Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. General Features of Cannabidiol
2.1. The Endocannabinoid System
2.2. Pharmacokinetics of Cannabidiol
3. Alzheimer’s Disease Pathology
3.1. Neuroinflammation in AD
3.2. Role of Microglia and the Proinflammatory Cascade in AD
3.3. Oxidative Stress
3.4. Role of Oxidative Stress in AD
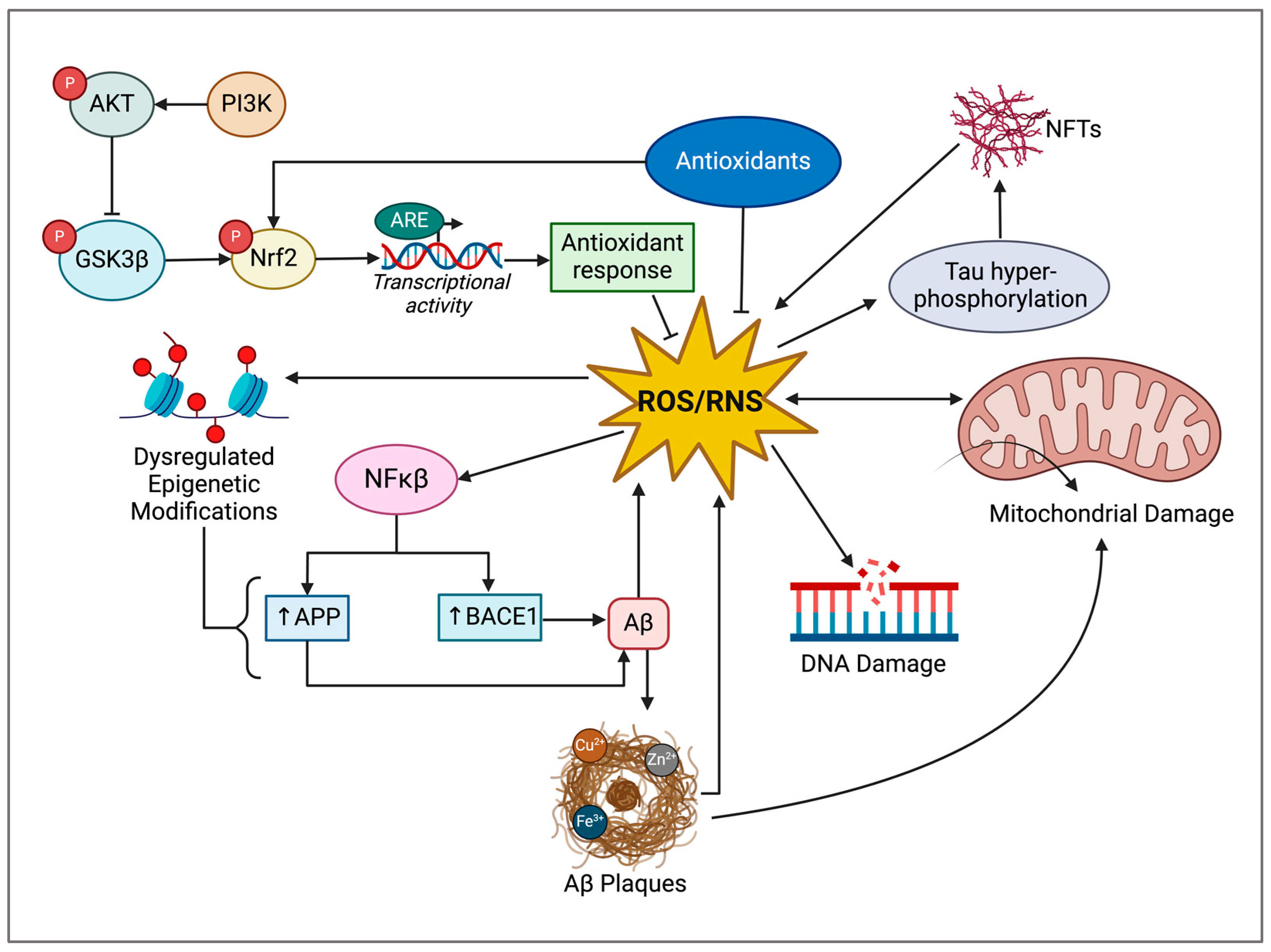
4. Cannabidiol in Preclinical Models of Alzheimer’s Disease
4.1. CBD’s Treatment of AD-Related Pathologies
4.1.1. Modulation of Neuroinflammation by CBD
4.1.2. Modulation of Oxidative Stress by CBD
5. Cannabidiol as a Potential Treatment for Alzheimer’s Disease
6. Future Directions and Limitations of Cannabidiol in Alzheimer’s Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Sun, Y.; Li, J.; Liu, S.; Ding, H.; Wang, G.; Li, X. Assessing Cannabidiol as a Therapeutic Agent for Preventing and Alleviating Alzheimer’s Disease Neurodegeneration. Cells 2023, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for Neurodegenerative Disorders: A Comprehensive Review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Collins, A.E.; Hickey, J.P.; Pfeifer, J.A.; Kalisch, B.E. Sex Differences in the Level of Homocysteine in Alzheimer’s Disease and Parkinson’s Disease Patients: A Meta-Analysis. Brain Sci. 2023, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.L.; Müller, N. Neuroinflammation, Microglia and Implications for Anti-Inflammatory Treatment in Alzheimer’s Disease. Int. J. Alzheimers Dis. 2010, 2010, 732806. [Google Scholar] [CrossRef]
- Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L.; Liu, Y. Targeting Neuroinflammation in Alzheimer’s Disease: From Mechanisms to Clinical Applications. Neural Regen. Res. 2023, 18, 708. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Campbell, V.A.; Gowran, A. Alzheimer’s Disease; Taking the Edge off with Cannabinoids? Br. J. Pharmacol. 2007, 152, 655–662. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Cooray, R.; Gupta, V.; Suphioglu, C. Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson’s and Alzheimer’s Diseases: A Review. Mol. Neurobiol. 2020, 57, 4878–4890. [Google Scholar] [CrossRef]
- Karl, T.; Garner, B.; Cheng, D. The Therapeutic Potential of the Phytocannabinoid Cannabidiol for Alzheimer’s Disease. Behav. Pharmacol. 2017, 28, 142–160. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Hu, S.S.-J.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. In Endocannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; pp. 59–93. ISBN 978-3-319-20825-1. [Google Scholar]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional Expression of Brain Neuronal CB2 Cannabinoid Receptors Are Involved in the Effects of Drugs of Abuse and in Depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the Cannabinoid CB2 Receptor in the Rat Cerebellum: An Immunohistochemical Study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 Receptors Are Expressed by Perivascular Microglial Cells in the Human Brain: An Immunohistochemical Study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Karl, T.; Cheng, D.; Garner, B.; Arnold, J.C. The Therapeutic Potential of the Endocannabinoid System for Alzheimer’s Disease. Expert Opin. Ther. Targets 2012, 16, 407–420. [Google Scholar] [CrossRef]
- Abate, G.; Uberti, D.; Tambaro, S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology 2021, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Brust, C.A.; Nikas, S.P.; Song, F.; et al. Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell 2020, 180, 655–665.e18. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A Comprehensive Profile of Brain Enzymes That Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to Anandamide and Enhanced Endogenous Cannabinoid Signaling in Mice Lacking Fatty Acid Amide Hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Ludányi, A.; Hu, S.S.-J.; Yamazaki, M.; Tanimura, A.; Piomelli, D.; Watanabe, M.; Kano, M.; Sakimura, K.; Maglóczky, Z.; Mackie, K.; et al. Complementary Synaptic Distribution of Enzymes Responsible for Synthesis and Inactivation of the Endocannabinoid 2-Arachidonoylglycerol in the Human Hippocampus. Neuroscience 2011, 174, 50–63. [Google Scholar] [CrossRef]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative Receptor Binding Analyses of Cannabinoid Agonists and Antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. [Google Scholar]
- Petitet, F.; Jeantaud, B.; Reibaud, M.; Imperato, A.; Dubroeucq, M.-C. Complex Pharmacology of Natural Cannabivoids: Evidence for Partial Agonist Activity of Δ9-Tetrahydrocannabinol and Antagonist Activity of Cannabidiol on Rat Brain Cannabinoid Receptors. Life Sci. 1998, 63, PL1–PL6. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.; Choudhuri, S.; Cournoyer, P.; Downey, J.; Muldoon Jacobs, K. Review of the Oral Toxicity of Cannabidiol (CBD). Food Chem. Toxicol. 2023, 176, 113799. [Google Scholar] [CrossRef]
