Exploring the Therapeutic Potential: Bioactive Molecules and Dietary Interventions in Multiple Sclerosis Management
Abstract
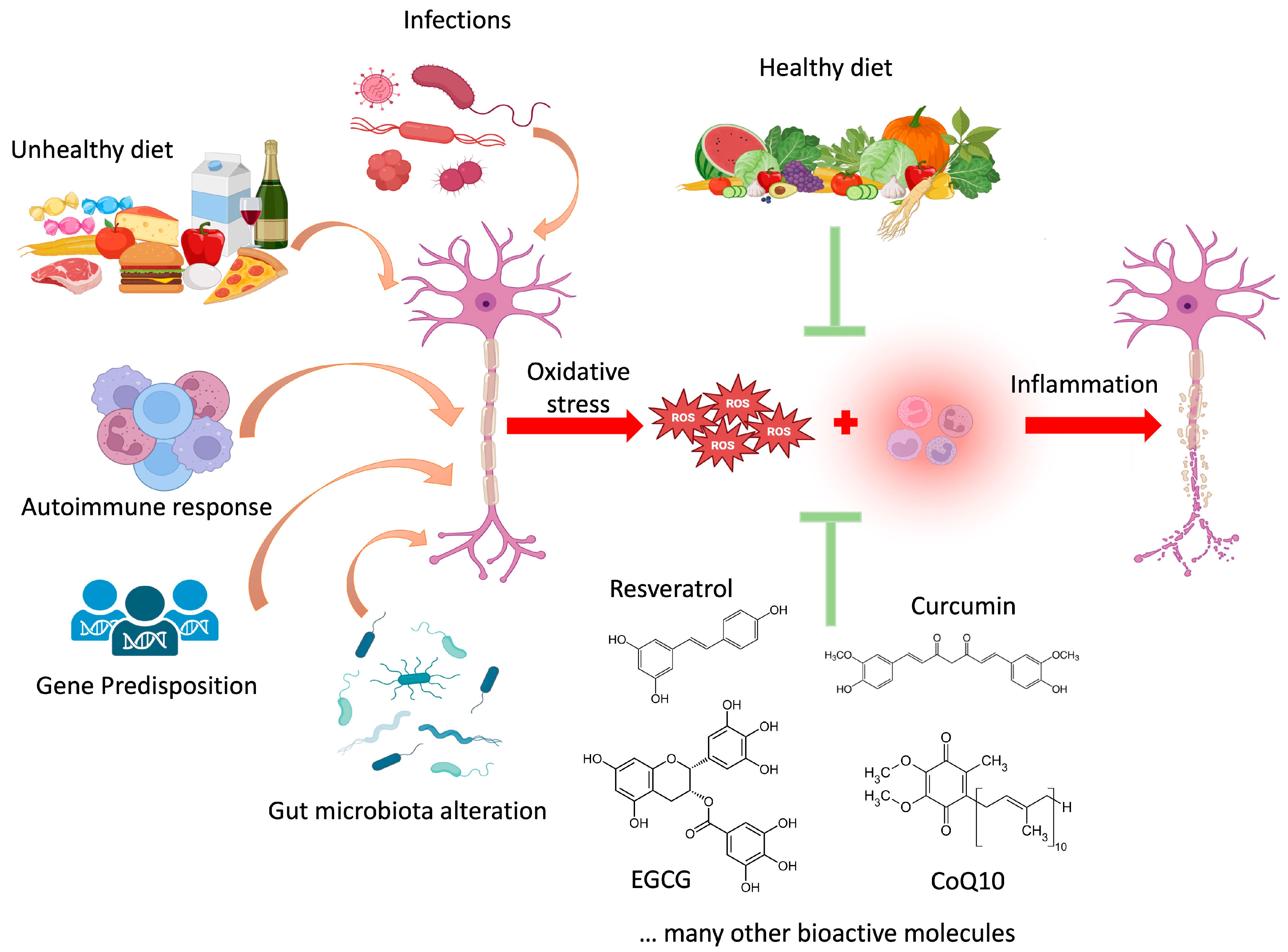
:1. Multiple Sclerosis: An Overview
2. Natural Compounds of Interest in MS
2.1. Ubiquinone (CoQ10)
2.2. Resveratrol
2.3. Curcumin
2.4. Epigallocatechin Gallate (EGCG)
2.5. Citicoline (DCI)
2.6. Ellagic Acid
2.7. Boswellic Acid (BA)
2.8. Withania Somnifera Extract
2.9. Ginseng Extract
2.10. Ginkgo biloba Extract
3. Diet and MS
3.1. Ketogenic or Mediterranean Diet
| Study Type | Population | Results | Reference |
|---|---|---|---|
| Prospective case-control study | 25 patients with MS | 6 months of KD intervention enhanced microbial mass and composition. | [99] |
| Prospective, mixed, and quasi-experimental pilot study | 27 patients with MS | 12 weeks of KD intervention following the Mediterranean diet food pattern determined enhanced satiation and improved body composition. | [96] |
| Single-arm, open-label study | 20 patients with RRMS | Positive results regarding safety, tolerability, and adherence to MAKD | [97] |
| 6-month prospective intervention | 65 patients with RRMS | 6 months of intervention with KD determined improvements in anthropometric measures, clinical outcomes, and laboratory biomarkers. | [98] |
| 3-month follow-up of a 6-month prospective intervention | 65 patients with RRMS | The KD intervention resulted in improvements in energy levels, cognitive functions, and mood of the patients involved. Adherence of patients during the follow-up varied among participants. | [100] |
| Randomized Controlled Trial | 60 patients with MS | After 6 months of adaptive KD, results highlight reduced serum neurofilament light chain protein (a biomarker of neuroaxonal damage) | [101] |
| Diet | Percent Total Daily Energy Intake | ||
|---|---|---|---|
| Fat (%) | Carbohydrate (%) | Proteins (%) | |
| Classic KD (4:1 kd) | 90 | 2 | 8 |
| 3:1 KD | 87 | 4 | 9 |
| 2:1 KD | 82 | 8 | 10 |
| 1:1 KD | 70 | 10 | 20 |
3.2. D-Galactose
3.3. β-Hydroxybutyrate
3.4. Other Dietary Supplements
- Omega-3 fatty acids, such as eicosapentaenoic acid and docosahexaenoic acid, exert anti-inflammatory and neuroprotective effects. It was demonstrated that supplementation with omega-3 fatty acids has been associated with reduced relapse rates, improved cognitive function, and enhanced quality of life in patients with MS.
- Vitamins A, C, and E display a positive effect on MS progression as antioxidant molecules, scavenging free radicals and reducing the oxidative stress production. However, they also mitigate inflammation, helping to preserve neuronal integrity and function in MS.
- A separate mention is deserved for vitamin D, the deficiency of which is associated with the onset and progression of MS, as this molecule is involved in the modulation of the immune response. Supplementation with vitamin D has shown promising results in reducing disease activity and progression.
- Probiotics. Since emerging evidence suggests a link between gut microbiota dysbiosis and MS pathogenesis, probiotic supplementation could act in immune response modulation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| CNS | Central Nervous System |
