R. Exploring Pyrrolo-Fused Heterocycles as Promising Anticancer Agents: An Integrated Synthetic, Biological, and Computational Approach. Pharmaceuticals 2023, 16, 865. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, X.; Zhang, L.; Gao, X.; Gui, Y. Norcantharidin in cancer therapy—A new approach to overcoming therapeutic resistance: A review. Medicine 2024, 103, e37394. [Google Scholar] [CrossRef]
- Kim, B.; Srivastava, S.K.; Kim, S.-H. Caspase-9 as a therapeutic target for treating cancer. Expert Opin. Ther. Targets 2014, 19, 113–127. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, F.; Du, Q.; Zhang, H.; Luo, X.; Song, X.; Zhao, X.; Zhang, W.; Tong, D. Swainsonine induces apoptosis through mitochondrial pathway and caspase activation in goat trophoblasts. Int. J. Biol. Sci. 2014, 10, 789–797. [Google Scholar] [CrossRef]
- Yigong, S. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wan, X.-W.; Du, Y.-T.; Feng, Y.; Yang, L.-S.; Liu, Y.-B.; Chen, T.; Zhu, Z.; Xu, Y.-T.; Wang, C.-C. Norcantharidin Enhances the Antitumor Effect of 5-Fluorouracil by Inducing Apoptosis of Cervical Cancer Cells: Network Pharmacology, Molecular Docking, and Experimental Validation. Curr. Issues Mol. Biol. 2024, 46, 3906-3918. https://doi.org/10.3390/cimb46050242
Huang Y, Wan X-W, Du Y-T, Feng Y, Yang L-S, Liu Y-B, Chen T, Zhu Z, Xu Y-T, Wang C-C. Norcantharidin Enhances the Antitumor Effect of 5-Fluorouracil by Inducing Apoptosis of Cervical Cancer Cells: Network Pharmacology, Molecular Docking, and Experimental Validation. Current Issues in Molecular Biology. 2024; 46(5):3906-3918. https://doi.org/10.3390/cimb46050242
Chicago/Turabian StyleHuang, Yong, Xin-Wei Wan, Yu-Tong Du, Yue Feng, Lin-Sen Yang, Yong-Bin Liu, Tian Chen, Zhuan Zhu, Yi-Ting Xu, and Cheng-Cheng Wang. 2024. "Norcantharidin Enhances the Antitumor Effect of 5-Fluorouracil by Inducing Apoptosis of Cervical Cancer Cells: Network Pharmacology, Molecular Docking, and Experimental Validation" Current Issues in Molecular Biology 46, no. 5: 3906-3918. https://doi.org/10.3390/cimb46050242
APA StyleHuang, Y., Wan, X. -W., Du, Y. -T., Feng, Y., Yang, L. -S., Liu, Y. -B., Chen, T., Zhu, Z., Xu, Y. -T., & Wang, C. -C. (2024). Norcantharidin Enhances the Antitumor Effect of 5-Fluorouracil by Inducing Apoptosis of Cervical Cancer Cells: Network Pharmacology, Molecular Docking, and Experimental Validation. Current Issues in Molecular Biology, 46(5), 3906-3918. https://doi.org/10.3390/cimb46050242