- Watanabe, K.; Kayano, Y.; Matsunaga, T.; Yamamoto, I.; Yoshimura, H. Inhibition of Anandamide Amidase Activity in Mouse Brain Microsomes by Cannabinoids. Biol. Pharm. Bull. 1996, 19, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Iuvone, T.; D’amico, A.; Esposito, G.; Steardo, L.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y.; De Man, J.G. Effect of Cannabidiol on Sepsis-Induced Motility Disturbances in Mice: Involvement of CB1 Receptors and Fatty Acid Amide Hydrolase. Neurogastroenterol. Motil. 2008, 20, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Samara, E.; Mechoulam, R. Comparative Metabolism of Cannabidiol in Dog, Rat and Man. Pharmacol. Biochem. Behav. 1991, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Zheng, R.; Lee, K.; Qi, Z.; Wang, Z.; Xu, Z.; Wu, X.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The Benefits of Neuroinflammation for the Repair of the Injured Central Nervous System. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune Cells Contribute to the Maintenance of Neurogenesis and Spatial Learning Abilities in Adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of Learning and Memory by Meningeal Immunity: A Key Role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef]
- Sokolova, A.; Hill, M.D.; Rahimi, F.; Warden, L.A.; Halliday, G.M.; Shepherd, C.E. Monocyte Chemoattractant Protein-1 Plays a Dominant Role in the Chronic Inflammation Observed in Alzheimer’s Disease. Brain Pathol. 2009, 19, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Mastrangelo, M.A.; Park, K.M.; Sudol, K.L.; Narrow, W.C.; Oddo, S.; LaFerla, F.M.; Callahan, L.M.; Federoff, H.J.; Bowers, W.J. Chronic Neuron-Specific Tumor Necrosis Factor-Alpha Expression Enhances the Local Inflammatory Environment Ultimately Leading to Neuronal Death in 3xTg-AD Mice. Am. J. Pathol. 2008, 173, 1768–1782. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Orellana, D.I.; González-Billault, C.; Maccioni, R.B. Interleukin-6 Induces Alzheimer-Type Phosphorylation of Tau Protein by Deregulating the Cdk5/P35 Pathway. Exp. Cell Res. 2004, 295, 245–257. [Google Scholar] [CrossRef]
- Griffin, W.S.T.; Sheng, J.G.; Roberts, G.W.; Mrak, R.E. Interleukin-1 Expression in Different Plaque Types in Alzheimer’s Disease: Significance in Plaque Evalution. J. Neuropathol. Exp. Neurol. 1995, 54, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Ten, M.-S.; Yu, J.-T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Rani, V.; Verma, R.; Kumar, K.; Chawla, R. Role of Pro-Inflammatory Cytokines in Alzheimer’s Disease and Neuroprotective Effects of Pegylated Self-Assembled Nanoscaffolds. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100149. [Google Scholar] [CrossRef]
- Morales, I.; Jiménez, J.M.; Mancilla, M.; Maccioni, R.B. Tau Oligomers and Fibrils Induce Activation of Microglial Cells. J. Alzheimers Dis. 2013, 37, 849–856. [Google Scholar] [CrossRef]
- Liu, C.; Cui, G.; Zhu, M.; Kang, X.; Guo, H. Neuroinflammation in Alzheimer’s Disease: Chemokines Produced by Astrocytes and Chemokine Receptors. Int. J. Clin. Exp. Pathol. 2014, 7, 8342–8355. [Google Scholar]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Lee, C.Y.D.; Landreth, G.E. The Role of Microglia in Amyloid Clearance from the AD Brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]
- Mandrekar, S.; Jiang, Q.; Lee, C.Y.D.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Landreth, G.E. Microglia Mediate the Clearance of Soluble Aβ through Fluid Phase Macropinocytosis. J. Neurosci. 2009, 29, 4252–4262. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.; Dou, Y.; Wu, H.; Liu, Y.; Lou, H.-F.; Zhang, J.; Li, X.; Wang, H.; Duan, S. P2Y4 Receptor-Mediated Pinocytosis Contributes to Amyloid Beta-Induced Self-Uptake by Microglia. Mol. Cell. Biol. 2013, 33, 4282–4293. [Google Scholar] [CrossRef]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef]
- Fu, H.; Liu, B.; Li, L.; Lemere, C.A. Microglia Do Not Take Up Soluble Amyloid-Beta Peptides, But Partially Degrade Them by Secreting Insulin-Degrading Enzyme. Neuroscience 2020, 443, 30–43. [Google Scholar] [CrossRef]
- Fu, H.; Liu, B.; Frost, J.L.; Hong, S.; Jin, M.; Ostaszewski, B.; Shankar, G.M.; Costantino, I.M.; Carroll, M.C.; Mayadas, T.N.; et al. Complement Component C3 and Complement Receptor Type 3 Contribute to the Phagocytosis and Clearance of Fibrillar Aβ by Microglia. Glia 2012, 60, 993–1003. [Google Scholar] [CrossRef]
- Kong, Y.; Ruan, L.; Qian, L.; Liu, X.; Le, Y. Norepinephrine Promotes Microglia to Uptake and Degrade Amyloid β Peptide through Upregulation of Mouse Formyl Peptide Receptor 2 and Induction of Insulin-Degrading Enzyme. J. Neurosci. 2010, 30, 11848–11857. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Jurado-Arjona, J.; Hernández, F.; Rábano, A.; Avila, J. Direct Evidence of Internalization of Tau by Microglia In Vitro and In Vivo. J. Alzheimers Dis. 2016, 50, 77–87. [Google Scholar] [CrossRef]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Phagocytosis of Full-Length Tau Oligomers by Actin-Remodeling of Activated Microglia. J. Neuroinflamm. 2020, 17, 10. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Frackowiak, J.; Wisniewski, H.M.; Wegiel, J.; Merz, G.S.; Iqbal, K.; Wang, K.C. Ultrastructure of the Microglia That Phagocytose Amyloid and the Microglia That Produce β-Amyloid Fibrils. Acta Neuropathol. 1992, 84, 225–233. [Google Scholar] [CrossRef]
