Network Pharmacology Analysis, Molecular Docking Integrated Experimental Verification Reveal the Mechanism of Gynostemma pentaphyllum in the Treatment of Type II Diabetes by Regulating the IRS1/PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction and UPLC-HRMS Analysis of Compounds from GP
2.3. Active Compounds Screening and Targets Fishing
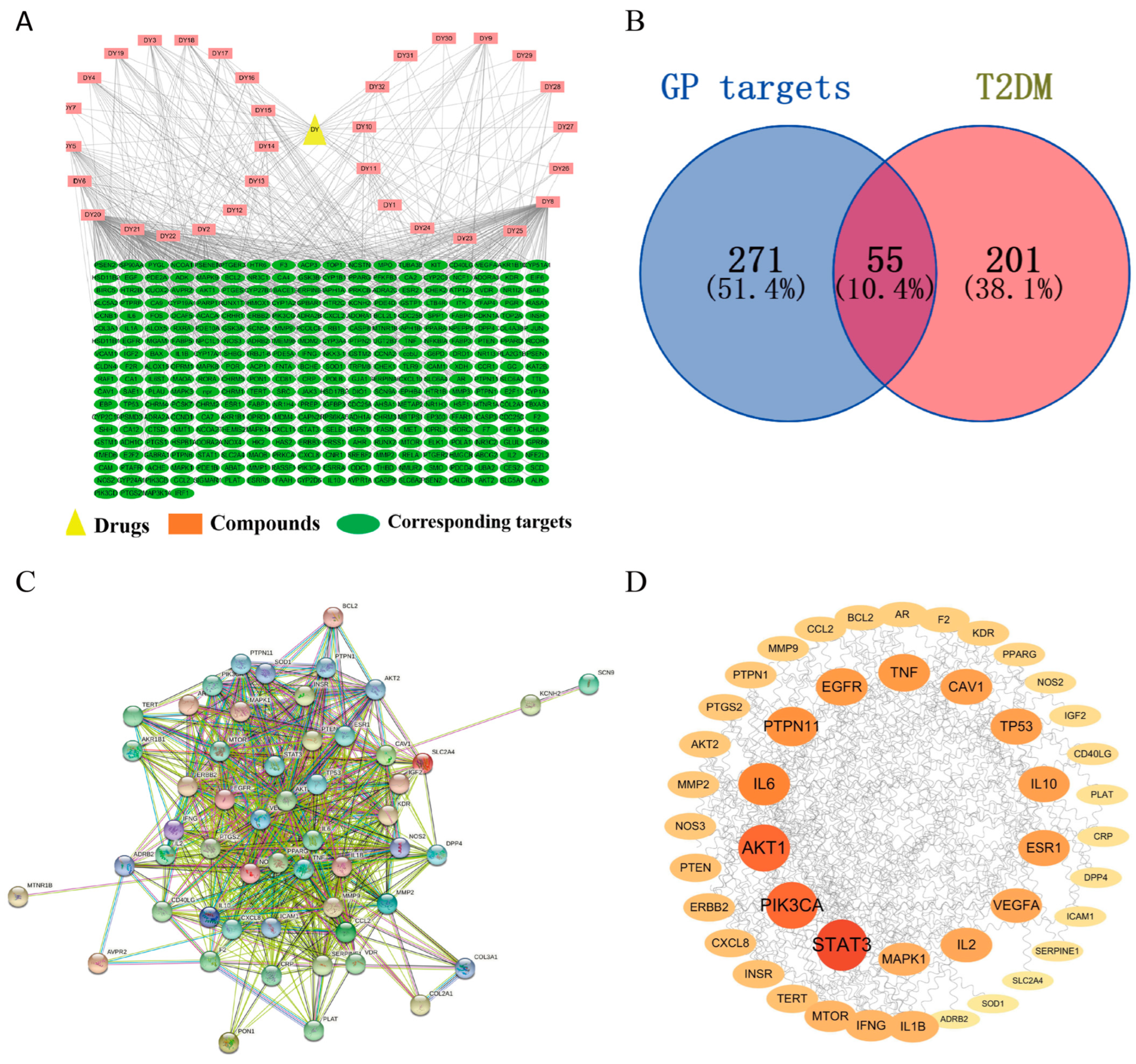
2.4. Construction and Analysis of Drug–Component–Target Interaction
2.5. Venny Analysis
2.6. Construction of the PPI Network
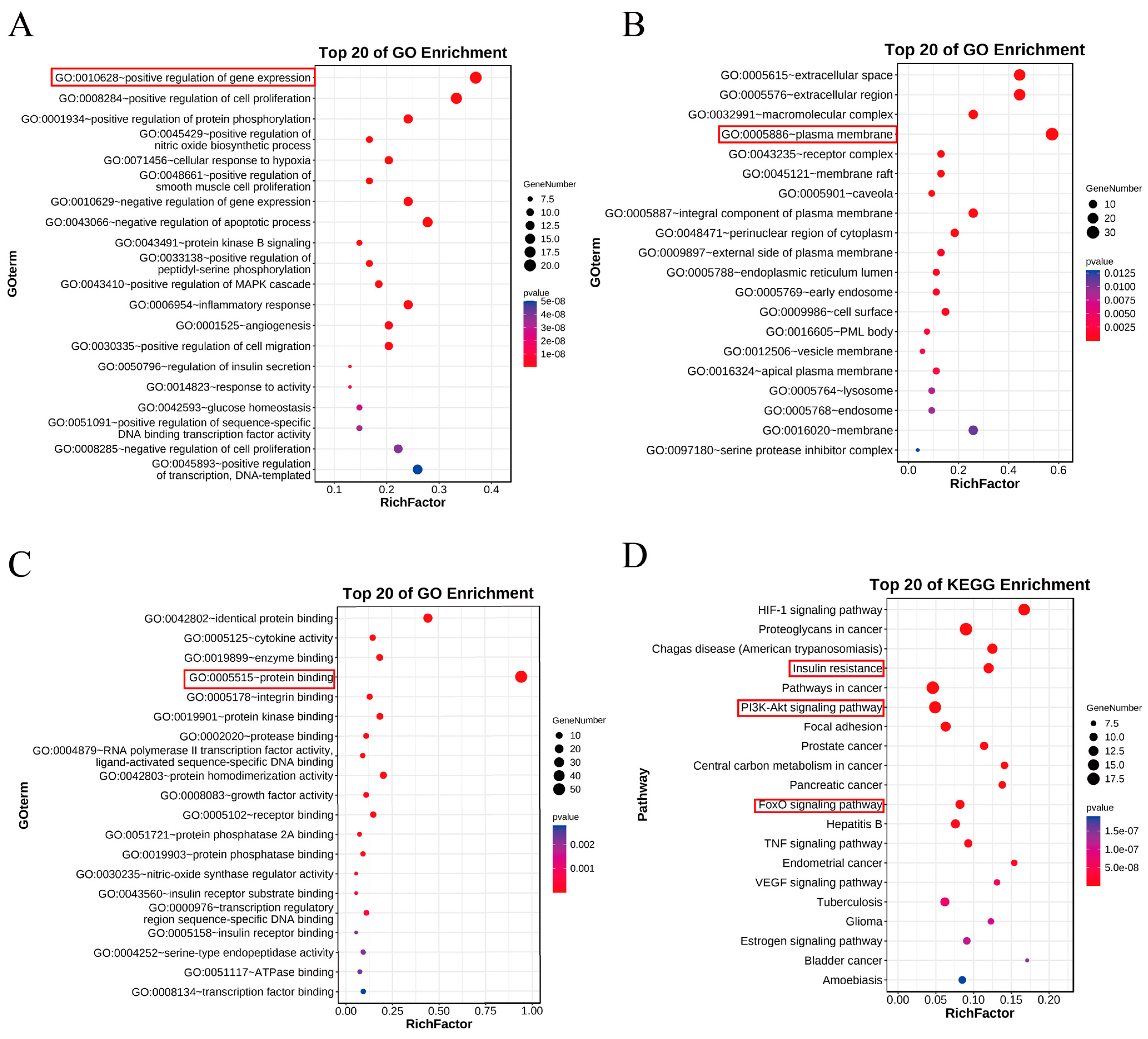
2.7. Functional Enrichment Analysis
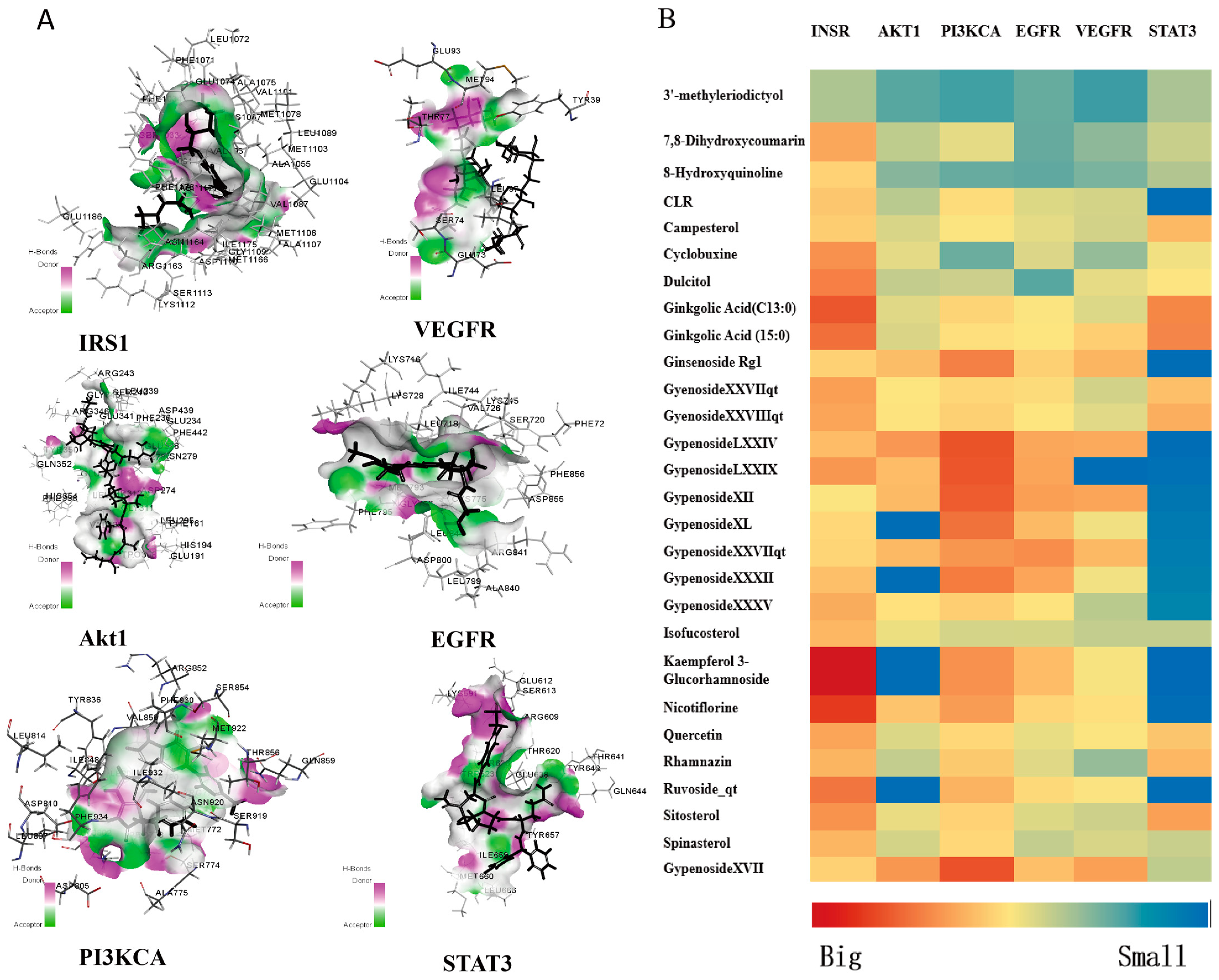
2.8. Component–Target Molecular Docking
2.9. Cell Culture
2.10. Cytotoxicity Was Detected by MTT Assay
2.11. Glucose Consumption Assay
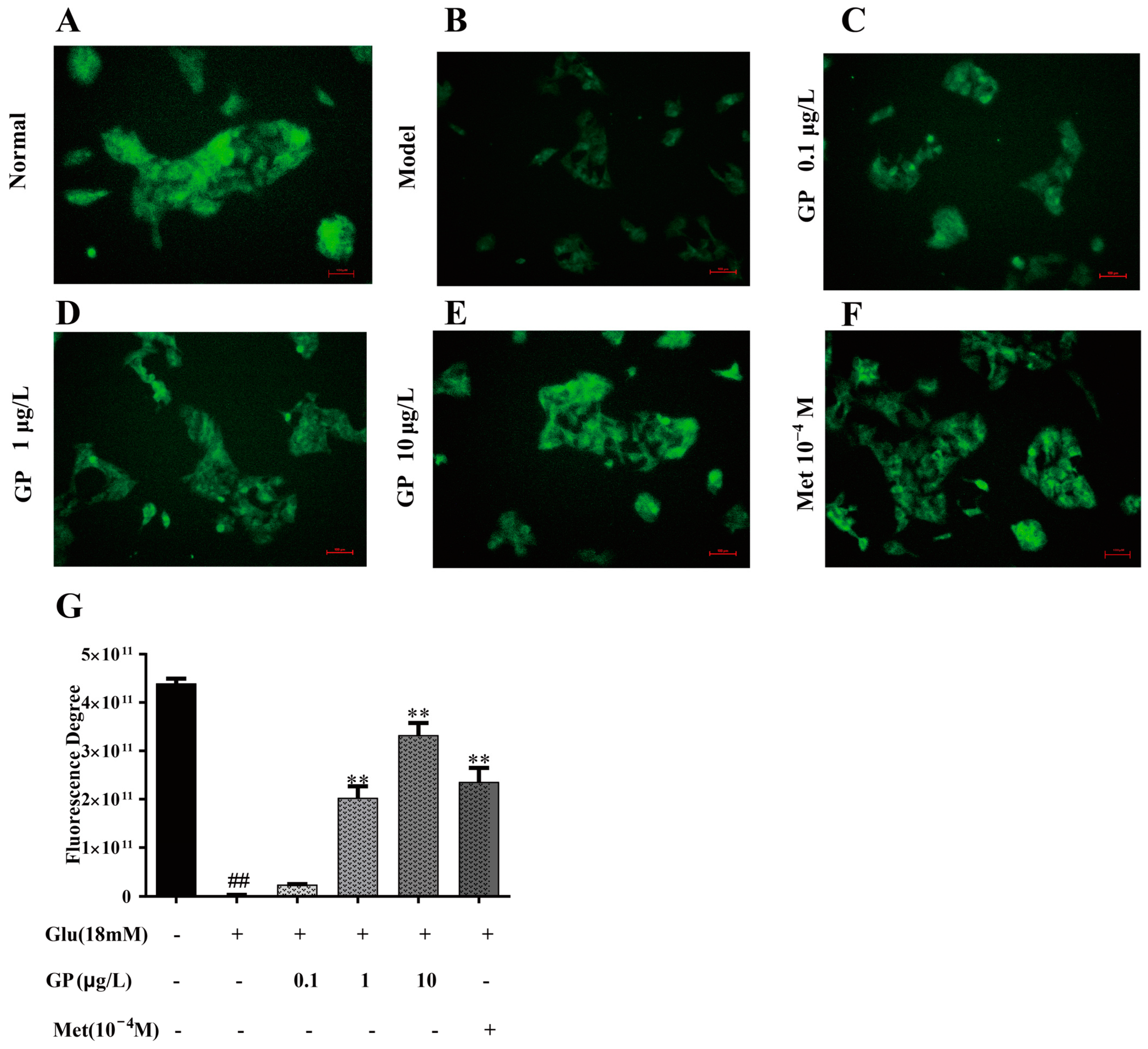
2.12. 2-NBDG Glucose Uptake Assay
2.13. Western Blot Assay
2.14. Statistic Analysis
3. Results
3.1. The Screening of Active Compounds and Targets Fishing
3.2. Construction and Analysis of Drug–Component–Target Interaction
3.3. Prediction of Gene Targets of T2DM
3.4. Construction of the PPI Network
3.5. GO and KEGG Enrichment Analysis
3.6. Molecular Docking Studies of Active Components with Key Targets
3.7. Effect of GP Extract on Glucose Consumption in IR HepG2 Cells
3.8. GP Extract Increased Glucose Uptake in Glucosamine-Induced HepG2 Cells
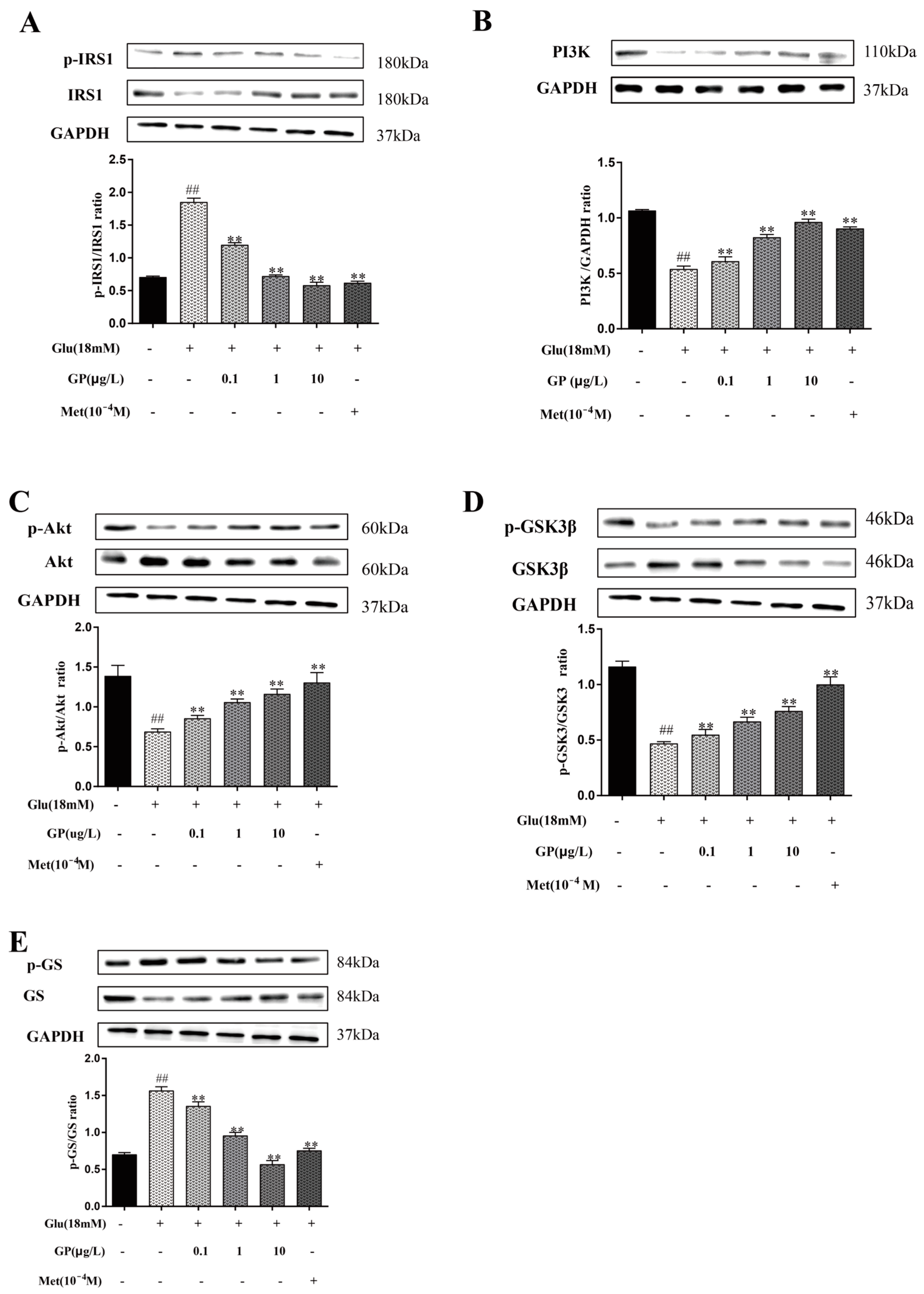
3.9. Effect of GP Extract on the IRS-1/PI3K/AKT Signaling Pathway in Glucosamine-Induced HepG2 Cells
3.10. Effect of GP Extract on the Expression of G6Pase and PEPCK in IR-HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogle, G.D.; James, S.; Dabelea, D.; Pihoker, C.; Svennson, J.; Maniam, J.; Klatman, E.L.; Patterson, C.C. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res. Clin. Pract. 