Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming
Abstract
:1. Introduction
2. Maternal Nutrition and Effects on Fetus and Newborns
3. Maternal Dietary Factors Influencing Metabolic and Endocrine Changes
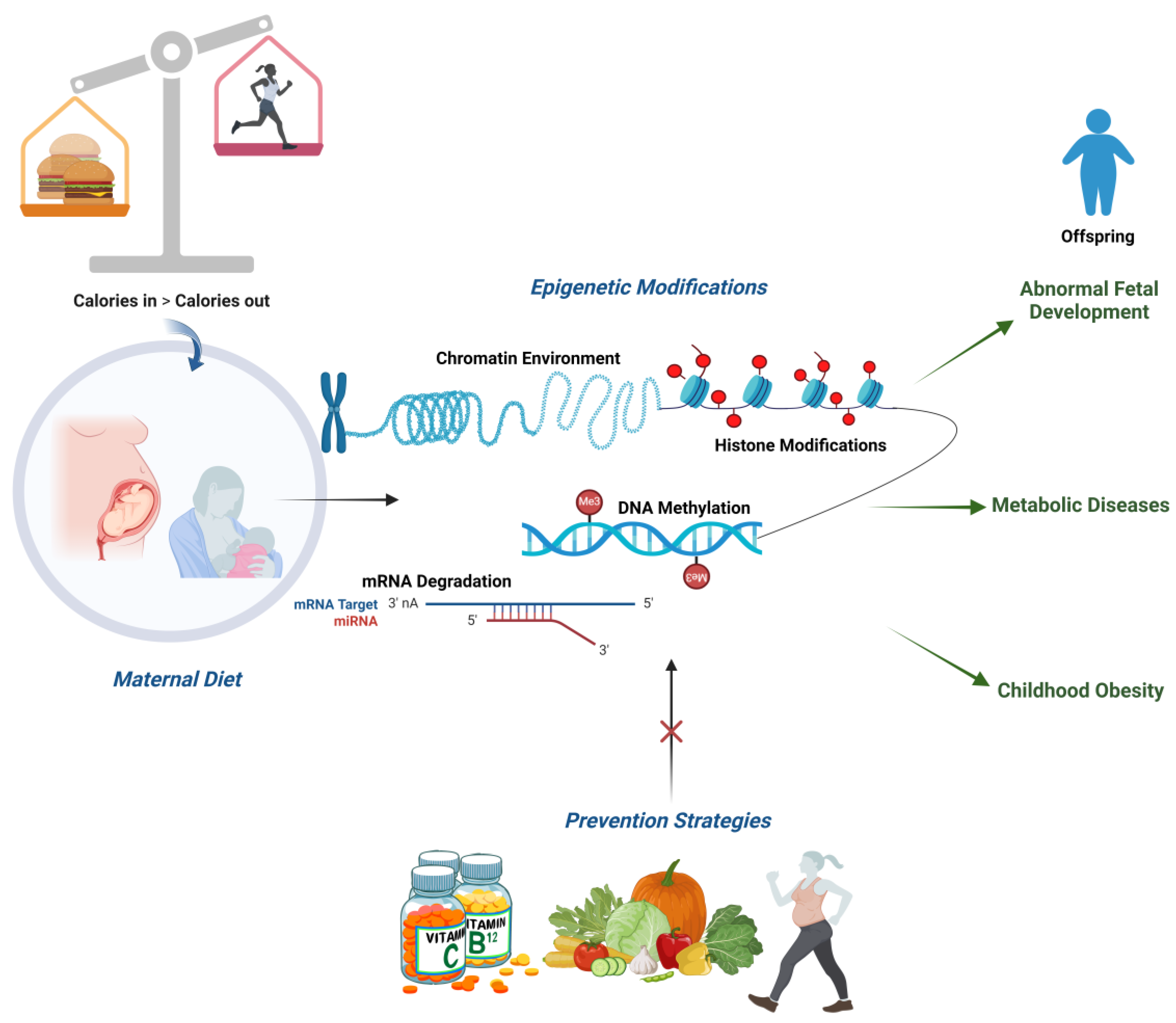
4. Maternal Dietary Factors Influencing Epigenetic Changes
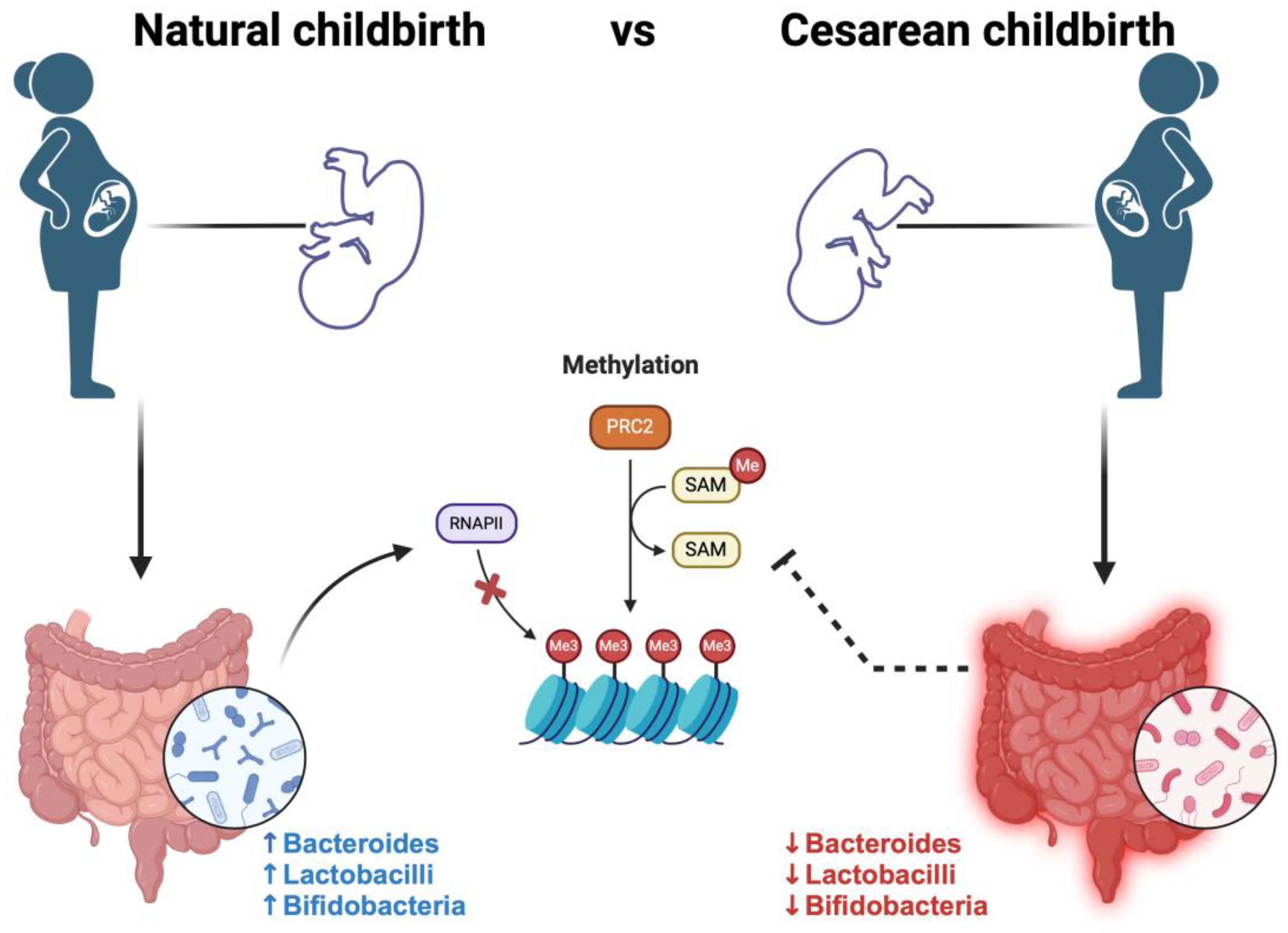
5. Maternal Diet–Epigenetics–Microbiome
6. Prevention Strategies and Epigenetics
7. Conclusions and Prevention Strategies for Future Health
Author Contributions
Funding
Conflicts of Interest
References
- Barker, D.J.P. Developmental origins of adult health and disease. J. Epidemiol. Community Health 2004, 58, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Martinez, D.H.; Bosquez-Mendoza, V.M.; Ruiz-Noa, Y.; Ibarra-Reynoso, L.D.R.; Barbosa-Sabanero, G.; Lazo-de-la-Vega-Monroy, M.L. Nutritional, pharmacological, and environmental programming of NAFLD in early life. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G99–G114. [Google Scholar] [CrossRef]
- He, S.; Stein, A.D. Early-Life Nutrition Interventions and Associated Long-Term Cardiometabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 461–489. [Google Scholar] [CrossRef] [PubMed]
- Kintossou, A.K.; Blanco-Lopez, J.; Iguacel, I.; Pisanu, S.; Almeida, C.C.B.; Steliarova-Foucher, E.; Sierens, C.; Gunter, M.J.; Ladas, E.J.; Barr, R.D.; et al. Early Life Nutrition Factors and Risk of Acute Leukemia in Children: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3775. [Google Scholar] [CrossRef]
- Ley, D.; Desseyn, J.L.; Gouyer, V.; Plet, S.; Tims, S.; Renes, I.; Mischke, M.; Gottrand, F. Early life nutrition influences susceptibility to chronic inflammatory colitis in later life. Sci. Rep. 2019, 9, 18111. [Google Scholar] [CrossRef]
- Cristian, A.; Tarry-Adkins, J.L.; Aiken, C.E. The Uterine Environment and Childhood Obesity Risk: Mechanisms and Predictions. Curr. Nutr. Rep. 2023, 12, 416–425. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Mei, H.; Zhang, Y.; Xu, K.; Yang, S.; Zhang, J. Early childhood body mass index trajectory and overweight/obesity risk differed by maternal weight status. Eur. J. Clin. Nutr. 2022, 76, 450–455. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Liang, J.F.; Rogers, C.J.; Zhao, J.X.; Zhu, M.J.; Du, M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 2013, 62, 3727–3735. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Zhang, L.; Maricelli, J.W.; Rodgers, B.D.; Zhu, M.J.; Du, M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016, 6, 34345. [Google Scholar] [CrossRef]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, H.; Wang, L.; Song, Q.; Sun, D.; Li, W.; Leng, J.; Gao, R.; Hu, G.; Qi, L. Maternal Gestational Diabetes Mellitus Modifies the Relationship Between Genetically Determined Body Mass Index During Pregnancy and Childhood Obesity. Mayo Clin. Proc. 2020, 95, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Hüls, A.; Wright, M.N.; Bogl, L.H.; Kaprio, J.; Lissner, L.; Molnár, D.; Moreno, L.A.; De Henauw, S.; Siani, A.; Veidebaum, T.; et al. Polygenic risk for obesity and its interaction with lifestyle and sociodemographic factors in European children and adolescents. Int. J. Obes. 2021, 45, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Marousez, L.; Pourpe, C.; Oger, F.; Lecoutre, S.; Catheline, D.; Görs, S.; Metges, C.C.; Guinez, C.; Laborie, C.; et al. Maternal high-fat diet during suckling programs visceral adiposity and epigenetic regulation of adipose tissue stearoyl-CoA desaturase-1 in offspring. Int. J. Obes. 2019, 43, 2381–2393. [Google Scholar] [CrossRef]
