Decreased Memory and Learning Ability Mediated by Bmal1/M1 Macrophages/Angptl2/Inflammatory Cytokine Pathway in Mice Exposed to Long-Term Blue Light Irradiation
Abstract
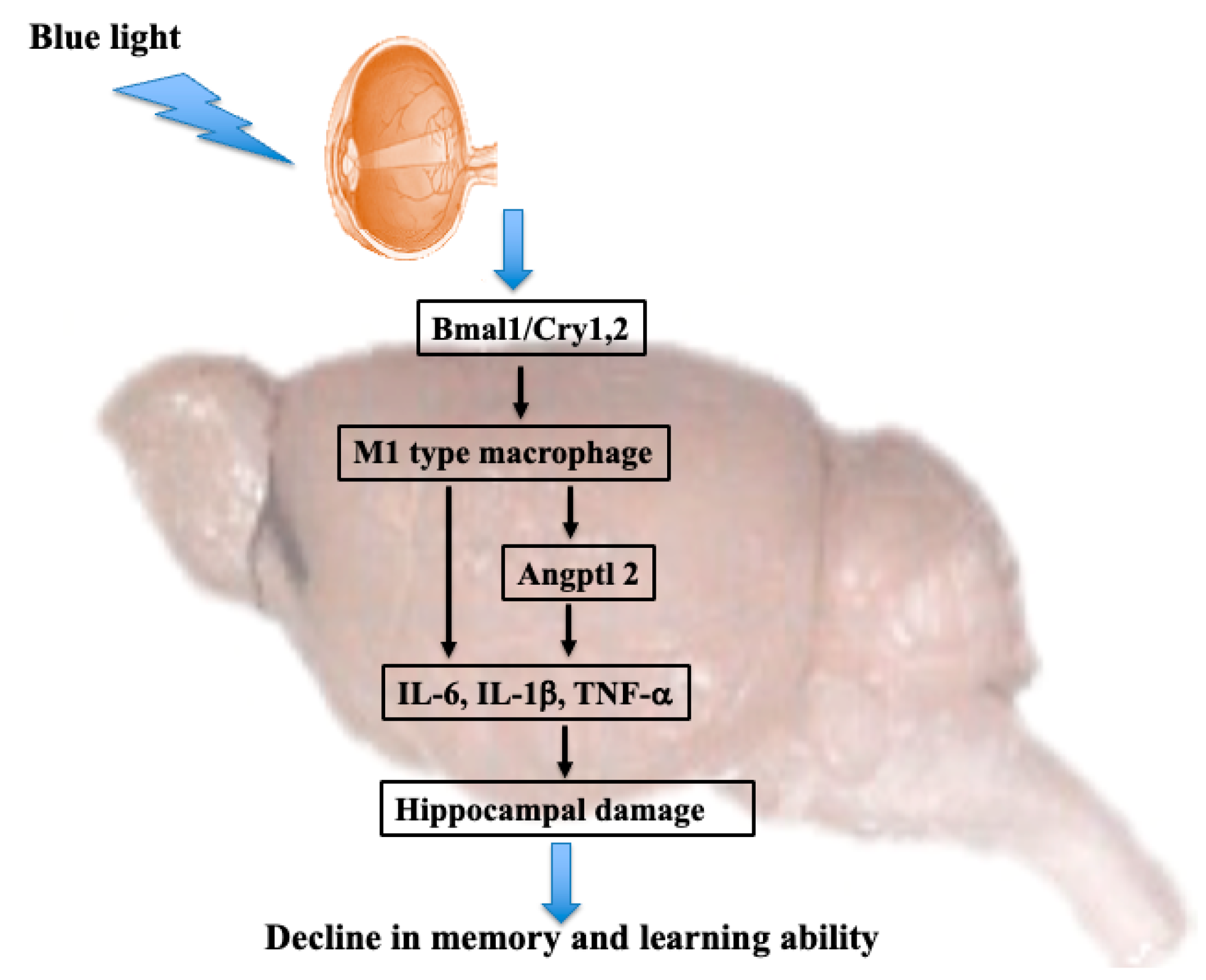
1. Introduction
2. Materials and Methods
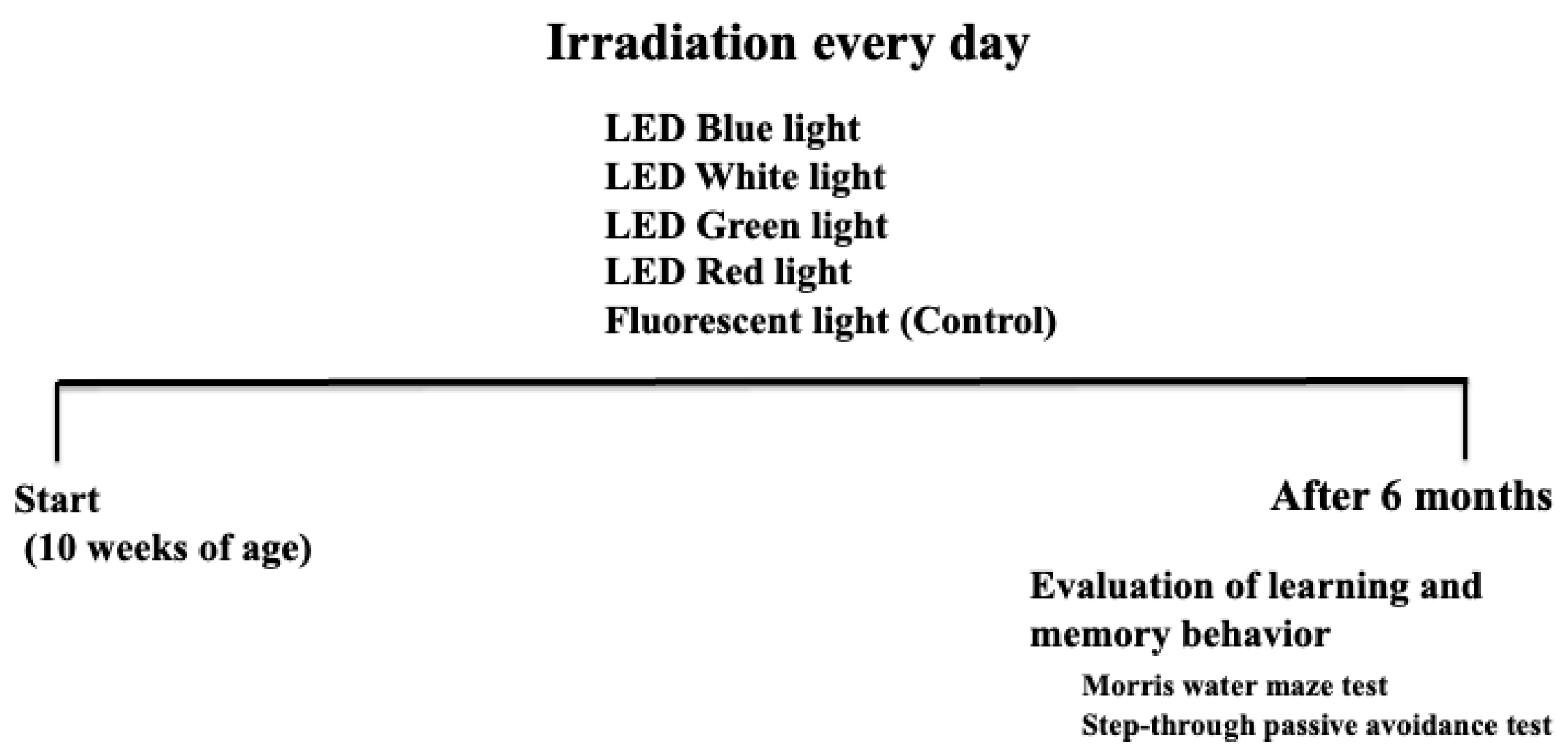
2.1. Animal Experiments
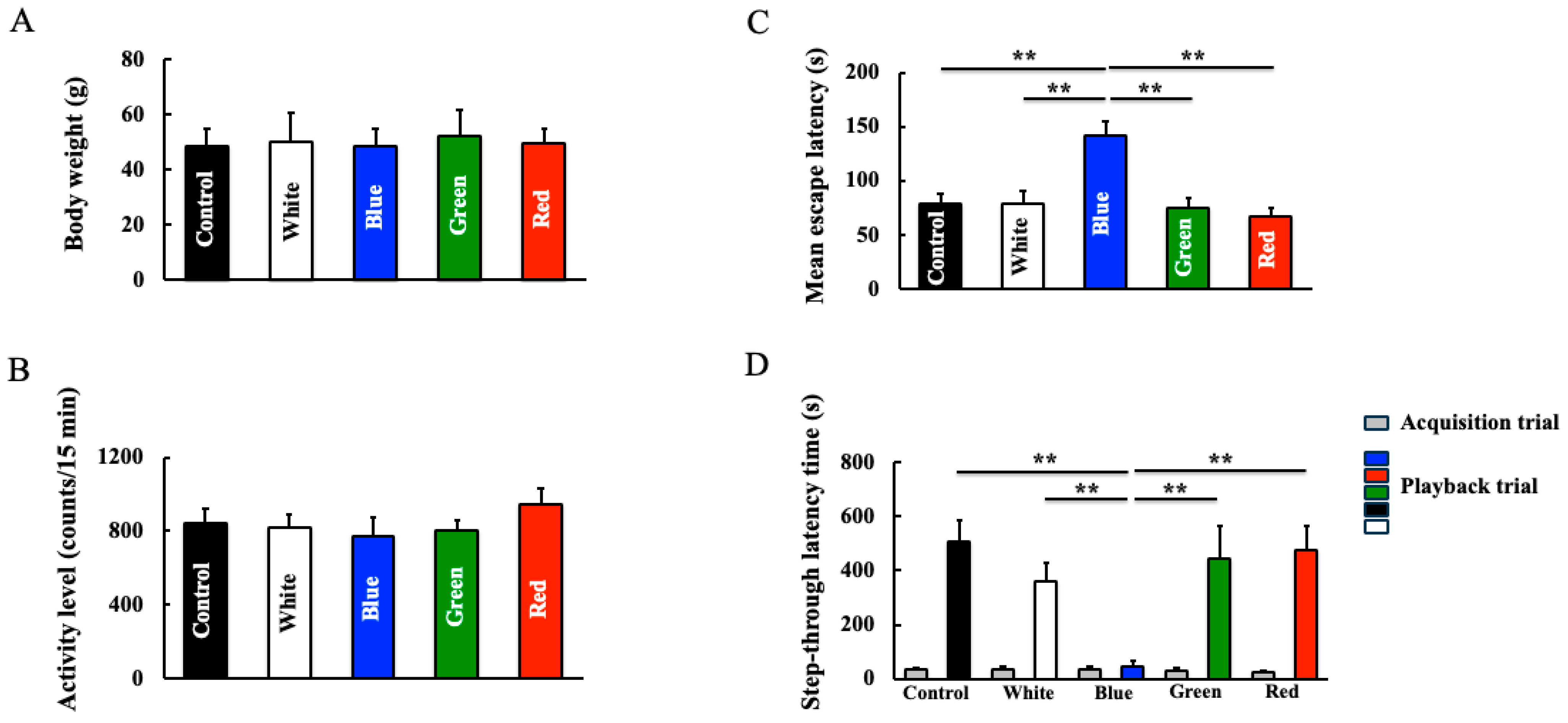
2.2. Spontaneous Locomotor Activity
2.3. Morris Water Maze Test
2.4. Step-Through Passive Avoidance Test
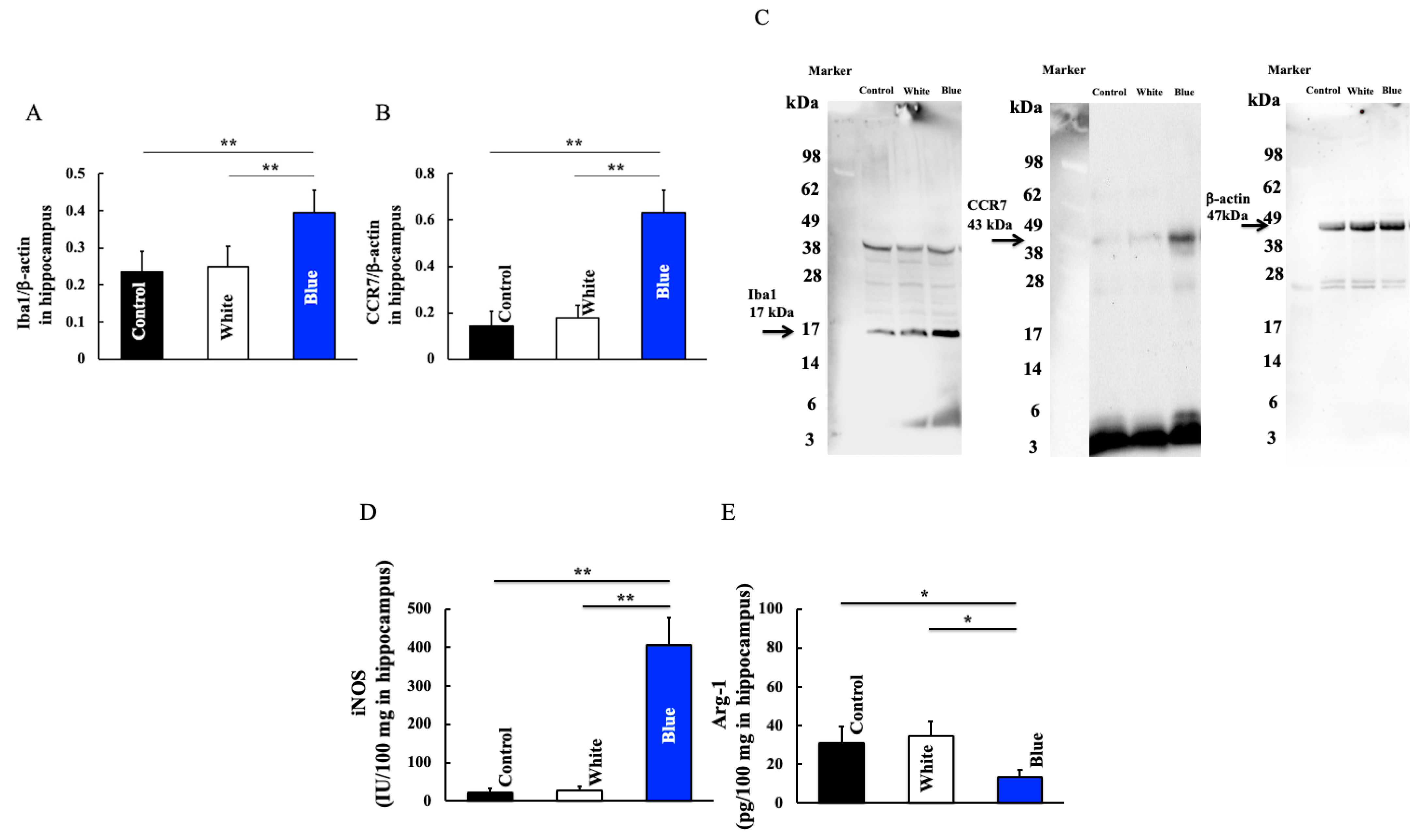
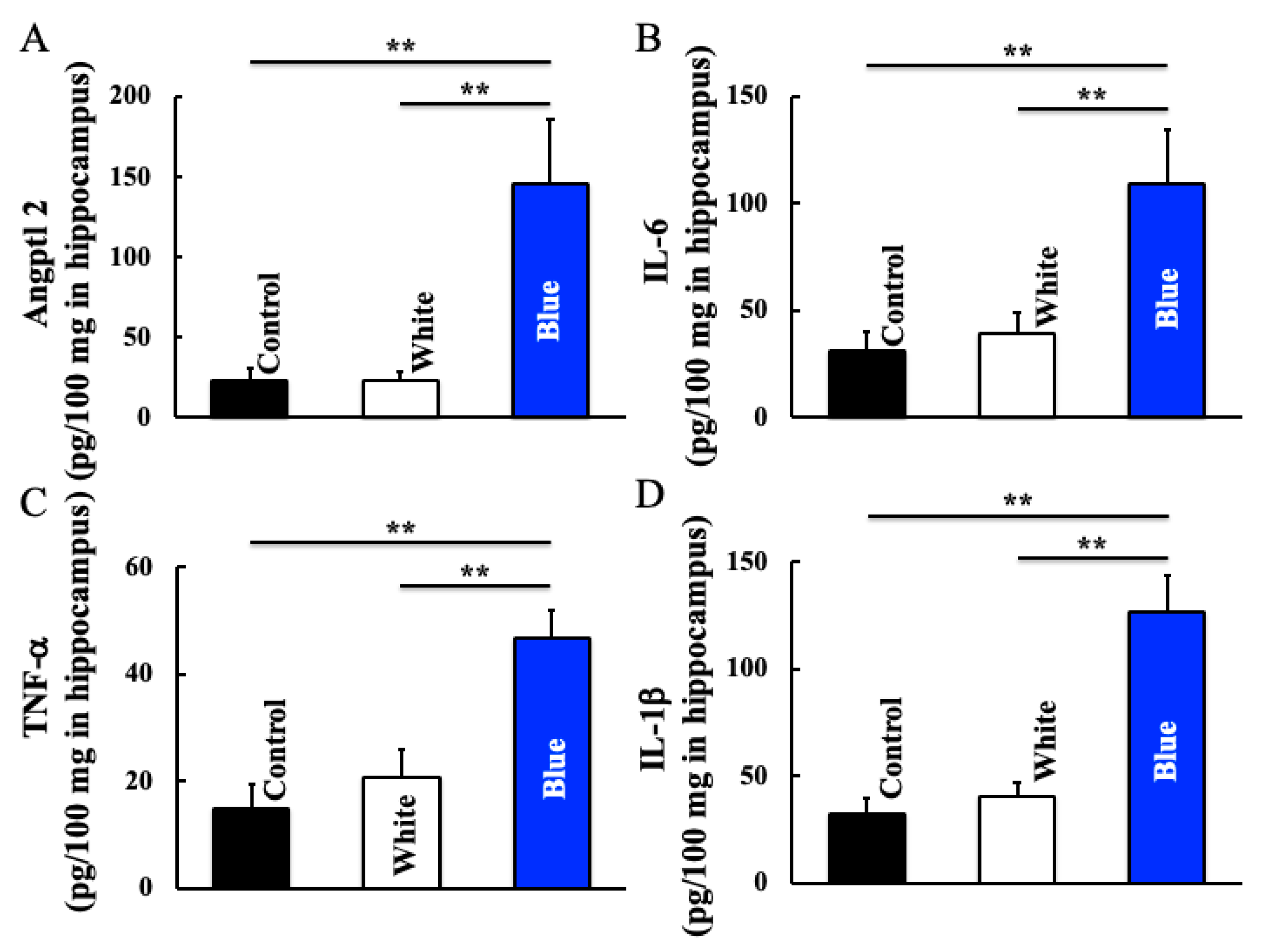
2.5. Measurement of Inducible Nitric Oxide Synthase (iNOS), Arginase-1 (Arg-1), Angiopoietin like Protein 2 (ANGPTL2), Interleukin (IL)-6, Tumor Necrosis Factor (TNF)-α, and IL-1-β Levels in Hippocampus
2.6. Western Blotting Analysis of the Hippocampus Tissue Specimens
2.7. Statistical Analysis
3. Results
3.1. Behavioral Effects of Long-Term Blue Light Irradiation in Mice
3.2. Effect of Long-Term Blue Light Irradiation on the Expression of Bmal1, Cry1, and Cry2 in the Hippocampus
3.3. Effect of Long-Term Blue Light Irradiation on Iba1, CCR7, iNOS, and Arg-1 Levels in the Hippocampus
3.4. Effect of Long-Term Blue Light Irradiation on Angptl2, IL-6, TNF-α, and IL-1β Levels in the Hippocampus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loh, K.; Redd, S. Understanding and preventing computer vision syndrome. Malays. Fam. Physician 2008, 3, 128–130. [Google Scholar] [PubMed]