| Relapsing-Remitting Multiple Sclerosis | RRMS |
| Disease-modifying therapies | DMT |
| Secondary Progressive Multiple Sclerosis | SPMS |
| Primary Progressive Multiple Sclerosis | PPMS |
| Nervous System | NS |
| Myelin Basic Protein | MBP |
| D-Galactose | Gal |
| Coenzyme Q10 | CoQ10 |
| Matrix Metallo Proteases | MMPs |
| Blood-Brain Barrier | BBB |
| Tumor Necrosis Factor Alfa | TNF-α |
| Interleukin-6 | IL-6 |
| Interleukin-4 | IL-4 |
| Superoxide Dismutase | SOD |
| Glutathione Peroxidase | GPx |
| Expanded Disability Status Scale | EDSS |
| Cuprizone | CPZ |
| Resveratrol | RV |
| Nuclear Factor Erythroid 2-Related Factor 2 | Nrf-2 |
| Interleukin-1β | IL-1β |
| Regulatory T cells | Treg |
| Experimental Autoimmune Encephalomyelitis | EAE |
| Epigallocatechin gallate | ECGC |
| Body Mass Index | BMI |
| Citicoline | DCI |
| Phosphatidylcholine | PC |
| Oligodendrocyte Precursor Cells | OPCs |
| Boswellic Acid | BA |
| Heme-oxygenase 1 | HO-1 |
| Ketogenic Diet | KD |
| Mediterranean Diet | MD |
| Reactive Oxygen Species | ROS |
| Modified Atkins Ketogenic Diet | MAKD |
| Healthy Subjects | HS |
| Hexose-6-phosphate dehydrogenase | H6PD |
References
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Chataway, J. Multiple Sclerosis, a Treatable Disease. Clin. Med. 2016, 16, s53. [Google Scholar]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, E.; Marriott, J.J.; Jetté, N.; Pringsheim, T.; Makhani, N.; Morrow, S.A.; Fisk, J.D.; Evans, C.; Béland, S.G.; Kulaga, S.; et al. Incidence and Prevalence of Multiple Sclerosis in Europe: A Systematic Review. BMC Neurol. 2013, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ravera, S.; Calzia, D.; Panfoli, I. Impairment of Heme Synthesis in Myelin as Potential Trigger of Multiple Sclerosis. Med. Hypotheses 2012, 78, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, D.; Tallantyre, E.; Kalincik, T.; Planchon, S.M.; Evangelou, N. Early Highly Effective versus Escalation Treatment Approaches in Relapsing Multiple Sclerosis. Lancet Neurol. 2019, 18, 973–980. [Google Scholar] [CrossRef]
- Morell, P.; Norton, W.T. Myelin. Sci. Am. 1980, 242, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Stassart, R.M.; Möbius, W.; Nave, K.A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [PubMed]
- Norton, W.T.; Autilio, L.A. The lipid composition of purified bovine brain myelin. J. Neurochem. 1966, 13, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, R.; Gilkerson, R.; Aggeler, R.; Yamagata, K.; Remington, S.J.; Capaldi, R.A. Energy Substrate Modulates Mitochondrial Structure and Oxidative Capacity in Cancer Cells. Cancer Res. 2004, 64, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Agranoff, B.W.; Goldberg, D. Diet and the geographical distribution of multiple sclerosis. Lancet 1974, 304, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Hartstein, J.; Ulett, G. Galactose Treatment of Multiple Sclerosis; a Preliminary Report. Dis. Nerv. Syst. 1957, 18, 255–258. [Google Scholar] [PubMed]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic Oligodendrocytes Maintain Myelin and Long-Term Axonal Integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold. Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef] [PubMed]