2022, 183, 109083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, F.; Liu, X.; Wang, L.; Chen, B.; Li, L.; Wang, M. Cucurbitane glycosides from the fruit of Siraitia grosvenori and their effects on glucose uptake in human HepG2 cells in vitro. Food Chem. 2017, 228, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-diabetic activity evaluation of a polysaccharide extracted from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Mu, J.; Li, D.; Ma, Y.; Meng, X. Role of Wnt signaling pathways in type 2 diabetes mellitus. Mol. Cell. Biochem. 2021, 476, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Vijan, S. Type 2 Diabetes. Ann. Intern. Med. 2020, 172, 705. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, H.; Li, X.; Peng, X.; Rao, G.; Xie, Z.; Yang, Q.; Du, L.; Xie, C. Modular characteristics and mechanism of action of herbs for type 2 diabetes treatment in Chinese medicine. Heliyon 2023, 9, e20106. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Han, L.; Zhao, L.; Tong, X. Exploring the Mechanisms of Berberine against Type 2 Diabetes Mellitus and Its Complications by Network Pharmacology. Diabetes 2019, 68, 2469-PUB. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Wang, Y.-S.; Liu, Y.-Y.; Jiang, C.-H.; Wang, J.; Jiang, X.-Y.; Liu, B.-W.; Wang, L.; Ye, W.-C.; Zhang, J.; et al. Chemical Characterization and Atherosclerosis Alleviation Effects of Gypenosides from Gynostemma pentaphyllum through Ameliorating Endothelial Dysfunction via the PCSK9/LOX-1 Pathway. J. Agric. Food Chem. 2022, 70, 11944–11957. [Google Scholar] [CrossRef]
- Xie, P.; Luo, H.-T.; Pei, W.-J.; Xiao, M.-Y.; Li, F.-F.; Gu, Y.-L.; Piao, X.-L. Saponins derived from Gynostemma pentaphyllum regulate triglyceride and cholesterol metabolism and the mechanisms: A review. J. Ethnopharmacol. 2024, 319, 117186. [Google Scholar] [CrossRef]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Antidiabetic Effect of Gynostemma pentaphyllum Tea in Randomly Assigned Type 2 Diabetic Patients. Horm. Metab. Res. 2010, 42, 353–357. [Google Scholar] [CrossRef]