- Jaiswal, S.; Asper, L.; Long, J.; Lee, A.; Harrison, K.; Gle-Biowski, B. Ocular and visual discomfort associated with smartphones, tablets and computers: What we do and do not know. Clin. Exp. Optom. 2019, 102, 463–477. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Erdinest, N.; Berkow, D. Computer vision syndrome. Harefuah 2021, 160, 386–392. [Google Scholar] [PubMed]
- Auffret, E.; Gomart, G.; Bourcier, T.; Gaucher, D.; Speeg-Schatz, C.; Sauer, A. Digital eye strain. Symptoms, prevalence, pathophysiology, and management. J. Fr. Ophtalmal. 2021, 44, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Toda, I.; Miki, E.; Tsubota, K. Effect of blue light-reducing eye glasses on critical flicker frequency. Asia Pac. J. Ophthalmal. 2015, 4, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Algvere, P.V.; Marshall, J.; Seregard, S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 2006, 84, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Kim, E.W.; St-Onge, M.P.; Westwood, A.J. Blocking nocturnal blue light for insomnia: A randomized controlled trial. J. Psychiatr. Res. 2018, 96, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.; Brown, E.; Czeisler, C. Sensitivity of the human circadian pavemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695–702. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Induction of skin cancer by long-term blue light irradiation. Biomedicines 2023, 11, 2321. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Dumpala, S.; Zele, A.J.; Feigl, B. Outer retinal structure and function deficits contribute to circadian disruption in patients with type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.A.; Blagonravov, M.L.; Goryachev, V.A.; Chibisov, S.M.; Azova, M.M.; Syatkin, S.P. Daytime exposure to blue light alters cardiovascular circadian rhythms, electrolyte excretion and melatonin production. Pathophysiology 2022, 29, 118–133. [Google Scholar] [CrossRef]
- Canazei, M.; Weninger, J.; Pohl, W.; Marksteiner, J.; Weiss, E.H. Effects of dynamic bedroom lighting on measures of sleep and circadian activity rhythm in inpatients with major depressive disorder. Sci. Rep. 2022, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, S.; Gui, Q.; Tang, K.; Xu, Q.; Tao, Y.; Chen, M.; Cheng, J.; Wang, L.; Zhang, L. Blue light insertion at night is involved in sleep and arousal-promoting response delays and depressive-like emotion in mice. Biosci. Rep. 2021, 41, BSR20204033. [Google Scholar] [CrossRef]
- Silva, E.H.A.; Santana, N.N.M.; Seixas, N.R.M.; Bezerra, L.L.F.; Silva, M.M.O.; Santos, S.F.; Cavalcante, J.S.; Leocadio-Miguel, M.A.; Engelberth, R.C. Blue light exposure-dependent improvement in robustness of circadian rest-activity rhythm in aged rats. PLoS ONE 2023, 18, e0292342. [Google Scholar] [CrossRef]