- Ronnenberg, A.G.; Wang, X.; Xing, H.; Chen, C.; Chen, D.; Guang, W.; Guang, A.; Wang, L.; Ryan, L.; Xu, X. Low preconception body mass index is associated with birth outcome in a prospective cohort of Chinese women. J. Nutr. 2003, 133, 3449–3455. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Delvecchio, M.; Zito, A.; De Palma, F.; Cortese, F.; Nitti, A.; Massari, E.; Gesualdo, M.; Ricci, G.; et al. Vascular Function and Myocardial Performance Indices in Children Born Small for Gestational Age. Circ. J. 2016, 80, 958–963. [Google Scholar] [CrossRef]
- Marzano, F.; Faienza, M.F.; Caratozzolo, M.F.; Brunetti, G.; Chiara, M.; Horner, D.S.; Annese, A.; D’Erchia, A.M.; Consiglio, A.; Pesole, G.; et al. Pilot study on circulating miRNA signature in children with obesity born small for gestational age and appropriate for gestational age. Pediatr. Obes. 2018, 13, 803–811. [Google Scholar] [CrossRef]
- Zamojska, J.; Niewiadomska-Jarosik, K.; Kierzkowska, B.; Gruca, M.; Wosiak, A.; Smolewska, E. Lipid Profile in Children Born Small for Gestational Age. Nutrients 2023, 15, 4781. [Google Scholar] [CrossRef]
- Harmancıoğlu, B.; Kabaran, S. Maternal high fat diets: Impacts on offspring obesity and epigenetic hypothalamic programming. Front. Genet. 2023, 11, 14–1158089. [Google Scholar] [CrossRef]
- Lowe, W.L.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, B.; Liang, X.; Mei, H.; Lu, H.; Xie, S.; Bei, W.; Mei, W.; Zhang, J. Effect of maternal glycemia and weight status on offspring birth measures and BMI-z among Chinese population in the first year. Sci. Rep. 2017, 7, 16030. [Google Scholar] [CrossRef]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatr. Obes. 2017, 12, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.P.; Ayres, L.F.A.; Barreto, D.S.; Henriques, B.D.; Prado, M.R.M.C.; Passos, C.M.D. Acquisition of microbiota according to the type of birth: An integrative review. Rev. Lat. Am. Enfermagem. 2021, 29, e3446. [Google Scholar] [CrossRef] [PubMed]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 16, 148. [Google Scholar] [CrossRef]
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The Epigenetic Connection Between the Gut Microbiome in Obesity and Diabetes. Front. Genet. 2020, 15, 1329. [Google Scholar] [CrossRef] [PubMed]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2019, 14, 47–50. [Google Scholar] [CrossRef]
- Brei, C.; Stecher, L.; Meyer, D.M.; Young, V.; Much, D.; Brunner, S.; Hauner, H. Impact of Dietary Macronutrient Intake During Early and Late Gestation on Offspring Body Composition at Birth, 1, 3, and 5 Years of Age. Nutrients 2018, 10, 579. [Google Scholar] [CrossRef]
- Montalvo-Martínez, L.; Maldonado-Ruiz, R.; Cárdenas-Tueme, M.; Reséndez-Pérez, D.; Camacho, A. Maternal Overnutrition Programs Central Inflammation and Addiction-Like Behavior in Offspring. BioMed Res. Int. 2018, 2018, 8061389. [Google Scholar] [CrossRef]