- Norberg, A.; Hoa, N.K.; Liepinsh, E.; Van Phan, D.; Thuan, N.D.; Jornvall, H.; Sillard, R.; Ostenson, C.-G. A novel insulin-releasing substance, phanoside, from the plant Gynostemma pentaphyllum. J. Biol. Chem. 2004, 279, 41361–41367. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.K.; Norberg, A.; Sillard, R.; Van Phan, D.; Thuan, N.D.; Dzung, D.T.N.; Jornvall, H.; Ostenson, C.-G. The possible mechanisms by which phanoside stimulates insulin secretion from rat islets. J. Endocrinol. 2007, 192, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. A Publ. Can. Soc. Pharm. Sci. Soc. Can. Des Sci. Pharm. 2006, 9, 281–291. [Google Scholar]
- Ji, X.; Shen, Y.; Guo, X. Isolation, Structures, and Bioactivities of the Polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A Review. Biomed Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, W.; Li, B.; Bao, P.; Wang, F.; Sun, J.; Song, G.; Yin, L.; Nan, Z. Efficacy and safety of acupuncture combined with Chinese Herbal Medicine for diabetic nephropathy A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27087. [Google Scholar] [CrossRef] [PubMed]
- Lokman, E.F.; Gu, H.F.; Mohamud, W.N.W.; Ostenson, C.-G. Evaluation of Antidiabetic Effects of the Traditional Medicinal Plant Gynostemma pentaphyllum and the Possible Mechanisms of Insulin Release. Evid.-Based Complement. Altern. Med. 2015, 2015, 120572. [Google Scholar] [CrossRef]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; Lopez, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Gan, L.; Jia, L.-Y.; Zhou, D.-C.; Bi, S.; Meng, Z.-Q.; Guan, G.-J.; Huang, M.-M.; He, X.; Zhang, C.-F.; et al. Screen of anti-migraine active compounds from Duijinsan by spectrum-effect relationship analysis and molecular docking. J. Ethnopharmacol. 2021, 279, 114352. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Fang, B.; Zhang, B.; Xu, B.; de la Cruz, C.B.V.; El-Seedi, H.; Simal-Gandara, J.; Wang, S.; et al. Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNK-IRS-1 and PI3K/Akt signaling pathways. Food Chem. 2021, 335, 127513. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Ren, D.; Li, T.; Niu, P.; Zhang, X.; Yang, X.; Xiao, J. Fu Brick Tea Manages HFD/STZ-Induced Type 2 Diabetes by Regulating the Gut Microbiota and Activating the IRS1/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2022, 70, 8274–8287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.