Drug-Resistance Biomarkers in Patient-Derived Colorectal Cancer Organoid and Fibroblast Co-Culture System
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Patient-Derived Organoids and CAFs
2.2. Co-Culture and Drug Treatment
2.3. Immunofluorescence Staining
2.4. RNA Sample Preparation and Library Construction
2.5. Data Processing and Analysis
2.6. Enrichment Analysis
3. Results
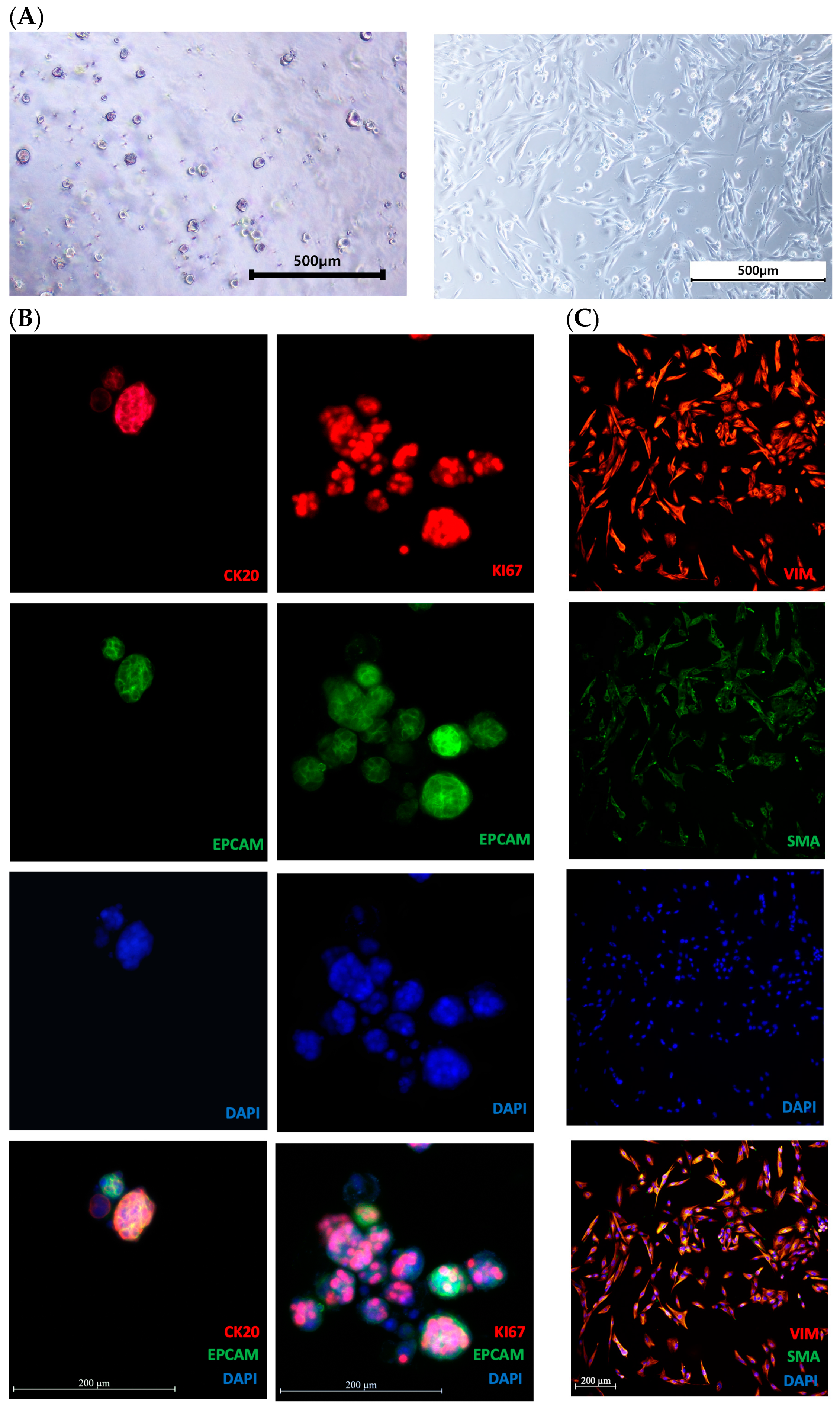
3.1. Morphological Analysis and Characteristics of Organoids and CAFs
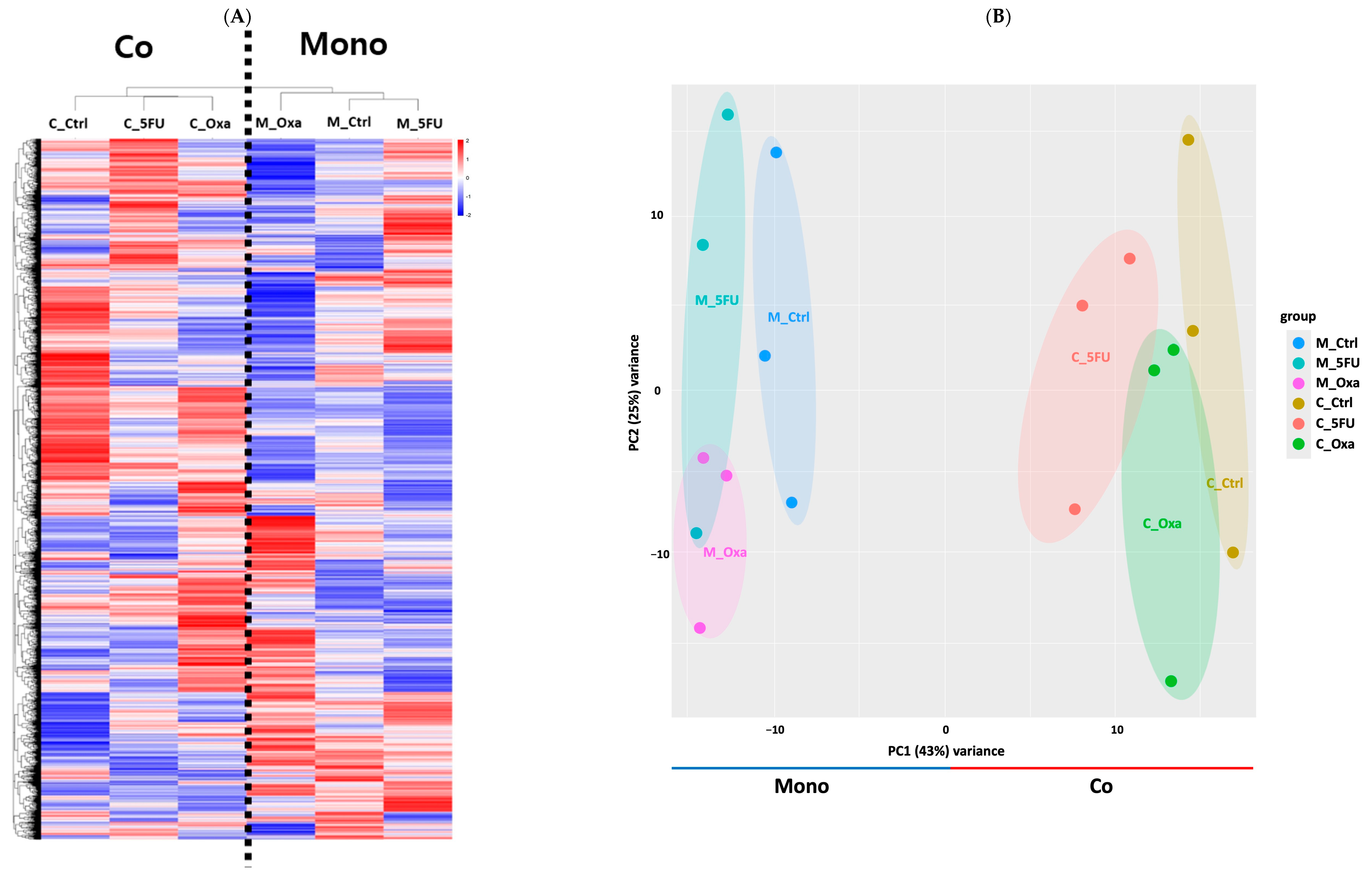