- Díaz-López, A.; Rodríguez Espelt, L.; Abajo, S.; Arija, V. Close Adherence to a Mediterranean Diet during Pregnancy Decreases Childhood Overweight/Obesity: A Prospective Study. Nutrients 2024, 16, 532. [Google Scholar] [CrossRef] [PubMed]
- Claesson, I.M.; Josefsson, A.; Olhager, E.; Oldin, C.; Sydsjö, G. Effects of a gestational weight gain restriction program for obese women: Sibling pairs’ weight development during the first five years of life. Sex. Reprod. Health 2018, 17, 65–74. [Google Scholar] [CrossRef] [PubMed]
- O’brien, C.M.; Louise, J.; Deussen, A.; Dodd, J.M. In Overweight or Obese Pregnant Women, Maternal Dietary Factors are not Associated with Fetal Growth and Adiposity. Nutrients 2018, 10, 870. [Google Scholar] [CrossRef]
- Foster, B.A.; Escaname, E.; Powell, T.L.; Larsen, B.; Siddiqui, S.K.; Menchaca, J.; Aquino, C.; Ramamurthy, R.; Hale, D.E. Randomized Controlled Trial of DHA Supplementation during Pregnancy: Child Adiposity Outcomes. Nutrients 2017, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, M.L.; Mensegue, M.F.; Pirola, C.J. The Association between High Fat Diet around Gestation and Metabolic Syndrome-related Phenotypes in Rats: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 5086. [Google Scholar] [CrossRef]
- Heerwagen, M.J.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental Programming of Fetal Growth and Development. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar] [CrossRef]
- Tie, H.T.; Xia, Y.Y.; Zeng, Y.S.; Zhang, Y.; Dai, C.L.; Guo, J.J.; Zhao, Y. Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: A meta-analysis. Arch. Gynecol. Obstet. 2014, 289, 247–257. [Google Scholar] [CrossRef]
- Diesel, J.C.; Eckhardt, C.L.; Day, N.L.; Brooks, M.M.; Arslanian, S.A.; Bodnar, L.M. Gestational Weight Gain and Offspring Longitudinal Growth in Early Life. Ann. Nutr. Metab. 2015, 67, 49–57. [Google Scholar] [CrossRef]
- Dietz, W.H. Periods of risk in childhood for the development of adult obesity–what do we need to learn? J. Nutr. 1997, 127, 1884S–1886S. [Google Scholar] [CrossRef] [PubMed]
- Rajamoorthi, A.; LeDuc, C.A.; Thaker, V.V. The metabolic conditioning of obesity: A review of the pathogenesis of obesity and the epigenetic pathways that “program” obesity from conception. Front. Endocrinol. 2022, 13, 1032491. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 2014, 64 (Suppl. S1), 26–34. [Google Scholar] [CrossRef]
- Armistead, B.; Johnson, E.; VanderKamp, R.; Kula-Eversole, E.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. Placental Regulation of Energy Homeostasis during Human Pregnancy. Endocrinol 2020, 161, bqaa076. [Google Scholar] [CrossRef]
- Parrettini, S.; Cavallo, M.; Gaggia, F.; Calafiore, R.; Luca, G. Adipokines: A Rainbow of Proteins with Metabolic and Endocrine Functions. Protein Pept. Lett. 2020, 27, 1204–1230. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M.; Stumvoll, M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014, 2, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ekstrand, Y.; Björn, C.; Wennergren, M.; Powell, T.L. Alterations in the activity of placental amino acid trans- porters in pregnancies complicated by diabetes. Diabetes 2002, 51, 2214–2219. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Kalhan, S.; Rossi, K.; Gruca, L.; Burkett, E.; O’Brien, A. Glucose turnover and gluconeogenesis in human pregnancy. J. Clin. Invest. 1997, 100, 1775–1781. [Google Scholar] [CrossRef]