-H. Activation of the PI3K/Akt Pathway by Oxidative Stress Mediates High Glucose-Induced Increase of Adipogenic Differentiation in Primary Rat Osteoblasts. J. Cell. Biochem. 2013, 114, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Shi, C.-X.; Gao, R.; Sun, H.-J.; Xiong, X.-Q.; Ding, L.; Chen, Q.; Li, Y.-H.; Wang, J.-J.; Kang, Y.-M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, Y.; Zeng, N.; Stiles, B.L. Regulation of basal expression of hepatic PEPCK and G6Pase by AKT2. Biochem. J. 2020, 477, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, M.; Guo, R.; Ding, Y.; Zhang, H.; He, Y. The improvement of sulforaphane in type 2 diabetes mellitus (T2DM) and related complications: A review. Trends Food Sci. Technol. 2022, 129, 397–407. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 2158–2171. [Google Scholar] [CrossRef]
- Lundqvist, L.C.E.; Rattigan, D.; Ehtesham, E.; Demmou, C.; Ostenson, C.-G.; Sandstrom, C. Profiling and activity screening of Dammarane-type triterpen saponins from Gynostemma pentaphyllum with glucose-dependent insulin secretory activity. Sci. Rep. 2019, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Merovci, A.; Abdul-Ghani, M.; Mari, A.; Solis-Herrera, C.; Xiong, J.; Daniele, G.; Tripathy, D.; DeFronzo, R.A. Effect of Dapagliflozin With and Without Acipimox on Insulin Sensitivity and Insulin Secretion in T2DM Males. J. Clin. Endocrinol. Metab. 2016, 101, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Khan, S.; Wani, S.A. Controlling diabetes with the aid of medicinal herbs: A critical compilation of a decade of research. Crit. Rev. Food Sci. Nutr. 2022, 63, 12552–12566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef] [PubMed]
- He, C.-J.; Ma, L.-Q.; Iqbal, M.S.; Huang, X.-J.; Li, J.; Yang, G.-Z.; Ihsan, A. Veratrilla baillonii Franch exerts anti-diabetic activity and improves liver injury through IRS/PI3K/AKT signaling pathways in type 2 diabetic db/db mice. J. Funct. Foods 2020, 75, 104204. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Sai, Z. Study on the Prognosis Effect of Traditional Chinese Medicine Treatment in DR Patients Based on the Perspective of Network Pharmacology. Contrast Media Mol. Imaging 2022, 2022, 3528732. [Google Scholar] [CrossRef] [PubMed]