- Velumian, A.A.; Samoilova, M.; Fehlings, M.G. Visualization of Cytoplasmic Diffusion within Living Myelin Sheaths of CNS White Matter Axons Using Microinjection of the Fluorescent Dye Lucifer Yellow. Neuroimage 2011, 56, 27–34. [Google Scholar] [CrossRef]
- Adriano, E.; Perasso, L.; Panfoli, I.; Ravera, S.; Gandolfo, C.; Mancardi, G.; Morelli, A.; Balestrino, M. A Novel Hypothesis about Mechanisms Affecting Conduction Velocity of Central Myelinated Fibers. Neurochem. Res. 2011, 36, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A. Myelination and Support of Axonal Integrity by Glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Panfoli, I.; Calzia, D.; Aluigi, M.G.; Bianchini, P.; Diaspro, A.; Mancardi, G.; Morelli, A. Evidence for Aerobic ATP Synthesis in Isolated Myelin Vesicles. Int. J. Biochem. Cell Biol. 2009, 41, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ravera, S.; Panfoli, I. Hypothesis of an Energetic Function for Myelin. Cell. Biochem. Biophys. 2011, 61, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Trevisiol, A.; Kusch, K.; Steyer, A.M.; Gregor, I.; Nardis, C.; Winkler, U.; Köhler, S.; Restrepo, A.; Möbius, W.; Werner, H.B.; et al. Structural Myelin Defects Are Associated with Low Axonal ATP Levels but Rapid Recovery from Energy Deprivation in a Mouse Model of Spastic Paraplegia. PLoS Biol. 2020, 18, e3000943. [Google Scholar] [CrossRef] [PubMed]
- Trevisiol, A.; Saab, A.S.; Winkler, U.; Marx, G.; Imamura, H.; Möbius, W.; Kusch, K.; Nave, K.A.; Hirrlinger, J. Monitoring ATP Dynamics in Electrically Active White Matter Tracts. eLife 2017, 6, e24241. [Google Scholar] [CrossRef] [PubMed]
- Zambonin, J.L.; Zhao, C.; Ohno, N.; Campbell, G.R.; Engeham, S.; Ziabreva, I.; Schwarz, N.; Lee, S.E.; Frischer, J.M.; Turnbull, D.M.; et al. Increased Mitochondrial Content in Remyelinated Axons: Implications for Multiple Sclerosis. Brain 2011, 134, 1901. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Nobbio, L.; Visigalli, D.; Bartolucci, M.; Calzia, D.; Fiorese, F.; Mancardi, G.; Schenone, A.; Morelli, A.; Panfoli, I. Oxydative Phosphorylation in Sciatic Nerve Myelin and Its Impairment in a Model of Dysmyelinating Peripheral Neuropathy. J. Neurochem. 2013, 126, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kiryu-Seo, S.; Ohno, N.; Kidd, G.J.; Komuro, H.; Trapp, B.D. Demyelination Increases Axonal Stationary Mitochondrial Size and the Speed of Axonal Mitochondrial Transport. J. Neurosci. 2010, 30, 6658. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Stys, P.K. Virtual Hypoxia and Chronic Necrosis of Demyelinated Axons in Multiple Sclerosis. Lancet Neurol. 2009, 8, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Meli, F.; Ysrraelit, C. Neuronal Injury in Multiple Sclerosis. Medicina 2006, 66, 472–485. [Google Scholar] [PubMed]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Murúa, S.R.; Farez, M.F.; Quintana, F.J. The Immune Response in Multiple Sclerosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary Interventions for Multiple Sclerosis-related Outcomes. Cochrane Database Syst. Rev. 2020, CD004192. [Google Scholar] [CrossRef] [PubMed]