- Nash, T.R.; Chow, E.S.; Law, A.D.; Fu, S.D.; Fuszara, E.; Bilska, A.; Bebas, P.; Kretzschmer, D.; Giebultowics, J.M. Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. NPJ Aging Mech. Dis. 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Vanuk, J.R.; Shane, B.R.; Weber, M.; Bajaj, S. A randomized, double-blind, placebo-controlled tried of blue wavelength light exposure on sleep and recovery of brain structure, function, and congnition following mild traumatic brain injury. Neurobiol. Dis. 2020, 134, 104679. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kasahara, E. Long-term UVA eye irradiation causes decreased learning ability in mice. Photodermatol. Photoimmunol. Photomed. 2016, 32, 129–135. [Google Scholar] [CrossRef]
- Hiramoto, K.; Okada, T.; Ito, M. Abnormalities in behavior, learning ability, and the cholinergic system induced by long-term ultraviolet A irradiation of mice. Neuropsychobiology 1996, 33, 182–185. [Google Scholar] [CrossRef]
- Jacobs, G.H.; Neitz, J.; Deegan, J.F. Retinal eceptors in rodents maximally sensitive to ultraviolet light. Nature 1991, 353, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, C.J.; Shin, M.S.; Lim, B.V. Treadmill exercise ameliorates memory impairment through ERK-Akt-CREB-BDNF signaling pathway in cerebral ischemia gerbils. J. Exerc. Rehabil. 2020, 16, 49–57. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yamate, Y.; Sugiyama, D.; Matsuda, K.; Iizuka, Y.; Yamaguchi, T. Tranexamic acid ameliorates nonmelanoma skin cancer induced by long-term ultraviolet A irradiation. Photochem. Photobiol. 