- Hopp, S.C.; Lin, Y.; Oakley, D.; Roe, A.D.; DeVos, S.L.; Hanlon, D.; Hyman, B.T. The Role of Microglia in Processing and Spreading of Bioactive Tau Seeds in Alzheimer’s Disease. J. Neuroinflamm. 2018, 15, 269. [Google Scholar] [CrossRef]
- Bhaskar, K.; Konerth, M.; Kokiko-Cochran, O.N.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T. Regulation of Tau Pathology by the Microglial Fractalkine Receptor. Neuron 2010, 68, 19–31. [Google Scholar] [CrossRef]
- Jiang, T.; Tan, L.; Zhu, X.-C.; Zhou, J.-S.; Cao, L.; Tan, M.-S.; Wang, H.-F.; Chen, Q.; Zhang, Y.-D.; Yu, J.-T. Silencing of TREM2 Exacerbates Tau Pathology, Neurodegenerative Changes, and Spatial Learning Deficits in P301S Tau Transgenic Mice. Neurobiol. Aging 2015, 36, 3176–3186. [Google Scholar] [CrossRef]
- Perea, J.R.; Ávila, J.; Bolós, M. Dephosphorylated Rather than Hyperphosphorylated Tau Triggers a Pro-Inflammatory Profile in Microglia through the P38 MAPK Pathway. Exp. Neurol. 2018, 310, 14–21. [Google Scholar] [CrossRef]
- MRC CFAS; Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. Microglial Immunophenotype in Dementia with Alzheimer’s Pathology. J. Neuroinflamm. 2016, 13, 135. [Google Scholar] [CrossRef]
- Brelstaff, J.H.; Mason, M.; Katsinelos, T.; McEwan, W.A.; Ghetti, B.; Tolkovsky, A.M.; Spillantini, M.G. Microglia Become Hypofunctional and Release Metalloproteases and Tau Seeds When Phagocytosing Live Neurons with P301S Tau Aggregates. Sci. Adv. 2021, 7, eabg4980. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Tichauer, J.E.; Eugenín, J. Aging-dependent Changes of Microglial Cells and Their Relevance for Neurodegenerative Disorders. J. Neurochem. 2010, 112, 1099–1114. [Google Scholar] [CrossRef]
- Sheng, J.G.; Zhu, S.G.; Jones, R.A.; Griffin, W.S.T.; Mrak, R.E. Interleukin-1 Promotes Expression and Phosphorylation of Neurofilament and Tau Proteins in Vivo. Exp. Neurol. 2000, 163, 388–391. [Google Scholar] [CrossRef]
- Blasko, I.; Marx, F.; Steiner, E.; Hartmann, T.; Grubeck-Loebenstein, B. TNFα plus IFNγ Induce the Production of Alzheimer Β-amyloid Peptides and Decrease the Secretion of APPs. FASEB J. 1999, 13, 63–68. [Google Scholar] [CrossRef]
- Liao, Y.-F.; Wang, B.-J.; Cheng, H.-T.; Kuo, L.-H.; Wolfe, M.S. Tumor Necrosis Factor-α, Interleukin-1β, and Interferon-γ Stimulate γ-Secretase-Mediated Cleavage of Amyloid Precursor Protein through a JNK-Dependent MAPK Pathway. J. Biol. Chem. 2004, 279, 49523–49532. [Google Scholar] [CrossRef]
- Nelson, M.L.; Pfeifer, J.A.; Hickey, J.P.; Collins, A.E.; Kalisch, B.E. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1042. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, R.; George, J.; Sanches, R.; Rodrigues, D.I.; Gonçalves, N.; Cunha, R.A. Activation of Microglial Cells Triggers a Release of Brain-Derived Neurotrophic Factor (BDNF) Inducing Their Proliferation in an Adenosine A2A Receptor-Dependent Manner: A2A Receptor Blockade Prevents BDNF Release and Proliferation of Microglia. J. Neuroinflamm. 2013, 10, 780. [Google Scholar] [CrossRef]
- Johansson, J.U.; Woodling, N.S.; Wang, Q.; Panchal, M.; Liang, X.; Trueba-Saiz, A.; Brown, H.D.; Mhatre, S.D.; Loui, T.; Andreasson, K.I. Prostaglandin Signaling Suppresses Beneficial Microglial Function in Alzheimer’s Disease Models. J. Clin. Investig. 2015, 125, 350–364. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Q.; Johansson, J.U.; Liang, X.; Woodling, N.S.; Priyam, P.; Loui, T.M.; Merchant, M.; Breyer, R.M.; Montine, T.J.; et al. Inflammatory Prostaglandin E2 Signaling in a Mouse Model of Alzheimer Disease. Ann. Neurol. 2012, 72, 788–798. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Li, Y.R.; Trush, M. Defining ROS in Biology and Medicine. React. Oxyg. Spec. 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Khajeh Dangolani, S.; Panahi, F.; Tavaf, Z.; Nourisefat, M.; Yousefi, R.; Khalafi-Nezhad, A. Synthesis and Antioxidant Activity Evaluation of Some Novel Aminocarbonitrile Derivatives Incorporating Carbohydrate Moieties. ACS Omega 2018, 3, 10341–10350. [Google Scholar] [CrossRef]
- Mohana, K.N.; Kumar, C.B.P. Synthesis and Antioxidant Activity of 2-Amino-5-Methylthiazol Derivatives Containing 1,3,4-Oxadiazole-2-Thiol Moiety. ISRN Org. Chem. 2013, 2013, 620718. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular Defenses against Damage from Reactive Oxygen Species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel Antioxidants in Food Quality Preservation and Health Promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic Acid Attenuates β-Amyloid-Induced Oxidative Stress via Akt/GSK-3β/Fyn-Mediated Nrf2 Activation in PC12 Cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin Reduces Oxidative Stress and Improves Mitochondrial Dysfunction via Activation of the Nrf2/ARE Signaling Pathway in Neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin Protects against 6-OHDA-Induced Neurotoxicity via Activation of the Nrf2/ARE Signaling Pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, X.; Zhang, N.; Li, G.; Zhang, L.-H.; Tan, L.-Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a D-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017, 20, 462–472. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.S.; Chen, J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Regulation of the Antioxidant Response Element by Protein Kinase C-Mediated Phosphorylation of NF-E2-Related Factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475–12480. [Google Scholar] [CrossRef]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-Mediated Transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef]