- Lewis, R.M.; Wadsack, C.; Desoye, G. Placental fatty acid transfer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Muhlhausler, B.S. The effect of n-3 LCPUFA supplementation on oxidative stress and inflammation in the placenta and maternal plasma during pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef]
- Desoye, G. The human placenta in diabetes and obesity: Friend or foe? The 2017 Norbert Freinkel award lecture. Diabetes Care 2018, 41, 1362–1369. [Google Scholar] [CrossRef]
- Tatone, E.H.; Duffield, T.F.; Capel, M.B.; DeVries, T.J.; LeBlanc, S.J.; Gordon, J.L. A randomized controlled trial of dexamethasone as an adjunctive therapy to propylene glycol for treatment of hyperketonemia in postpartum dairy cattle. J. Dairy Sci. 2016, 99, 8991–9000. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced gly- cation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflam- matory response. J. Mol. Med. 2009, 87, 235–247. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. New Insights into the Role of Insulin and Hypothalamic-Pituitary-Adrenal (HPA) Axis in the Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8178. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Oken, E. Maternal obesity and associated offspring diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Khant Aung, Z.; Grattan, D.R.; Ladyman, S.R. Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol. Cell. Endocrinol. 2020, 516, 110933. [Google Scholar] [CrossRef]
- Nuamah, M.A.; Yura, S.; Sagawa, N.; Itoh, H.; Mise, H.; Korita, D.; Kakui, K.; Takemura, M.; Ogawa, Y.; Nakao, K.; et al. Significant increase in maternal plasma leptin concentration in induced delivery: A possible contribution of pro-inflammatory cytokines to placental leptin secretion. Endocr. J. 2004, 51, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Hoch, D.; Gauster, M.; Hauguel-de Mouzon, S.; Desoye, G. Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol. Asp. Med. 2019, 66, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cabalin, C.; Villalobos-Labra, R.; Toledo, F.; Sobrevia, L. Involvement of A2B adenosine receptors as anti-inflammatory in gestational diabesity. Mol. Asp. Med. 2019, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Sanchez, R.; Calderón, J.C.; Vega, G.; Trillos, M.C.; Ospina, S. Skeletal muscle as a protagonist in the pregnancy metabolic syndrome. Med. Hypotheses. 2019, 126, 26–37. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Pardo, F.; Subiabre, M.; Fuentes, G.; Toledo, F.; Silva, L.; Villalobos-Labra, R.; Sobrevia, L. Altered foetoplacental vascular endothelial signalling to insulin in diabesity. Mol. Aspects Med. 2019, 66, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lesage, J.; Hahn, D.; Léonhardt, M.; Blondeau, B.; Bréant, B.; Dupouy, J.P. Maternal undernutrition during late gestation induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. J. Endocrinol. 2002, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Tavares, L.; Duarte, A.; Baldeiras, I.; Cunha-Oliveira, T.; Martins, J.D.; Santos, M.S.; Maloyan, A.; Moreno, A.J.; Cox, L.A.; et al. Sex dependent vulnerability of fetal nonhuman primate cardiac mitochondria to moderate maternal nutrient reduction. Clin. Sci. 2021, 135, 1103–1126. [Google Scholar] [CrossRef]