2019, 95, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Du, L.; Xu, Y.; Chen, L.; Wu, Y. The effect of blue light exposure on the expression of circadian genes: Bmal1 and cryptochrome 1 in peripheral blood mononuclear cells of jaundiced neonates. Pediatr. Res. 2005, 58, 1180–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, R.K.; Liou, Y.H.; Knudsen, N.H.; Starost, K.A.; Xu, C.; Hyde, A.L.; Liu, S.; Jacobi, D.; Liao, N.S.; Lee, C.H. Bmal1 integrates mitochondrial metabolism and macrophage activation. eLife 2020, 9, e54090. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Moraes, C.T.; Murphy, M.P.; Mudinger, G.R.; Chandel, N.S. The qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007, 177, 1029–1036. [Google Scholar] [CrossRef]
- Lens, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Early, J.D.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ruan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Motokawa, I.; Endo, M.; Terada, K.; Horiguchi, H.; Miyata, K.; Kadomatsu, T.; Morinaga, J.; Sugizaki, T.; Ito, T.; Araki, K.; et al. Interstitial pneumonia induced by bleomycin treatment is exacerbated in Angptl2-deficient mice. Am. J. Phusiol. Lung Cell Mol. Physiol. 2016, 311, L704–L713. [Google Scholar] [CrossRef]
- Horiguchi, H.; Motoyoshi, E.; Kawane, K.; Kadomatsu, T.; Terada, K.; Morinaga, J.; Araki, K.; Miyata, K.; Oike, Y. ANGPTL2 expression in the intestinal stem cell niche controls epithelial regeneration and homeostasis. EMBO J. 2017, 36, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, T.; Endo, M.; Miyata, K.; Oike, Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol. Metab. 2014, 25, 245–254. [Google Scholar] [CrossRef]