- Shemirani, F.; Pingel, W.R.; Titcomb, T.J.; Salari-Moghaddam, A.; Arsalandeh, F.; Saxby, S.M.; Snetselaar, L.G.; Wahls, T.L. The Effect of Dietary Interventions on Inflammatory Biomarkers among People with Multiple Sclerosis: A Protocol for Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2024, 19, e0297510. [Google Scholar] [CrossRef] [PubMed]
- Rauchová, H. Coenzyme Q10 Effects in Neurological Diseases. Physiol. Res. 2021, 70, 683–714. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Avolio, C.; Ruggieri, M.; Giuliani, F.; Liuzzi, G.M.; Leante, R.; Riccio, P.; Livrea, P.; Trojano, M. Serum MMP-2 and MMP-9 Are Elevated in Different Multiple Sclerosis Subtypes. J. Neuroimmunol. 2003, 136, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 Supplementation Ameliorates Inflammatory Markers in Patients with Multiple Sclerosis: A Double Blind, Placebo, Controlled Randomized Clinical Trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Jazayeri, S.; Reza Gohari, M. Coenzyme Q10 Supplementation Reduces Oxidative Stress and Increases Antioxidant Enzyme Activity in Patients with Relapsing–Remitting Multiple Sclerosis. Int. J. Neurosci. 2013, 123, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of Depression and Anxiety in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a Treatment for Fatigue and Depression in Multiple Sclerosis Patients: A Double Blind Randomized Clinical Trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, B.; Madadi, S.; Fattahi, N.; Abouhamzeh, B. Coenzyme Q10 Enhances Remyelination and Regulate Inflammation Effects of Cuprizone in Corpus Callosum of Chronic Model of Multiple Sclerosis. J. Mol. Histol. 2021, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [PubMed]
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.S.; Dine, K.; Dutt, M.; Shindler, K.S. Resveratrol Neuroprotection in a Chronic Mouse Model of Multiple Sclerosis. Front. Neurol. 2012, 3, 25846. [Google Scholar]
- Mokni, M.; Elkahoui, S.; Limam, F.; Amri, M.; Aouani, E. Effect of Resveratrol on Antioxidant Enzyme Activities in the Brain of Healthy Rat. Neurochem. Res. 2007, 32, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Choi, H.J.; Zhu Bao Ting, B.T. Mechanism for the Protective Effect of Resveratrol against Oxidative Stress-Induced Neuronal Death. Free Radic. Biol. Med. 2010, 49, 800. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.M.; Martins, L.A.M.; Souza, D.O.; Quincozes-Santos, A. Glioprotective Effect of Resveratrol: An Emerging Therapeutic Role for Oligodendroglial Cells. Mol. Neurobiol. 2018, 55, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, S.; Mellemkjaer, A.; Iversen, M.B.; Pedersen, S.B.; Kragstrup, T.W. Resveratrol Displays Anti-Inflammatory Properties in an Ex Vivo Model of Immune Mediated Inflammatory Arthritis. BMC Rheumatol. 2018, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.-G. Resveratrol as a Protective Molecule for Neuroinflammation: A Review of Mechanisms. Curr. Pharm. Biotechnol. 2014, 15, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Petro, T.M. Regulatory Role of Resveratrol on Th17 in Autoimmune Disease. Int. Immunopharmacol. 