- De Plano, L.M.; Calabrese, G.; Rizzo, M.G.; Oddo, S.; Caccamo, A. The Role of the Transcription Factor Nrf2 in Alzheimer’s Disease: Therapeutic Opportunities. Biomolecules 2023, 13, 549. [Google Scholar] [CrossRef]
- Talebi, M.; Sadoughi, M.M.; Ayatollahi, S.A.; Ainy, E.; Kiani, R.; Zali, A.; Miri, M. Therapeutic Potentials of Cannabidiol: Focus on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2023, 168, 115805. [Google Scholar] [CrossRef]
- Pithadia, A.S.; Lim, M.H. Metal-Associated Amyloid-β Species in Alzheimer’s Disease. Curr. Opin. Chem. Biol. 2012, 16, 67–73. [Google Scholar] [CrossRef]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Tiiman, A.; Palumaa, P.; Tõugu, V. The Missing Link in the Amyloid Cascade of Alzheimer’s Disease—Metal Ions. Neurochem. Int. 2013, 62, 367–378. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Strodel, B.; Coskuner-Weber, O. Transition Metal Ion Interactions with Disordered Amyloid-β Peptides in the Pathogenesis of Alzheimer’s Disease: Insights from Computational Chemistry Studies. J. Chem. Inf. Model. 2019, 59, 1782–1805. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef]
- Kepp, K.P. Alzheimer’s Disease: How Metal Ions Define β-Amyloid Function. Coord. Chem. Rev. 2017, 351, 127–159. [Google Scholar] [CrossRef]
- Tõugu, V.; Tiiman, A.; Palumaa, P. Interactions of Zn(II) and Cu(II) Ions with Alzheimer’s Amyloid-Beta Peptide. Metal Ion Binding, Contribution to Fibrillization and Toxicity. Metallomics 2011, 3, 250. [Google Scholar] [CrossRef]
- Kandel, N.; Matos, J.O.; Tatulian, S.A. Structure of Amyloid Β25–35 in Lipid Environment and Cholesterol-Dependent Membrane Pore Formation. Sci. Rep. 2019, 9, 2689. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Zlokovic, B.V. The Role of the Cell Surface LRP and Soluble LRP in Blood-Brain Barrier Abeta Clearance in Alzheimer’s Disease. Curr. Pharm. Des. 2008, 14, 1601–1605. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood–Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.C.F.; Lemos, R.; Ferreiro, E.; Martins, M.; De Mendonça, A.; Santana, I.; Outeiro, T.F.; Pereira, C.M.F. Epigenetic Regulation of BACE1 in Alzheimer’s Disease Patients and in Transgenic Mice. Neuroscience 2012, 220, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Hemmelgarn, B.T.; Chuang, C.-C.; Best, T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-β Production in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 604658. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Oelze, B.; Schumacher, A. Age-Specific Epigenetic Drift in Late-Onset Alzheimer’s Disease. PLoS ONE 2008, 3, e2698. [Google Scholar] [CrossRef]
- Scarpa, S.; Cavallaro, R.A.; D’Anselmi, F.; Fuso, A. Gene Silencing through Methylation: An Epigenetic Intervention on Alzheimer Disease. J. Alzheimers Dis. 2006, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lithner, C.U.; Hernandez, C.; Sweatt, J.D.; Nordberg, A. O3-05-05: Epigenetic Effects of Aβ and the Implication on the Pathophysiology in Alzheimer’s Disease. Alzheimers Dement. 2011, 7, S508. [Google Scholar] [CrossRef]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.M.; Coleman, P.D.; Rutten, B.P.F.; Van Den Hove, D.L.A. Consistent Decrease in Global DNA Methylation and Hydroxymethylation in the Hippocampus of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Sun, J.; Li, S.; Wu, X.; Li, L. Oxidative Stress Induces DNA Demethylation and Histone Acetylation in SH-SY5Y Cells: Potential Epigenetic Mechanisms in Gene Transcription in Aβ Production. Neurobiol. Aging 2013, 34, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Readnower, R.D.; Sauerbeck, A.D.; Sullivan, P.G. Mitochondria, Amyloid β, and Alzheimer’s Disease. Int. J. Alzheimers Dis. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. β-Amyloid: The Key Peptide in the Pathogenesis of Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef]