- Phi Hung, N.; Gauhar, R.; Hwang, S.L.; Trong Tuan, D.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorganic Med. Chem. 2011, 19, 6254–6260. [Google Scholar]
- Song, M.; Tan, D.; Li, B.; Wang, Y.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Wellness 2022, 11, 1347–1354. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Y.; Wu, W.X. Analysis on the improvement of blood sugar and myocardial cells in patients with type 2 diabetes treated with Gynostemma pentaphyllum combined with repaglinide. Diabetes New World 2023, 26, 75–77+81. [Google Scholar]
- Yeo, J.; Kang, Y.-J.; Jeon, S.-M.; Jung, U.J.; Lee, M.-K.; Song, H.; Choi, M.-S. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J. Med. Food 2008, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhao, M.; Qi, X.; Liu, Y.; Li, N.; Liu, Z.; Bian, Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch. Pharmacal Res. 2016, 39, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Elmoghayer, M.E.; Saleh, N.M.; Abu Hashim, I.I. Enhanced oral delivery of hesperidin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for augmenting its hypoglycemic activity: In vitro-in vivo assessment study. Drug Deliv. Transl. Res. 2023, 14, 895–917. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.-Y.; Kwon, H.C.; Kwon, J.; Jang, D.S.; Kang, K.S. Dual Beneficial Effects of α-Spinasterol Isolated from Aster pseudoglehnii on Glucose Uptake in Skeletal Muscle Cells and Glucose-Stimulated Insulin Secretion in Pancreatic β-Cells. Plants 2022, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, M.-Y.; Li, T.; Jin, Z.-M.; Liu, Z.; Liu, Q.-Y.; Liu, Y.; Ding, J.-Y.; Jiang, H.; Hou, X. Study on Optimal Extraction and Hypoglycemic Effect of Quercetin. Evid.-Based Complement. Altern. Med. Ecam 2023, 2023, 8886503. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Zhang, H.; Li, L.; Wang, J.; Su, S.; Zhang, Y.; Song, L.; Zhou, K. Gypenoside XVII attenuates renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-triggered pyroptosis. Eur. J. Pharmacol. 2024, 962, 176187. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, R.; Cai, F.-F.; Zhou, W.-J.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Su, S.-B. Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FoxO3 signaling pathways based on network pharmacology analysis. J. Ethnopharmacol. 2021, 264, 113021. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Lai, Z.; Huang, J.; Li, C.; Zhang, Y.; Gong, X.; Deng, J.; Ye, X.; Li, X. An integrated network pharmacology and transcriptomic method to explore the mechanism of the total Rhizoma Coptidis alkaloids in improving diabetic nephropathy. J. Ethnopharmacol. 2021, 270, 113806. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, H.; Zhan, W.; Song, L.; Zhang, D.; Chen, X.; Lin, Z.; Wang, W.; Yang, Y.; Wang, L.; et al. Molecular mechanism of Fufang Zhenzhu Tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs. J. Ethnopharmacol. 2021, 274, 114056. [Google Scholar] [CrossRef]
- An, W.; Huang, Y.; Chen, S.; Teng, T.; Shi, Y.; Sun, Z.; Xu, Y. Mechanisms of Rhizoma Coptidis against type 2 diabetes mellitus explored by network pharmacology combined with molecular docking and experimental validation. Sci. Rep. 2021, 11, 20849. [Google Scholar] [CrossRef]
- Himanshu, D.; Ali, W.; Wamique, M. Type 2 diabetes mellitus: Pathogenesis and genetic diagnosis. J. Diabetes Metab. Disord. 2020, 19, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Y.; Sun, L.; Fang, Z.; Chen, L.; Zhao, P.; Wang, Z.; Guo, Y. Effect of young apple (Malus domestica Borkh. cv. Red Fuji) polyphenols on alleviating insulin resistance. Food Biosci. 2020, 36, 100637. [Google Scholar] [CrossRef]