2011, 11, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Boi, M.; Poddighe, L.; Melis, T.; Lai, Y.; Carta, G.; Quartu, M. Resveratrol Regulates BDNF, TrkB, PSA-NCAM, and Arc Expression in the Rat Cerebral Cortex after Bilateral Common Carotid Artery Occlusion and Reperfusion. Nutrients 2019, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Gachpazan, M.; Habbibirad, S.; Kashani, H.; Jamialahmadi, T.; Rahimi, H.R.; Sahebkar, A. Targeting Nuclear Factor-Kappa B Signaling Pathway by Curcumin: Implications for the Treatment of Multiple Sclerosis. Adv. Exp. Med. Biol. 2021, 1291, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Dehnavi, S.; Asadirad, A.; Xu, S.; Majeed, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Curcumin and Chemokines: Mechanism of Action and Therapeutic Potential in Inflammatory Diseases. Inflammopharmacology 2023, 31, 1069–1093. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Lee, J.D.; Park, C.; Choi, Y.H.; Kim, G.Y. Curcumin Attenuates the Release of Pro-Inflammatory Cytokines in Lipopolysaccharide-Stimulated BV2 Microglia. Acta Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Ahmadi, A.; Hassanzadeh, K.; Soleimani, Z.; Sathyapalan, T.; Mohammadi, A.; Sahebkar, A. Targeting the Balance of T Helper Cell Responses by Curcumin in Inflammatory and Autoimmune States. Autoimmun. Rev. 2019, 18, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ghoushi, E.; Poudineh, M.; Parsamanesh, N.; Jamialahmadi, T.; Sahebkar, A. Curcumin as a Regulator of Th17 Cells: Unveiling the Mechanisms. Food Chem. Mol. Sci. 2024, 8, 100198. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, Q.; Joe, Y.; Lee, B.H.; Ryu, D.G.; Kwon, K.B.; Ryter, S.W.; Chung, H.T. Curcumin Induces Apoptotic Cell Death of Activated Human CD4+ T Cells via Increasing Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Int. Immunopharmacol. 2013, 15, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2019, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin Induces a Fatal Energetic Impairment in Tumor Cells In Vitro and In Vivo by Inhibiting ATP-Synthase Activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin Promotes Oligodendrocyte Differentiation and Their Protection against TNF-α through the Activation of the Nuclear Receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Quarantelli, M.; Moccia, M.; Vacca, G.; Satelliti, B.; D’Ambrosio, G.; Carotenuto, A.; Ragucci, M.; Assogna, F.; Capacchione, A.; et al. ProspeCtive Study to Evaluate Efficacy, Safety and TOlerability of Dietary SupplemeNT of Curcumin (BCM95) in Subjects with Active Relapsing MultIple Sclerosis Treated with SubcutaNeous Interferon Beta 1a 44 Mcg TIW (CONTAIN): A Randomized, Controlled Trial. Mult. Scler. Relat. Disord. 2021, 56, 103274. [Google Scholar] [CrossRef] [PubMed]
- Merck KGaA. Dietary Supplement of Curcumin in Subjects with Active Relapsing Multiple Sclerosis Treated with Subcutaneous Interferon Beta 1a; Merck KGaA: Darmstadt, Germany, 2016; Available online: https://clinicaltrials.merckgroup.com/en/trial-details/?id=EMR200136-549 (accessed on 23 May 2024).