- Tabata, M.; Kadomatsu, T.; Fukuhara, S.; Miyata, K.; Ito, Y.; Endo, M.; Urano, T.; Zhu, H.J.; Tsukano, H.; Tazume, H.; et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009, 10, 178–188. [Google Scholar] [CrossRef]
- Odagiri, H.; Kadomatsu, T.; Endo, M.; Masuda, T.; Morioka, M.S.; Fukuhara, S.; Miyamoto, T.; Kobayashi, E.; Miyata, K.; Aoi, J.; et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci. Signal 2014, 7, ra7. [Google Scholar] [CrossRef]
- Doi, Y.; Ninamiya, T.; Hirakawa, Y.; Takahashi, O.; Mukai, N.; Hata, J.; Iwase, M.; Kitazono, T.; Oike, Y.; Kiyohara, T. Angiopoietin-like protein 2 and risk of type 2 diabetes in a general Japanease population: The hisayama study. Diabetes Care 2013, 36, 98–100. [Google Scholar] [CrossRef]
- Aoi, J.; Endo, M.; Kadomatsu, T.; Miyata, K.; Nakano, M.; Horiguchi, H.; Ogata, A.; Odagiri, H.; Yano, M.; Araki, K.; et al. Angiopoietin-loke protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011, 71, 7502–7512. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; de Water, J.V. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Enstrom, A.M.; Onore, C.E.; de Water, J.A.V.; Ashwood, P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 2010, 24, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shiwaku, H.; Tanaka, H.; Obita, T.; Ohuchi, S.; Yoshioka, Y.; Jin, X.; Kondo, K.; Fujita, K.; Homma, H.; et al. Tau activates microglia via the PQBPI-cGAS-STING pathway to promote brain inflammation. Nat. Commun. 2021, 12, 6565. [Google Scholar] [CrossRef]