- Reddy, P.H.; Tripathi, R.; Troung, Q.; Tirumala, K.; Reddy, T.P.; Anekonda, V.; Shirendeb, U.P.; Calkins, M.J.; Reddy, A.P.; Mao, P.; et al. Abnormal Mitochondrial Dynamics and Synaptic Degeneration as Early Events in Alzheimer’s Disease: Implications to Mitochondria-Targeted Antioxidant Therapeutics. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 639–649. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, S.D. Amyloid-β-Induced Mitochondrial Dysfunction. J. Alzheimers Dis. 2007, 12, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Adlard, P.A.; Morten, K.; Johnson, F.; Golden, T.R.; Hinerfeld, D.; Schilling, B.; Mavros, C.; Masters, C.L.; Volitakis, I.; et al. Mitochondrial Oxidative Stress Causes Hyperphosphorylation of Tau. PLoS ONE 2007, 2, e536. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary Lycopene Supplementation Improves Cognitive Performances in Tau Transgenic Mice Expressing P301L Mutation via Inhibiting Oxidative Stress and Tau Hyperphosphorylation. J. Alzheimers Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, X.; Lee, H.; Tabaton, M.; Perry, G.; Smith, M.A.; Zhu, X. Chronic Oxidative Stress Causes Increased Tau Phosphorylation in M17 Neuroblastoma Cells. Neurosci. Lett. 2010, 468, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Valenti, D.; Latina, V.; Amadoro, G. Role of Oxygen Radicals in Alzheimer’s Disease: Focus on Tau Protein. Oxygen 2021, 1, 96–120. [Google Scholar] [CrossRef]
- Steinhilb, M.L.; Dias-Santagata, D.; Fulga, T.A.; Felch, D.L.; Feany, M.B. Tau Phosphorylation Sites Work in Concert to Promote Neurotoxicity In Vivo. Mol. Biol. Cell 2007, 18, 5060–5068. [Google Scholar] [CrossRef]
- Alonso, A.D.C.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s Disease Hyperphosphorylated Tau Sequesters Normal Tau into Tangles of Filaments and Disassembles Microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The Marijuana Component Cannabidiol Inhibits Beta-Amyloid-Induced Tau Protein Hyperphosphorylation through Wnt/Beta-Catenin Pathway Rescue in PC12 Cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol Inhibits Inducible Nitric Oxide Synthase Protein Expression and Nitric Oxide Production in Beta-Amyloid Stimulated PC12 Neurons through P38 MAP Kinase and NF-κB Involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective Effect of Cannabidiol, a Non-Psychoactive Component from Cannabis sativa, on Beta-Amyloid-Induced Toxicity in PC12 Cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Harvey, B.S.; Ohlsson, K.S.; Mååg, J.L.; Musgrave, I.F.; Smid, S.D. Contrasting Protective Effects of Cannabinoids against Oxidative Stress and Amyloid-β Evoked Neurotoxicity in Vitro. Neurotoxicology 2012, 33, 138–146. [Google Scholar] [CrossRef]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5YAPP+ Cells through PPARγ Involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef]
- Raja, A.; Ahmadi, S.; de Costa, F.; Li, N.; Kerman, K. Attenuation of Oxidative Stress by Cannabinoids and Cannabis Extracts in Differentiated Neuronal Cells. Pharmaceuticals 2020, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, P.; Nagaratnam, N.; Solowij, N.; Huang, X.F. Parkin Mediates Cannabidiol Prevention of Amyloid-Beta-Induced Senescence in Human Astrocytes. Cannabis Cannabinoid Res. 2023, 8, 309–320. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Kim, J.; Choi, P.; Park, Y.T.; Kim, T.; Ham, J.; Kim, J.C. The Cannabinoids, CBDA and THCA, Rescue Memory Deficits and Reduce Amyloid-Beta and Tau Pathology in an Alzheimer’s Disease-like Mouse Model. Int. J. Mol. Sci. 2023, 24, 6827. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Du, Y.; Zhao, X.; Tang, Q.; Su, W.; Hu, Y.; Yu, P. Cannabidiol Enhances Microglial Beta-Amyloid Peptide Phagocytosis and Clearance via Vanilloid Family Type 2 Channel Activation. Int. J. Mol. Sci. 2022, 23, 5367. [Google Scholar] [CrossRef]
- Schubert, D.; Kepchia, D.; Liang, Z.; Dargusch, R.; Goldberg, J.; Maher, P. Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina (Kaunas) 2019, 55, 747. [Google Scholar] [CrossRef]
- Patel, V.; Abu-Hijleh, F.; Rigg, N.; Mishra, R. Cannabidiol Protects Striatal Neurons by Attenuating Endoplasmic Reticulum Stress. Cannabis Cannabinoid Res. 2023, 8, 299–308. [Google Scholar] [CrossRef]
- Alali, S.; Riazi, G.; Ashrafi-Kooshk, M.R.; Meknatkhah, S.; Ahmadian, S.; Hooshyari Ardakani, M.; Hosseinkhani, B. Cannabidiol Inhibits Tau Aggregation In Vitro. Cells 2021, 10, 3521. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Schröder, N. Memory-Rescuing Effects of Cannabidiol in an Animal Model of Cognitive Impairment Relevant to Neurodegenerative Disorders. Psychopharmacology 2012, 219, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- de Paula Faria, D.; Estessi de Souza, L.; Duran, F.L.S.; Buchpiguel, C.A.; Britto, L.R.; Crippa, J.A.S.; Filho, G.B.; Real, C.C. Cannabidiol Treatment Improves Glucose Metabolism and Memory in Streptozotocin-Induced Alzheimer’s Disease Rat Model: A Proof-of-Concept Study. Int. J. Mol. Sci. 2022, 23, 1076. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo, L.J.R.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in Vivo Blunts Beta-Amyloid Induced Neuroinflammation by Suppressing IL-1beta and iNOS Expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and Other Cannabinoids Reduce Microglial Activation in Vitro and in Vivo: Relevance to Alzheimer’s Disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef]