- Gong, P.; Xiao, X.; Wang, S.; Shi, F.; Liu, N.; Chen, X.; Yang, W.; Wang, L.; Chen, F. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway (vol 281, 114558, 2021). J. Ethnopharmacol. 2023, 313, 116629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Nakayama, M.; Hisatsune, J.; Yamasaki, E.; Isomoto, H.; Kurazono, H.; Hatakeyama, M.; Azuma, T.; Yamaoka, Y.; Yahiro, K.; Moss, J.; et al. Helicobacter pylori VacA-induced Inhibition of GSK3 through the PI3K/Akt Signaling Pathway. J. Biol. Chem. 2009, 284, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Biggs, W.H., 3rd; Kitamura, T.; Cavenee, W.K.; Wright, C.V.E.; Arden, K.C.; Accili, D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Shi, J.; Miao, D.; Tan, D.; Zhao, C.; Xiong, Z.; Zhang, X. miR-1182-mediated ALDH3A2 inhibition affects lipid metabolism and progression in ccRCC by activating the PI3K-AKT pathway. Transl. Oncol. 2024, 40, 101835. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-Akt signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]




| NO | Molecular Name | CAS | OB (%) | DL | Molecular Weight |
|---|---|---|---|---|---|
| DY1 | Homoeriodictyol | 446-71-9 | 51.61 | 0.27 | 302.28 |
| DY2 | Rhamnazin | 552-54-5 | 47.14 | 0.34 | 330.31 |
| DY3 | Sitosterol | 68555-08-8 | 36.91 | 0.75 | 414.79 |
| DY4 | Ruvoside_qt | 6859-20-7 | 36.12 | 0.76 | 390.57 |
| DY5 | Spinasterol | 481-18-5 | 42.98 | 0.76 | 412.77 |
| DY6 | Campesterol | 474-62-4 | 37.58 | 0.71 | 400.76 |
| DY7 | Isofucosterol | 481-14-1 | 43.78 | 0.76 | 412.77 |
| DY8 | Ginsenoside f2 | 62025-49-4 | 36.43 | 0.25 | 785.14 |
| DY9 | CLR | 80356-14-5 | 37.87 | 0.68 | 386.73 |
| DY10 | Quercetin | 73123-10-1 | 46.43 | 0.28 | 302.25 |
| DY11 | Cyclobuxine | 2241-90-9 | 84.48 | 0.70 | 386.69 |
| DY12 | Gypenoside LXXIV | 110261-97-7 | 34.21 | 0.24 | 801.14 |
| DY13 | Gypenoside LXXIX | 110282-46-7 | 37.75 | 0.25 | 785.14 |
| DY14 | Gypenoside XII | 80321-64-8 | 36.43 | 0.25 | 785.14 |
| DY15 | Gypenoside XI | 94987-10-7 | 4.89 | 0.10 | 799.12 |
| DY16 | Gypenoside XXXV | 90069-02-6 | 5.85 | 0.04 | 444.77 |
| DY17 | Gypenoside XXVII_qt | 81474-82-0 | 30.21 | 0.74 | 418.73 |
| DY18 | Gypenoside XXVIII_qt | 81474-80-8 | 32.08 | 0.74 | 416.71 |
| DY19 | Gypenoside XXXII | 176182-04-0 | 34.24 | 0.25 | 787.11 |