- Petrik, J.; Reusens, B.; Arany, E.; Remacle, C.; Coelho, C.; Hoet, J.J.; Hill, D.J. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 1999, 140, 4861–4873. [Google Scholar] [CrossRef]
- Pereira, S.P.; Tavares, L.C.; Duarte, A.I.; Baldeiras, I.; Cunha-Oliveira, T.; Martins, J.D.; Santos, M.S.; Maloyan, A.; Moreno, A.J.; Cox, L.A.; et al. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 2007, 50, 2495–2503. [Google Scholar]
- Busada, J.T.; Cidlowski, J.A. Mechanisms of Glucocorticoid Action During Development. Curr. Top. Dev. Biol. 2017, 125, 147–170. [Google Scholar] [PubMed]
- Zhu, P.; Wang, W.; Zuo, R.; Sun, K. Mechanisms for establishment of the placental glucocorticoid barrier, a guard for life. Cell. Mol. Life Sci. 2019, 76, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Tomlinson, J.W. Glucocorticoids in pregnancy. Obstet. Med. 2020, 13, 62–69. [Google Scholar] [CrossRef]
- Jung, C.; Ho, J.T.; Torpy, D.J.; Rogers, A.; Doogue, M.; Lewis, J.G.; Czajko, R.J.; Inder, W.J. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J. Clin. Endocrinol. Metab. 2011, 96, 1533–1540. [Google Scholar] [CrossRef]
- Dy, J.; Guan, H.; Sampath-Kumar, R.; Richardson, B.S.; Yang, K. Placental 11β-Hydroxysteroid Dehydrogenase Type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: Evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta 2008, 29, 193–200. [Google Scholar] [CrossRef]
- Jimeno, B.; Hau, M.; Gómez-Díaz, E.; Verhulst, S. Developmental conditions modulate DNA methylation at the glucocorticoid receptor gene with cascading effects on expression and corticosterone levels in zebra finches. Sci. Rep. 2019, 1, 15869. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Furigo, I.C.; Teixeira, P.D.S.; Ramos-Lobo, A.M.; Peroni, C.N.; Bartolini, P.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Growth Hormone Receptor Deletion Reduces the Density of Axonal Projections from Hypothalamic Arcuate Nucleus Neurons. Neuroscience 2020, 10, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Loh, J.; Ang, R.S.E.; Chong, M.F. Tracking of Maternal Diet from Pregnancy to Postpregnancy: A Systematic Review of Observational Studies. Curr. Dev. Nutr. 2020, 4, nzaa118. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Bustamante-Sánchez, Á.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Plata-SanJuan, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Infancy Dietary Patterns, Development, and Health: An Extensive Narrative Review. Children 2022, 9, 1072. [Google Scholar] [CrossRef]
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human Milk Oligosaccharides and Bacterial Profile Modulate Infant Body Composition during Exclusive Breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Ebers, G.C.; Ramagopalan, S.V. Epigenetics: Molecular mechanisms and implications for disease. Trends Mol. Med. 2010, 16, 7–16. [Google Scholar] [CrossRef]