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; de la Rubia Ortí, J.E. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2016, 42, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J.; Gareri, P. Citicoline: Pharmacological and clinical review, 2022 update. Rev. Neurol 2022, 75 (Suppl. 5), S1–S89. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Manzel, A.; Gropengießer, K.; Schäfer, N.; Gudi, V.; Singh, V.; Tejedor, L.S.; Jörg, S.; Hammer, A.; Voss, E.; et al. Pivotal Role of Choline Metabolites in Remyelination. Brain 2015, 138, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P.; Świątkiewicz, M.; Kamińska, A.; Jünemann, A.; Rejdak, R.; Rejdak, K. Citicoline: A Candidate for Adjunct Treatment of Multiple Sclerosis. Pharmaceuticals 2021, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Busto, R.; Serna, J.; Perianes-Cachero, A.; Quintana-Portillo, R.; García-Seisdedos, D.; Canfrán-Duque, A.; Paino, C.L.; Lerma, M.; Casado, M.E.; Martín-Hidalgo, A.; et al. Ellagic Acid Protects from Myelin-Associated Sphingolipid Loss in Experimental Autoimmune Encephalomyelitis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, A.; Farzanehfar, M.; Hosseini, H.; Arab, F.L.; Nikkhah, A. Boswellic Acids as Promising Agents for the Management of Brain Diseases. Life Sci. 2023, 312, 121196. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Shahpiri, Z.; Bahramsoltani, R.; Nia, M.M.; Najafi, F.; Rahimi, R. Efficacy and Tolerability of Phytomedicines in Multiple Sclerosis Patients: A Review. CNS Drugs 2017, 31, 867–889. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kaczmarek, L. MMP-9 Inhibition: A Therapeutic Strategy in Ischemic Stroke. Mol. Neurobiol. 2014, 49, 563. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Effects of Ashwagandha (Roots of Withania somnifera) on Neurodegenerative Diseases. Biol. Pharm. Bull. 2014, 37, 892–897. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Banerjee, S.; Biswas, S.; Das, B.; Kar, A.; Katiyar, C.K. Withania somnifera (L.) Dunal—Modern Perspectives of an Ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021, 264, 113157. [Google Scholar] [CrossRef] [PubMed]
- Polumackanycz, M.; Petropoulos, S.A.; Śledziński, T.; Goyke, E.; Konopacka, A.; Plenis, A.; Viapiana, A. Withania somnifera L.: Phenolic Compounds Composition and Biological Activity of Commercial Samples and Its Aqueous and Hydromethanolic Extracts. Antioxidants 2023, 12, 550. [Google Scholar] [PubMed]
- Bhattacharya, S.K.; Bhattacharya, A.; Sairam, K.; Ghosal, S. Anxiolytic-Antidepressant Activity of Withania somnifera Glycowithanolides: An Experimental Study. Phytomedicine 2000, 7, 463–469. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D. An Investigation into the Stress-Relieving and Pharmacological Actions of an Ashwagandha (Withania somnifera) Extract: A Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-W.; Kim, Y.-C.; Rhee, Y.K.; Lee, Y.-C.; Kim, K.-T.; Hong, H.-D. Chemical Composition Characteristics of Korean Straight Ginseng Products. J. Ethn. Foods 2014, 1, 24–28. [Google Scholar] [CrossRef]