- Griffin, W.S.; Sheng, J.G.; Royston, M.C.; Gentleman, S.M.; McKenzie, J.E.; Graham, D.I.; Roberts, G.W.; Mrak, R.E. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Bahtiyar, S.; Karaca, K.G.; Henckens, M.J.A.G.; Roozendaal, B. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol. Cell Neurosci. 2020, 108, 103537. [Google Scholar] [CrossRef]
- Joels, M.; Sarabdjitsingh, R.A.; Karst, H. Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacol. Rev. 2012, 64, 901–938. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic mediators of synapse development and plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Morris, G.P.; Clark, I.A.; Zinn, R.; Vissel, B. Microglia: A new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol. Learn. Mem. 2013, 105, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimming, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011, 31, 16241–16250. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Wang, Q.; Zhang, D.; Zhao, Q.; Zhang, J.; Xie, L.; Liu, G.; You, Z. Minocycline inhibits microlial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, J.L.; Tarr, A.J.; Wohleb, E.S.; Godbout, J.P.; Mo, X.; Sheridan, J.F.; Eubank, T.D.; Marsh, C.B. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE 2013, 8, e58488. [Google Scholar] [CrossRef]
- Huberman, A.D.; Niell, C.M. What can mice tell us about how vision works? Trends Neurosci. 2011, 34, 464–473. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Decreased Memory and Learning Ability Mediated by Bmal1/M1 Macrophages/Angptl2/Inflammatory Cytokine Pathway in Mice Exposed to Long-Term Blue Light Irradiation. Curr. Issues Mol. Biol. 2024, 46, 4924-4934. https://doi.org/10.3390/cimb46050295
Hiramoto K, Kubo S, Tsuji K, Sugiyama D, Hamano H. Decreased Memory and Learning Ability Mediated by Bmal1/M1 Macrophages/Angptl2/Inflammatory Cytokine Pathway in Mice Exposed to Long-Term Blue Light Irradiation. Current Issues in Molecular Biology. 2024; 46(5):4924-4934. https://doi.org/10.3390/cimb46050295
Chicago/Turabian StyleHiramoto, Keiichi, Sayaka Kubo, Keiko Tsuji, Daijiro Sugiyama, and Hideo Hamano. 2024. "Decreased Memory and Learning Ability Mediated by Bmal1/M1 Macrophages/Angptl2/Inflammatory Cytokine Pathway in Mice Exposed to Long-Term Blue Light Irradiation" Current Issues in Molecular Biology 46, no. 5: 4924-4934. https://doi.org/10.3390/cimb46050295
APA StyleHiramoto, K., Kubo, S., Tsuji, K., Sugiyama, D., & Hamano, H. (2024). Decreased Memory and Learning Ability Mediated by Bmal1/M1 Macrophages/Angptl2/Inflammatory Cytokine Pathway in Mice Exposed to Long-Term Blue Light Irradiation. Current Issues in Molecular Biology, 46(5), 4924-4934. https://doi.org/10.3390/cimb46050295