- Gonnella, F.; Konstantinidou, F.; Di Berardino, C.; Capacchietti, G.; Peserico, A.; Russo, V.; Barboni, B.; Stuppia, L.; Gatta, V. A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View. Int. J. Mol. Sci. 2022, 23, 8890. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Larios, T.C.; Ortega-Márquez, A.L.; Rodríguez-Aguilera, J.R.; Vázquez-Martínez, E.R.; Domínguez-López, A.; Morimoto, S. A low-protein maternal diet during gestation affects the expression of key pancreatic β-cell genes and the methylation status of the regulatory region of the MafA gene in the offspring of Wistar rats. Front. Vet. Sci. 2023, 10, 1138564. [Google Scholar] [CrossRef] [PubMed]
- Koemel, N.A.; Skilton, M.R. Epigenetic Aging in Early Life: Role of Maternal and Early Childhood Nutrition. Curr. Nutr. Rep. 2022, 11, 318–328. [Google Scholar] [CrossRef]
- Amorín, R.; Liu, L.; Moriel, P.; DiLorenzo, N.; Lancaster, P.A.; Peñagaricano, F. Maternal diet induces persistent DNA methylation changes in the muscle of beef calves. Sci. Rep. 2023, 13, 1587. [Google Scholar] [CrossRef]
- Espinoza García, A.S.; Martínez Moreno, A.G.; Reyes Castillo, Z. The role of ghrelin and leptin in feeding behavior: Genetic and molecular evidence. Endocrinol. Diabetes Nutr. 2021, 68, 654–663. [Google Scholar] [CrossRef]
- Wróblewski, A.; Strycharz, J.; Świderska, E.; Drewniak, K.; Drzewoski, J.; Szemraj, J.; Kasznicki, J.; Śliwińska, A. Molecular Insight into the Interaction between Epigenetics and Leptin in Metabolic Disorders. Nutrients 2019, 11, 1872. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.E.; Sadovnikoff, A.I.; Ridout, K.K.; Lesseur, C.; Marsit, C.J.; Tyrka, A.R. Associations of maternal diet and placenta leptin methylation. Mol. Cell. Endocrinol. 2020, 505, 110739. [Google Scholar] [CrossRef] [PubMed]
- Küpers, L.K.; Fernández-Barrés, S.; Nounu, A.; Friedman, C.; Fore, R.; Mancano, G.; Dabelea, D.; Rifas-Shiman, S.L.; Mulder, R.H.; Oken, E.; et al. Maternal Mediterranean diet in pregnancy and newborn DNA methylation: A meta-analysis in the PACE Consortium. Epigenetics 2022, 17, 1419–1431. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease: A paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Lumey, L.H.; Stein, A.D.; Kahn, H.S.; van der Pal-de Bruin, K.M.; Blauw, G.J.; Zybert, P.A.; Susser, E.S. Cohort profile: The Dutch Hunger Winter families study. Int. J. Epidemiol. 2007, 36, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Yarde, F.; Broekmans, F.J.; van der Pal-de Bruin, K.M.; Schönbeck, Y.; te Velde, E.R.; Stein, A.D.; Lumey, L.H. Prenatal famine, birthweight, reproductive performance and age at menopause: The Dutch hunger winter families study. Hum. Reprod. 2013, 28, 3328–3336. [Google Scholar] [CrossRef]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M. Biobank-based Integrative Omics Studies Consortium, Slagboom, P.E.; van Zwet, E.W.; et al. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef]
- Kelishadi, R.; Farajian, S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: A review of evidence. Adv. Biomed. Res. 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Symons, L.; Vanautgaerden, E.-L.