- Lee, M.J.; Chang, B.J.; Oh, S.; Nah, S.Y.; Cho, I.H. Korean Red Ginseng Mitigates Spinal Demyelination in a Model of Acute Multiple Sclerosis by Downregulating P38 Mitogen-Activated Protein Kinase and Nuclear Factor-ΚB Signaling Pathways. J. Ginseng Res. 2018, 42, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of Ginsenosides, the Main Active Components of Panax Ginseng, in Inflammatory Responses and Diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Ahn, G.; Park, E.; Ha, D.; Song, J.Y.; Jee, Y. An Acidic Polysaccharide of Panax Ginseng Ameliorates Experimental Autoimmune Encephalomyelitis and Induces Regulatory T Cells. Immunol. Lett. 2011, 138, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, X.; Yang, Q.; Li, M.; Ran, Q.; Liu, Q.; Yang, L.; Sun, L.; Guo, Y.; Li, Y.; et al. Ginsenoside Rg1 Promotes Remyelination and Functional Recovery in Demyelinating Disease by Enhancing Oligodendrocyte Precursor Cells-Mediated Myelin Repair. Phytomedicine 2022, 106, 154309. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.E.; Cahill, L.; Wenk, G.L. Ginkgo Biloba: A Cognitive Enhancer? Psychol. Sci. Public Interest 2002, 3, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.K.; Diamond, B.J.; Rausch, S.; Kaufman, M.; Shiflett, S.C.; Graves, L. The Effect of Ginkgo Biloba on Functional Measures in Multiple Sclerosis: A Pilot Randomized Controlled Trial. Explore 2006, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, E.; Barthel, B. A Review of Ketogenic Diet and Lifestyle. Mo Med. 2022, 119, 84. [Google Scholar]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Lehner-Gulotta, D.; Woolbright, E.; Banwell, B.; Bergqvist, A.G.C.; Chen, S.; Coleman, R.; Conaway, M.; Goldman, M.D. Phase II Study of Ketogenic Diets in Relapsing Multiple Sclerosis: Safety, Tolerability and Potential Clinical Benefits. J. Neurol. Neurosurg. Psychiatry 2022, 93, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 283641. [Google Scholar]
- Benlloch, M.; López-Rodríguez, M.M.; Cuerda-Ballester, M.; Drehmer, E.; Carrera, S.; Ceron, J.J.; Tvarijonaviciute, A.; Chirivella, J.; Fernández-García, D.; de la Rubia Ortí, J.E. Satiating Effect of a Ketogenic Diet and Its Impact on Muscle Improvement and Oxidation State in Multiple Sclerosis Patients. Nutrients 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Banwell, B.; Christina Bergqvist, A.G.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot Study of a Ketogenic Diet in Relapsing-Remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e565. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, E.; Lehner-Gulotta, D.; Florenzo, B.; Banwell, B.; Bergqvist, A.G.C.; Coleman, R.; Conaway, M.; Goldman, M.D.; Brenton, J.N. Ketogenic Diet in Relapsing Multiple Sclerosis: Patient Perceptions, Post-Trial Diet Adherence & Outcomes. Clin. Nutr. 2023, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.; Steffen, F.; Zipp, F.; Bittner, S. Impact of Dietary Intervention on Serum Neurofilament Light Chain in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1102. [Google Scholar] [CrossRef] [PubMed]
- Lutfullin, I.; Eveslage, M.; Bittner, S.; Antony, G.; Flaskamp, M.; Luessi, F.; Salmen, A.; Gisevius, B.; Klotz, L.; Korsukewitz, C.; et al. Original Research: Association of Obesity with Disease Outcome in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 57. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Del Balzo, V.; Diolordi, L.; Pinto, A.; Giusti, A.M.; Vitiello, V.; Cannella, C.; Dernini, S.; Donini, L.M.; Berry, E.M. Mediterranean Diet Pyramids: Towards the Italian Model. Ann. Ig. Med. Prev. Comunità 2012, 24, 443–447. [Google Scholar] [PubMed]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern Vision of the Mediterranean Diet. J. Prev. Med. Hyg. 2022, 63, E36. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, 16295–16296. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, P.; Mosti, S.; Romano, M.R.; Lombardo, F.; Moscato, G.; Meucci, G. Increased T-Lymphocyte Interleukin-6 Binding in Patients with Multiple Sclerosis. Eur. J. Neurol. 