- Arnanz, M.A.; Ruiz de Martín Esteban, S.; Martínez Relimpio, A.M.; Rimmerman, N.; Tweezer Zaks, N.; Grande, M.T.; Romero, J. Effects of Chronic, Low-Dose Cannabinoids, Cannabidiol, Delta-9-Tetrahydrocannabinol and a Combination of Both, on Amyloid Pathology in the 5xFAD Mouse Model of Alzheimer’s Disease. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-Term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Shang, K.; Zieba, J.; Olaya, J.; Li, H.; Garner, B.; Karl, T. Chronic Treatment with 50 Mg/Kg Cannabidiol Improves Cognition and Moderately Reduces Aβ40 Levels in 12-Month-Old Male AβPPswe/PS1ΔE9 Transgenic Mice. J. Alzheimers Dis. 2020, 74, 937–950. [Google Scholar] [CrossRef]
- Coles, M.; Watt, G.; Kreilaus, F.; Karl, T. Medium-Dose Chronic Cannabidiol Treatment Reverses Object Recognition Memory Deficits of APP(Swe)/PS1ΔE9 Transgenic Female Mice. Front. Pharmacol. 2020, 11, 587604. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic APPswe/PS1∆E9 Mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- García-Baos, A.; Puig-Reyne, X.; García-Algar, Ó.; Valverde, O. Cannabidiol Attenuates Cognitive Deficits and Neuroinflammation Induced by Early Alcohol Exposure in a Mice Model. Biomed. Pharmacother. 2021, 141, 111813. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Yang, G.; Hu, N.; Zhao, Z.; Zhao, L.; Li, S. Cannabidiol Alleviates the Damage to Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease Mice Via Regulating Neuronal Apoptosis and Neuroinflammation. Neuroscience 2022, 498, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.; Narayanasamy, P. Effect of Cannabidiol on the Neural Glyoxalase Pathway Function and Longevity of Several C. elegans Strains Including a C. elegans Alzheimer’s Disease Model. ACS Chem. Neurosci. 2022, 13, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Jin, S.; Lu, Y.; Peng, Y.; Zhao, L.; Wang, X. Cannabidiol Protects against Alzheimer’s Disease in C. Elegans via ROS Scavenging Activity of Its Phenolic Hydroxyl Groups. Eur. J. Pharmacol. 2022, 919, 174829. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; Dalle, S.; Mantini, D.; Koppo, K. Cannabidiol and Brain Function: Current Knowledge and Future Perspectives. Front. Pharmacol. 2024, 14, 1328885. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhushan, B.; Chanchal, D.K.; Sharma, S.K.; Rani, K.; Yadav, M.K.; Porwal, P.; Kumar, S.; Sharma, A.; Virmani, T.; et al. Emerging Therapeutic Potential of Cannabidiol (CBD) in Neurological Disorders: A Comprehensive Review. Behav. Neurol. 2023, 2023, 8825358. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, Efficacy, and Mechanisms of Action of Cannabinoids in Neurological Disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Tambe, S.M.; Mali, S.; Amin, P.D.; Oliveira, M. Neuroprotective Potential of Cannabidiol: Molecular Mechanisms and Clinical Implications. J. Integr. Med. 2023, 21, 236–244. [Google Scholar] [CrossRef]
- Liu, Z.; Martin, J.H. Gaps in Predicting Clinical Doses for Cannabinoids Therapy: Overview of Issues for Pharmacokinetics and Pharmacodynamics Modelling. Br. J. Clin. Pharmacol. 2018, 84, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D.; Abrams, D.I.; Gust, S.; Salicrup, A.; Throckmorton, D.C. Challenges for Clinical Cannabis and Cannabinoid Research in the United States. JNCI Monogr. 2021, 2021, 114–122. [Google Scholar] [CrossRef]
- Hossain, K.R.; Alghalayini, A.; Valenzuela, S.M. Current Challenges and Opportunities for Improved Cannabidiol Solubility. Int. J. Mol. Sci. 2023, 24, 14514. [Google Scholar] [CrossRef]
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Breward, N.; Duff, W.; Absher, N.; Patten, S.B.; Alcorn, J.; Mousseau, D.D. Cannabinoids in the Management of Behavioral, Psychological, and Motor Symptoms of Neurocognitive Disorders: A Mixed Studies Systematic Review. J. Cannabis Res. 2022, 4, 11. [Google Scholar] [CrossRef]
- Aziz, A.I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Sumsuzzman, D.M.; Ashraf, G.M.; Perveen, A.; Bungau, S.G.; Mousa, S.A.; El-Seedi, H.R.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Emerging Promise of Cannabinoids for the Management of Pain and Associated Neuropathological Alterations in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 1097. [Google Scholar] [CrossRef]
- Yau, G.T.Y.; Tai, W.; Arnold, J.C.; Chan, H.-K.; Kwok, P.C.L. Cannabidiol for the Treatment of Brain Disorders: Therapeutic Potential and Routes of Administration. Pharm. Res. 2023, 40, 1087–1114. [Google Scholar] [CrossRef] [PubMed]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Polizio, A.H.; Abbate, A.; Toldo, S.; Mezzaroma, E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules 2024, 29, 473. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical Implications of Trials Investigating Drug-drug Interactions between Cannabidiol and Enzyme Inducers or Inhibitors or Common Antiseizure Drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef]
- Trojan, V.; Landa, L.; Šulcová, A.; Slíva, J.; Hřib, R. The Main Therapeutic Applications of Cannabidiol (CBD) and Its Potential Effects on Aging with Respect to Alzheimer’s Disease. Biomolecules 2023, 13, 1446. [Google Scholar] [CrossRef]
- Leszko, M.; Meenrajan, S. Attitudes, Beliefs, and Changing Trends of Cannabidiol (CBD) Oil Use among Caregivers of Individuals with Alzheimer’s Disease. Complement. Ther. Med. 2021, 57, 102660. [Google Scholar] [CrossRef]