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; et al. The Influence of the Duration of Breastfeeding on the Infant’s Metabolic Epigenome. Nutrients 2019, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, W.B.; Bion, V.; Lockett, G.A.; Ziyab, A.H.; Soto-Ramírez, N.; Mukherjee, N.; Kurukulaaratchy, R.J.; Ewart, S.; Zhang, H.; Arshad, S.H.; et al. Duration of breastfeeding is associated with leptin (LEP) DNA methylation profiles and BMI in 10-year-old children. Clin. Epigenetics 2019, 11, 128. [Google Scholar] [CrossRef]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. MBio 2014, 5, e02113–e02114. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Raoult, D. The Microbiological Memory, an Epigenetic Regulator Governing the Balance Between Good Health and Metabolic Disorders. Front. Microbiol. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chen, Y.; Nian, Z.; Su, L.; Yu, H.; Chen, F.J.; Zhang, X.; Xu, W.; Zhou, L.; Liu, J.; et al. HDAC6- mediated acetylation of lipid droplet-binding protein CIDEC regulates fat- induced lipid storage. J. Clin. Invest. 2017, 127, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Mischke, M.; Plosch, T. More than just a gut instinct-the potential interplay between a baby’s nutrition, its gut microbiome, and the epigenome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1065–R1069. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, V.P.; Mellott, T.J.; Davison, J.M.; Wagner, N.; Lopez-Coviella, I.; Schnitzler, A.C.; Blusztajn, J.K. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Kok, D.E.; Dhonukshe-Rutten, R.A.; Lute, C.; Heil, S.G.; Uitterlinden, A.G.; van der Velde, N.; van Meurs, J.B.; van Schoor, N.M.; Hooiveld, G.J.; de Groot, L.C.; et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin. Epigenet. 2015, 7, 121. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, Other Methyl Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature. 2019, 568, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PloS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Zydecka, K.; Dabos, K.J.; Loniewski, I.; Koulaouzidis, A. Emerging concepts in non-invasive monitoring of Crohn’s disease. Ther. Adv. Gastroenterol. 2018, 11, 1756284818769076. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Pammi, M. Necrotizing enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators. Semin. Fetal Neonatal Med. 2018, 23, 400–405. [Google Scholar] [CrossRef]
- Tsuji, H.; Oozeer, R.; Matsuda, K.; Matsuki, T.; Ohta, T.; Nomoto, K.; Tanaka, R.; Kawashima, M.; Kawashima, K.; Nagata, S.; et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef. Microbes 2012, 3, 113–125. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Sensitive Quantitative Analysis of the Meconium Bacterial Microbiota in Healthy Term Infants Born Vaginally or by Cesarean Section. Front. Microbiol. 2016, 7, 1997. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Gut dysbiosis following C-section instigates higher colonisation of toxigenic Clostridium perfringens in infants. Benef. Microbes 2017, 8, 353–365. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Helve, O.; Kolho, K.L.; Saisto, T.; Skogberg, K.; Dikareva, E.; Stefanovic, V.; Salonen, A.; Andersson, S.; de Vos, W.M. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 2020, 183, 324–334.e5. [Google Scholar] [CrossRef]