| DY20 | Gypentonoside A_qt | 157752-01-7 | 36.13 | 0.80 | 472.78 |
| DY21 | Pyrocatechuic acid | 875-28-5 | 28.55 | 0.04 | 176.102 |
| DY22 | 8-Hydroxyquinoline | 148-24-3 | NA | NA | 145.158 |
| DY23 | 7,8-Dihydroxycoumarin | 486-35-1 | NA | NA | 178.14 |
| DY24 | Nicotiflorin | 6130-58-1 | 3.64 | 0.73 | 594.518 |
| DY25 | Kaempferol 3-Glucorhamnoside | 40437-72-7 | NA | NA | 594.518 |
| DY26 | Ginsenoside Rg1 | 22427-39-0 | NA | NA | 801.013 |
| DY27 | Gypenoside XVII | 80321-69-3 | 3.51 | 0.10 | 947.154 |
| DY28 | Ginkgolic acid (C13:0) | 20261-38-5 | NA | NA | 320.466 |
| DY29 | Ginkgolic Acid (C15:1) | 22910-60-7 | NA | NA | 306.504 |
| DY30 | Isorhamnetin-3-O-nehesperidine | 55033-90-4 | NA | NA | 624.54 |
| DY31 | Isoquercitrin | 21637-25-2 | 1.86 | 0.77 | 464.094 |
| DY32 | Dulcitol | 608-66-2 | NA | NA | 182.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhao, M.; Lu, M.; Feng, Y.; Zhang, X.; Wang, D.; Jiang, W. Network Pharmacology Analysis, Molecular Docking Integrated Experimental Verification Reveal the Mechanism of Gynostemma pentaphyllum in the Treatment of Type II Diabetes by Regulating the IRS1/PI3K/Akt Signaling Pathway. Curr. Issues Mol. Biol. 2024, 46, 5561-5581. https://doi.org/10.3390/cimb46060333
Yang S, Zhao M, Lu M, Feng Y, Zhang X, Wang D, Jiang W. Network Pharmacology Analysis, Molecular Docking Integrated Experimental Verification Reveal the Mechanism of Gynostemma pentaphyllum in the Treatment of Type II Diabetes by Regulating the IRS1/PI3K/Akt Signaling Pathway. Current Issues in Molecular Biology. 2024; 46(6):5561-5581. https://doi.org/10.3390/cimb46060333
Chicago/Turabian StyleYang, Songqin, Mao Zhao, Mingxing Lu, Yuhan Feng, Xia Zhang, Daoping Wang, and Wenwen Jiang. 2024. "Network Pharmacology Analysis, Molecular Docking Integrated Experimental Verification Reveal the Mechanism of Gynostemma pentaphyllum in the Treatment of Type II Diabetes by Regulating the IRS1/PI3K/Akt Signaling Pathway" Current Issues in Molecular Biology 46, no. 6: 5561-5581. https://doi.org/10.3390/cimb46060333