- Troup, L.J.; Erridge, S.; Ciesluk, B.; Sodergren, M.H. Perceived Stigma of Patients Undergoing Treatment with Cannabis-Based Medicinal Products. Int. J. Environ. Res. Public. Health 2022, 19, 7499. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Joaquim, H.P.G.; Pedrazzi, J.F.C.; Pain, A.O.; Duque, G.; Aprahamian, I. Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges. Brain Sci. 2022, 12, 1596. [Google Scholar] [CrossRef]
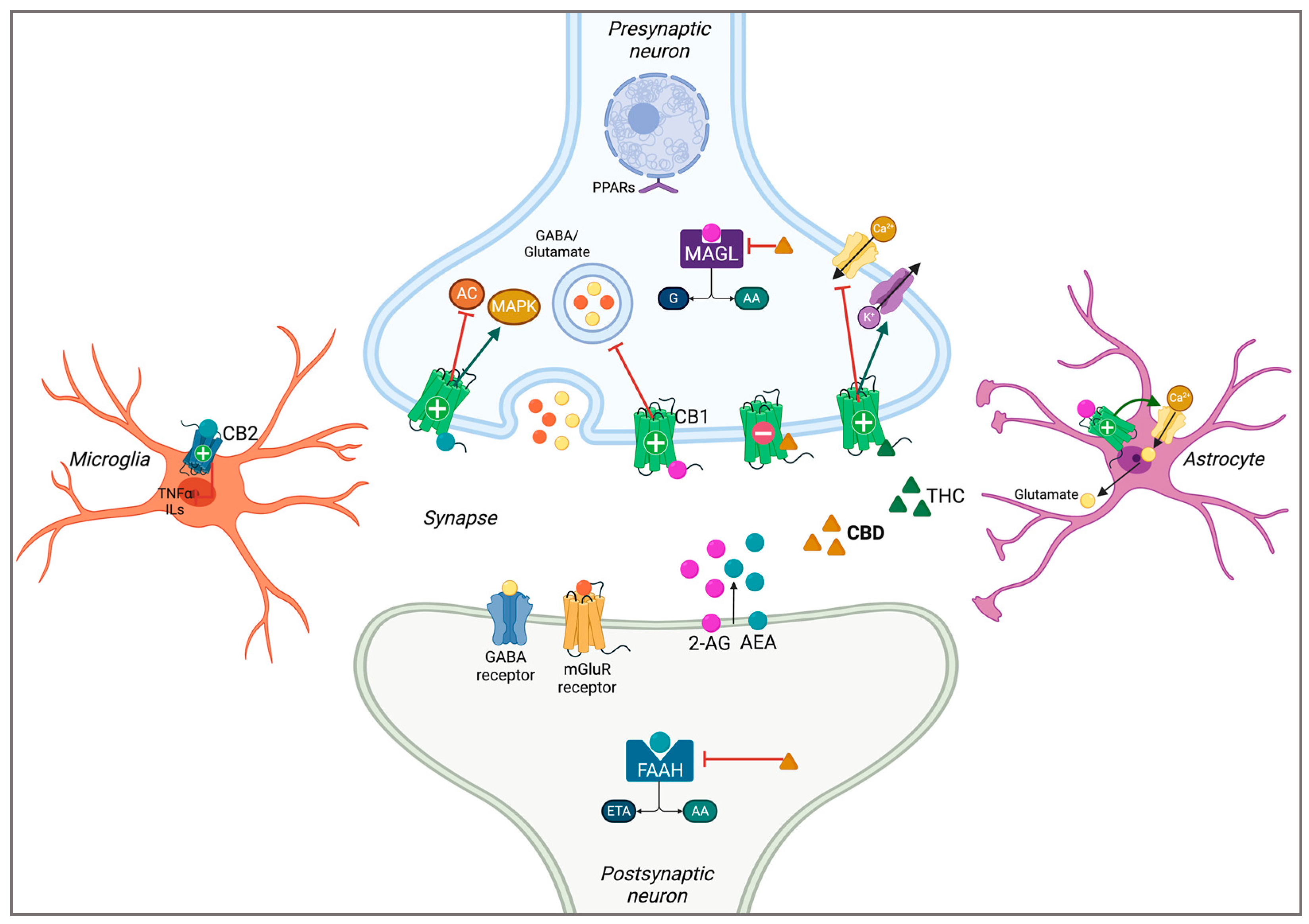
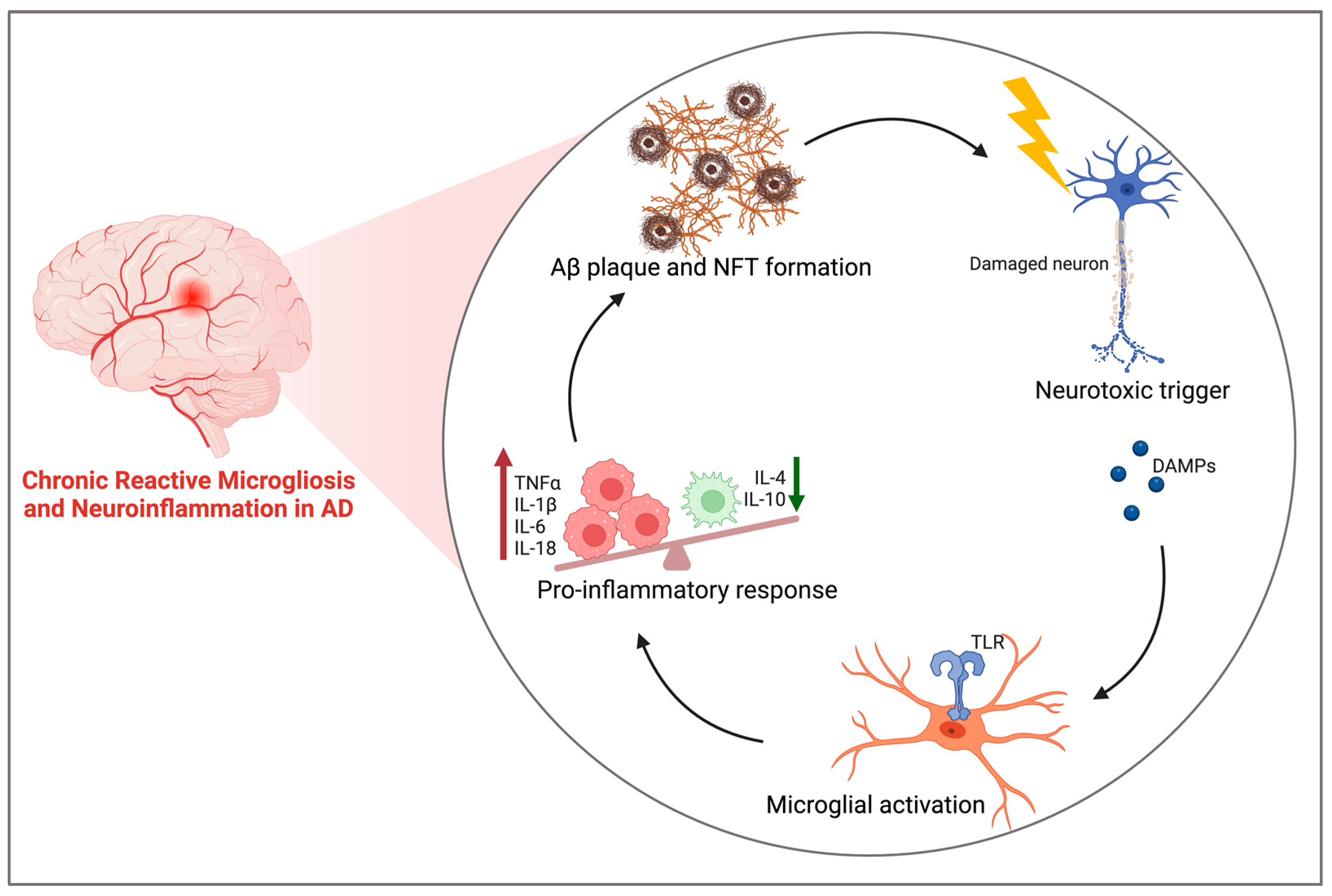
| First Author and Year | Model | Treatment (CBD) | Primary Outcome Measures | Main Results |
|---|---|---|---|---|
| Esposito 2006 [129] | PC12 cells (NGF diff.) | CBD 15 min pre-treatment (10−7–10−5 M); Aβ1–42 (1 μg/mL) 24 h | Tau hyperphosphorylation modulated through the Wnt/β-catenin pathway | CBD: ↓ Aβ (1–42)-induced p-GSK-3β; ↑ β-catenin; ↓ p-tau |
| Esposito 2006 [130] | PC12 cells (NGF-diff.) | CBD 15 min pre-treatment (10−6–10−4 M); Aβ1–42 (1 μg/mL) 24-hour | iNOS expression and NO production through p38 MAPK and NF-κB action | CBD: ↓ nitrite and iNOS expression; ↓ p-p38 MAPK; ↓ NF-κB activation |
| Iuvone 2004 [131] | PC12 cells | CBD (10−7–10−4 M) and Aβ1–42 (1 μg/mL) | Neuroprotection against Aβ-induced neurotoxicity by modulating ROS levels, lipoperoxidation, and apoptosis | CBD: ↓ cell death; ↓ ROS accumulation and lipid peroxidation; ↑ procaspase–total caspase 3 |
| Harvey 2012 [132] | PC12 and SH-SY5Y cells | PC12: CBD (1 or 10 μM) & Aβ1–40 or H2O2 or t-BHP; SH-SY5Y: CBD (0.01–10 μM) and H2O2 or t-BHP | Oxidative stress and Aβ-induced neurotoxicity: CBD compared to known antioxidants and anandamide | No effect of CBD against H2O2 or Aβ1–40 in PC12 cell viability; CBD (10 μM) ↑ cell viability against t-BHP in both cell lines |
| Scuderi 2014 [133] | SH-SY5YAPP+ cells | CBD (10−9–10−6 M) | Modulation of APP in APP-overexpressing cells and the involvement of PPARγ | CBD: ↓ APP and Aβ peptide levels and ↑ APP ubiquitination through PPARγ activation; ↑ cell viability |
| Raja 2020 [134] | SH-SY5Y cells (RA-diff.) | CBD (0.01–74 μg/mL) and Aβ1–42 (10 μM) or H2O2 (100 μM) | H2O2-induced oxidative stress and Aβ1–42-Cu(II) simulated AD-like oxidative stress | CBD: inhibits H2O2-induced ROS (IC50 = 42.7 μg/mL); displays no neurotoxicity (<1 μg/mL) |
| Wang 2023 [135] | 1’ human astrocytes | CBD (2.5 μM); Aβ1–42 (2 μM) | Aβ-induced cellular senescence and apoptosis with the involvement of Parkin | CBD: ↓ Aβ-induced astrocyte senescence and rescues Aβ-induced mitophagy deficits; ↓ mitochondrial ROS |
| Esposito 2011 [136] | 1’ rat astroglial cultures | CBD (10−9–10−7 M); Aβ1–42 (1 μg/mL) | Role of PPARγ receptor activity in CBD-mediated neuroprotection | CBD: ↓ release of NO, IL-1β, TNF-α, and S100B; ↓ Aβ-induced iNOS, GFAP, and S100B protein; ↓ p50 and p65 through selective PPARγ-dependent NF-κB inhibition |
| Kim 2023 [137] | 1’ mouse cortical neurons | CBDA (3 & 6 μM); Aβ1–42 (5 μM) | Aβ-induced AD-like characteristics | CBDA: ↓ Aβ and p-tau levels; alleviated calcium dysfunction; ↑ cell viability |
| Yang 2022 [138] | 1’ mouse microglial cultures | CBD (5 μM); Aβ1–42 (1 μg/mL) | Effects of CBD on TRPV2 expression and microglial Aβ phagocytosis | CBD: ↑ TRPV2-activation dependent microglial Aβ phagocytosis, mitochondrial function, and ATP production |
| Schubert 2019 [139] | MC65 cells | CBD (0.1 μM) | Neuroprotective capacity of 11 cannabinoids against Aβ-induced neurotoxicity and aggregation | CBD: inhibited amyloid toxicity and ↑ degradation and removal of Aβ |
| Mammana 2019 [140] | NSC-34 (serum deprived and RA-diff.) | CBD and/or CBG (2.5, 5, 10, 20, 40, and 80 μM) | Effect of CBG and CBD, alone and in combination, on neuroinflammation through cytokine, NF-κB, and Nrf2 involvement | CBG w/CBD: ↓ neuroinflammation (2.5 and 5 μM); ↓ TNF-α levels and NF-κB activation; ↑ IL10 and IL37 expression (5 μM); ↓ iNOS and ↑ Nrf2 levels (5 μM) |
| Patel 2023 [141] | STHdhQ7/Q7 cells | CBD (1 μM) pre-treatment, co-treatment, and post-treatment w/TG | Neuroprotection against TG-induced ER stress through the modulation of pro-survival and pro-apoptotic factors | CBD pre-treatment: ↑ cell-viability; ↑ pro-survival UPR mRNA expression (GRP78, MANF, and BCL-2) and protein levels (GRP78); ↓ pro-apoptotic mRNA expression (BIM and Caspase-12) |
| Alali 2021 [142] | E. coli BL21 | CBD (0, 10, 20, and 40 μM) in tau protein solution (20 μM) | Aggregation of recombinant human His-tagged tau protein expressed through pET-21a (+) vector | CBD: ↓ heparin-induced tau protein aggregation rate and levels |
| First Author and Year | Model | Treatment (CBD) | Primary Outcome Measures | Main Results |
|---|---|---|---|---|
| Esposito 2011 [136] | Aβ-treated male Sprague Dawley rats | 15-day intraperitoneal CBD (10 mg/kg); hippocampal Aβ1–42 (1 μg/mL) | Involvement of PPARγ in the neuroprotective effects of CBD following intrahippocampal injection of Aβ (1–42) | CBD: ↓ iNOS, GFAP, and S100B through PPARγ-dependent inhibition of NF-κB; ↓ reactive gliosis and ↑ neuron survival in rat hippocampus |