- Chaitman, J.; Gaschen, F. Fecal Microbiota Transplantation in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.; Anokhin, A.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Fernandez-Navarro, T.; Arboleya, S.; De Los Reyes-Gavilan, C.G.; Salazar, N.; Gueimonde, M. Fermented dairy foods: Impact on intestinal microbiota and health-linked biomarkers. Front. Microbiol. 2019, 10, 1046. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Lee, D.; Choi, S.; Chang, J.; Park, Y.J.; Kim, J.H.; Park, S.M. Association of antibiotics exposure within the first 2 years after birth with subsequent childhood type 1 diabetes. Endocrine 2022, 77, 21–29. [Google Scholar] [CrossRef]
- Di Berardino, C.; Peserico, A.; Capacchietti, G.; Zappacosta, A.; Bernabò, N.; Russo, V.; Mauro, A.; El Khatib, M.; Gonnella, F.; Konstantinidou, F.; et al. High-Fat Diet and Female Fertility across Lifespan: A Comparative Lesson from Mammal Models. Nutrients 2022, 14, 4341. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef]
- Menichini, D.; Longo, M.; Facchinetti, F. Maternal interventions to improve offspring outcomes in rodent models of diet-induced obesity: A review. J. Matern. Fetal Neonatal Med. 2019, 32, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Panagiotidou, A.; Chatzakis, C.; Ververi, A.; Eleftheriades, M.; Sotiriadis, A. The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring. Genes 2024, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Kusuyama, J.; Alves-Wagner, A.B.; Makarewicz, N.S.; Goodyear, L.J. Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab. 2020, 2, 858–872. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, V.; Palomera-Ávalos, V.; López-Ruiz, S.; Canudas, A.M.; Pallàs, M.; Griñán-Ferré, C. Maternal Resveratrol Supplementation Prevents Cognitive Decline in Senescent Mice Offspring. Int. J. Mol. Sci. 2019, 20, 1134. [Google Scholar] [CrossRef]
- McCullough, L.E.; Mendez, M.A.; Miller, E.E.; Murtha, A.P.; Murphy, S.K.; Hoyo, C. Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics 2015, 10, 597–606. [Google Scholar] [CrossRef]
- Kusuyama, J.; Makarewicz, N.S.; Albertson, B.G.; Alves-Wagner, A.B.; Conlin, R.H.; Prince, N.B.; Alves, C.R.R.; Ramachandran, K.; Kozuka, C.; Xiudong, Y.; et al. Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation. Diabetes 2022, 71, 1170–1181. [Google Scholar] [CrossRef]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faienza, M.F.; Urbano, F.; Anaclerio, F.; Moscogiuri, L.A.; Konstantinidou, F.; Stuppia, L.; Gatta, V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Curr. Issues Mol. Biol. 2024, 46, 4358-4378. https://doi.org/10.3390/cimb46050265
Faienza MF, Urbano F, Anaclerio F, Moscogiuri LA, Konstantinidou F, Stuppia L, Gatta V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Current Issues in Molecular Biology. 2024; 46(5):4358-4378. https://doi.org/10.3390/cimb46050265
Chicago/Turabian StyleFaienza, Maria Felicia, Flavia Urbano, Federico Anaclerio, Luigi Antonio Moscogiuri, Fani Konstantinidou, Liborio Stuppia, and Valentina Gatta. 2024. "Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming" Current Issues in Molecular Biology 46, no. 5: 4358-4378. https://doi.org/10.3390/cimb46050265
APA StyleFaienza, M. F., Urbano, F., Anaclerio, F., Moscogiuri, L. A., Konstantinidou, F., Stuppia, L., & Gatta, V. (2024). Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Current Issues in Molecular Biology, 46(5), 4358-4378. https://doi.org/10.3390/cimb46050265