2000, 7, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; Slaets, H.; Hellings, N. Immunomodulatory Properties of the IL-6 Cytokine Family in Multiple Sclerosis. Ann. N. Y. Acad. Sci. 2015, 1351, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Kappelmann, N.; Ye, Z.; Lamers, F.; Moser, S.; Jones, P.B.; Burgess, S.; Penninx, B.W.J.H.; Khandaker, G.M. Association of Inflammation with Depression and Anxiety: Evidence for Symptom-Specificity and Potential Causality from UK Biobank and NESDA Cohorts. Mol. Psychiatry 2021, 26, 7393. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [PubMed]
- Datta, I.; Zahoor, I.; Ata, N.; Rashid, F.; Cerghet, M.; Rattan, R.; Poisson, L.M.; Giri, S. Utility of an Untargeted Metabolomics Approach Using a 2D GC-GC-MS Platform to Distinguish Relapsing and Progressive Multiple Sclerosis. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bartolucci, M.; Calzia, D.; Morelli, A.; Panfoli, I. Galactose and Hexose 6-Phosphate Dehydrogenase Support the Myelin Metabolic Role. Indian J. Res. 2015, 4, 21–24. Available online: https://www.semanticscholar.org/paper/Galactose-and-Hexose-6-Phosphate-Dehydrogenase-the-Ravera-Bartolucci/d4886c3fe4bd4e2079285ca160d06a9dee5cf7fa (accessed on 19 April 2024).
- Alcina, A.; Ramagopalan, S.V.; Fernández, Ó.; Catalá-Rabasa, A.; Fedetz, M.; Ndagire, D.; Leyva, L.; Arnal, C.; Delgado, C.; Lucas, M.; et al. Hexose-6-Phosphate Dehydrogenase: A New Risk Gene for Multiple Sclerosis. Eur. J. Hum. Genet. 2010, 18, 618–620. [Google Scholar] [CrossRef]
- Watson, G.S.; Craft, S. Insulin Resistance, Inflammation, and Cognition in Alzheimer’s Disease: Lessons for Multiple Sclerosis. J. Neurol. Sci. 2006, 245, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Tryfonos, C.; Mantzorou, M.; Fotiou, D.; Vrizas, M.; Vadikolias, K.; Pavlidou, E.; Giaginis, C. Dietary Supplements on Controlling Multiple Sclerosis Symptoms and Relapses: Current Clinical Evidence and Future Perspectives. Medicines 2019, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tao, Y.; Wang, M.; Huang, F.; Wu, X. Natural Compounds as Potential Therapeutic Candidates for Multiple Sclerosis: Emerging Preclinical Evidence. Phytomedicine 2024, 123, 155248. [Google Scholar] [CrossRef] [PubMed]
| Foods | Frequency |
|---|---|
| Olive oil | Every meal |
| Vegetables | >2 serves every meal (one raw) |
| Legumes | ≥2 serves weekly |
| Nuts | 1–2 serves daily |
| Fruits | 1–2 serves every meal |
| Dairy foods (low fat) | 2 serves daily |
| Red meat | <2 serves/week |
| Fish/shellfish | ≥2 serves weekly |
| Eggs/poultry | 2–4 serves weekly |
| Cereals (whole grain) | 1–2 serves every meal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancreda, G.; Ravera, S.; Panfoli, I. Exploring the Therapeutic Potential: Bioactive Molecules and Dietary Interventions in Multiple Sclerosis Management. Curr. Issues Mol. Biol. 2024, 46, 5595-5613. https://doi.org/10.3390/cimb46060335
Tancreda G, Ravera S, Panfoli I. Exploring the Therapeutic Potential: Bioactive Molecules and Dietary Interventions in Multiple Sclerosis Management. Current Issues in Molecular Biology. 2024; 46(6):5595-5613. https://doi.org/10.3390/cimb46060335
Chicago/Turabian StyleTancreda, Gabriele, Silvia Ravera, and Isabella Panfoli. 2024. "Exploring the Therapeutic Potential: Bioactive Molecules and Dietary Interventions in Multiple Sclerosis Management" Current Issues in Molecular Biology 46, no. 6: 5595-5613. https://doi.org/10.3390/cimb46060335