| Fagherazzi 2012 [143] | Iron-induced model of ND in male Wistar rats | Intraperitoneal CBD (5 and 10 mg/kg); oral Fe2+ (30 mg/kg; 3 days) | Effects of CBD in iron overload-induced memory impaired rats | CBD: acute highest dose ↓ memory impairment w/chronic treatment; ↑ recognition memory w/chronic treatment; no effect on memory in CBD-treated control rats |
| de Paula Faria 2022 [144] | STZ-induced AD male Wistar rats | 7-day intraperitoneal CBD (20 mg/kg); STZ (3 mg/kg) | Effect of CBD on brain glucose metabolism and cognitive function measured through PET imaging | CBD: ↓ brain glucose hypometabolism and memory damage; ↓ total weight loss |
| Esposito 2007 [145] | Aβ-treated C57BL/6J mice | 7-day intraperitoneal CBD (2.5 or 10 mg/kg); hippocampal Aβ1–42 (10 ng) | Anti-inflammatory and antioxidant effects of CBD in mice with Aβ-induced neuroinflammation | CBD: ↓ GFAP mRNA and protein expression; ↓ iNOS and IL-1β protein levels, and related NO and IL-1β release |
| Kim 2023 [137] | Aβ-treated female ICR mice | Hippocampal CBDA (6 μM); hippocampal Aβ1–42 (3 μM) | Effect of CBDA on Aβ-induced AD-like symptoms and pathology | CBDA: ↓ hippocampal Aβ and p-tau levels; ↑ cognitive function; ↑ hippocampal BDNF, p-TrkB, and p-CREB levels |
| Martín-Moreno 2011 [146] | Aβ-treated C57/Bl6 mice | Intraperitoneal CBD (20 mg/kg); intraventricular Aβ1–40 (2.5 μg) | Effects of CBD compared to other cannabinoids in Aβ-induced memory-deficits and inflammatory cytokine expression | CBD: ↓ Aβ-induced cognitive impairments; ↓ IL6 expression but no effects on TNF-α |
| Arnanz 2023 [147] | 5xFAD mice | 28-day CBD (0.273 mg/kg) and CBD:THC (0.273:0.205 mg/kg) | Neuroprotective effects of chronic low-dose cannabinoid treatment in 5xFAD mice | CBD:THC: ↑ spatial memory All treatments: ↑ cortical insoluble Aβ |
| Cheng 2014 [148] | Male AβPP × PS1 mice | 8-month oral CBD (20 mg/kg) | Effect of chronic CBD treatment on memory, anxiety, Aβ load, oxidative damage, cholesterol, and neuroinflammation in transgenic model of AD | CBD: ↓ social recognition deficits; no effect on anxiety, learning, Aβ load, or oxidative damage; ↑ cholesterol in WT mice; non-sig. ↓ in cytokines |
| Watt 2020 [149] | Male AβPP × PS1 mice | 3-week intraperitoneal CBD (50 mg/kg) | Behavioural and anti-inflammatory effects of chronic CBD administration in transgenic model of AD | CBD: restored social recognition memory and spatial learning deficits; ↓ Aβ in hippocampus; no effect on neuro-inflammation or PPARγ |
| Coles 2020 [150] | Female AβPP × PS1 mice | Chronic intraperitoneal CBD (5 mg/kg) | Behavioural effects of medium-dose chronic CBD treatment administration in transgenic model of AD | CBD: restored object recognition and spatial learning deficits |
| Cheng 2014 [151] | Male AβPP × PS1 mice | 3-week intraperitoneal CBD (20 mg/kg) | Behavioural effects of chronic CBD treatment administration in transgenic model of AD | CBD: restored novel object recognition and social recognition impairments; no changes to anxiety-related behaviours |
| Garcìa-Baos 2021 [152] | PLAE C57BL/6 mice | CBD (20 mg/kg; 10 days) | Anti-inflammatory effects of CBD in a mouse model of FASD | CBD: ↓ cognitive deficits; ↓ PLAE-induced increases in TNF-α and IL6 in hippocampus |
| Wang 2022 [153] | MPTP-induced PD C57BL/6 male mice | 14-day oral CBD (100 mg/kg); intraperitoneal MPTP (30 mg/kg) | Neuroprotective effects of CBD on MPTP-induced PD mice | CBD: ↓ TNF-α, IL6 and IL-1β; ↑ IL-10; ↓ expression of NLRP3, caspase-1, and IL-1β inflammasome |
| Frandsen 2022 [154] | C. elgans transgenic model of AD | CBD (100 μM; 24 h) | Modulation of the glyoxalase pathway and the involvement of Nrf2 and NF-κB in Aβ-expressing C. elgans | CBD: ↑ survival; ↓ Aβ fluorescence; ↑ Nrf2 mediated protein levels through NF-κB inhibition |
| Zhang 2022 [155] | Aβ1–42-treated C. elgans | CBD (100 μM) | Effect of CBD on Aβ aggregation and Aβ-induced AD-like characteristics in C. elgans | CBD: ↓ Aβ aggregation; ↓ ROS independent of antioxidative genes |
| Wang 2023 [135] | C. elgans transgenic model of AD | CBD (5 μM) | Effects of CBD treatment on lifespan, ROS, and pumping rate in Aβ-expressing C. elgans | CBD: ↑ lifespan, ↓ ROS and restored pumping rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hickey, J.P.; Collins, A.E.; Nelson, M.L.; Chen, H.; Kalisch, B.E. Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Curr. Issues Mol. Biol. 2024, 46, 4379-4402. https://doi.org/10.3390/cimb46050266
Hickey JP, Collins AE, Nelson ML, Chen H, Kalisch BE. Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Current Issues in Molecular Biology. 2024; 46(5):4379-4402. https://doi.org/10.3390/cimb46050266
Chicago/Turabian StyleHickey, Jordan P., Andrila E. Collins, Mackayla L. Nelson, Helen Chen, and Bettina E. Kalisch. 2024. "Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease" Current Issues in Molecular Biology 46, no. 5: 4379-4402. https://doi.org/10.3390/cimb46050266
APA StyleHickey, J. P., Collins, A. E., Nelson, M. L., Chen, H., & Kalisch, B. E. (2024). Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Current Issues in Molecular Biology, 46(5), 4379-4402. https://doi.org/10.3390/cimb46050266