3.2. Co-Culture and Transcriptome Profiling
3.3. DEG Profiling in CRC Organoids in a Co-Culture
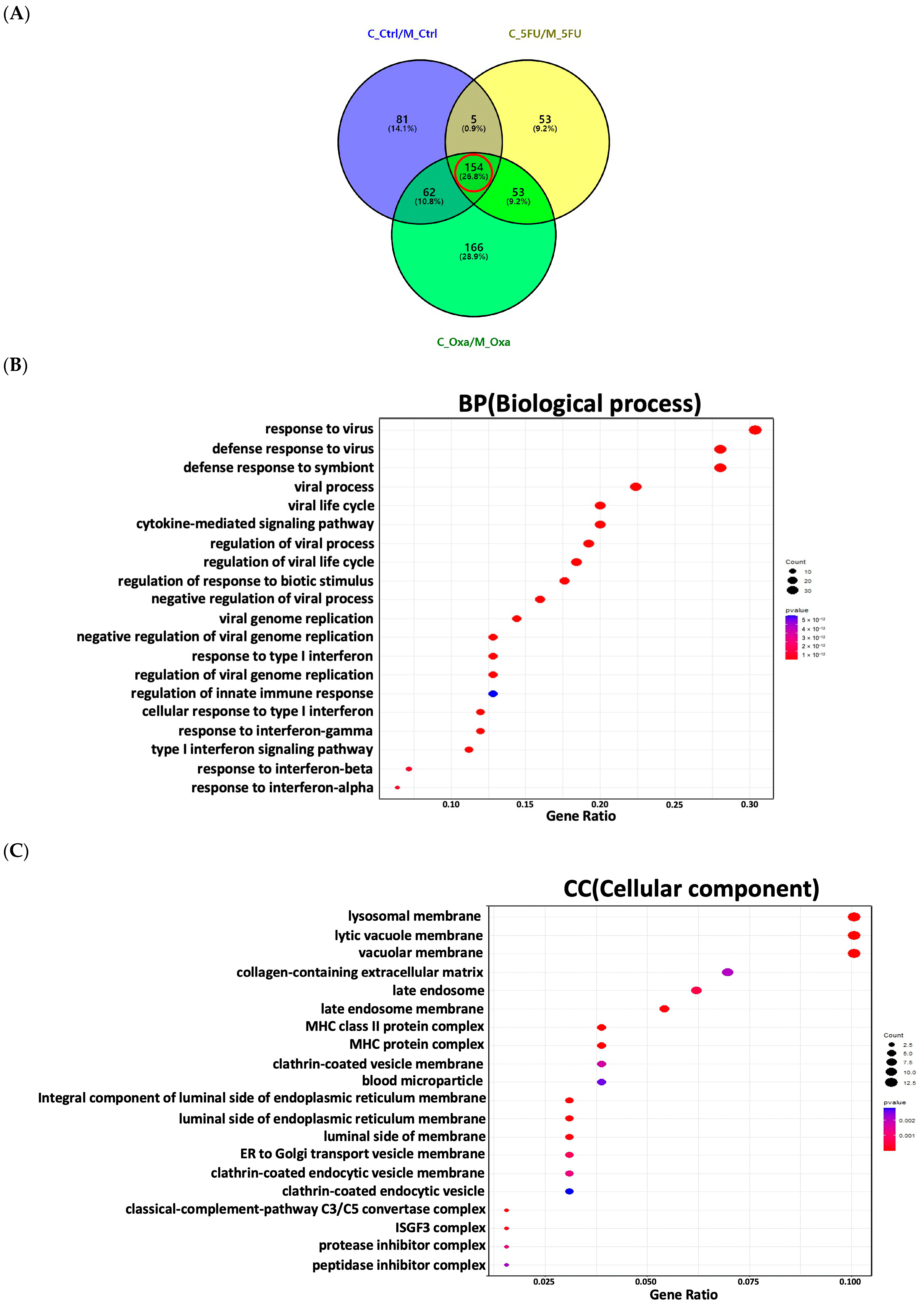
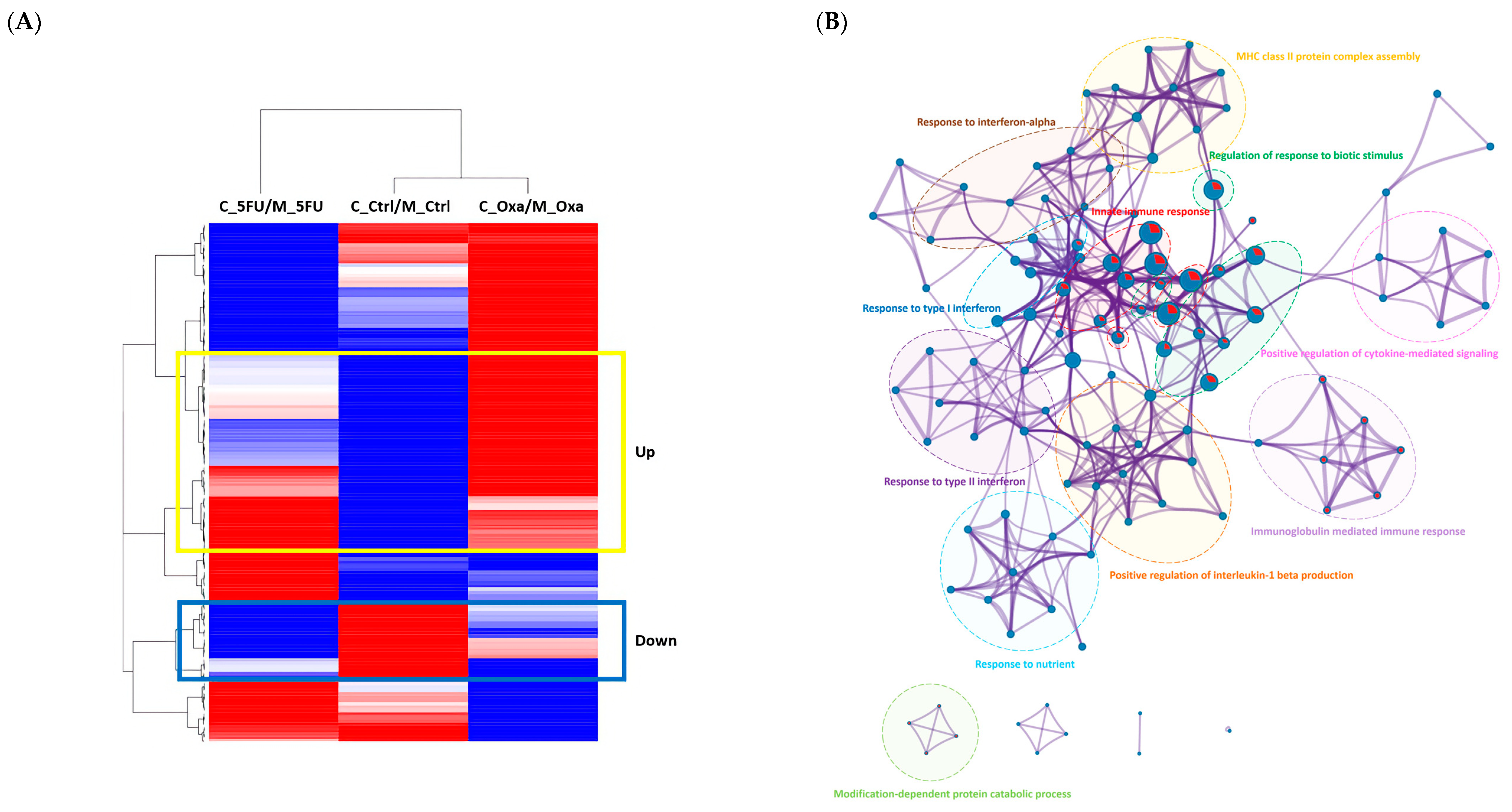
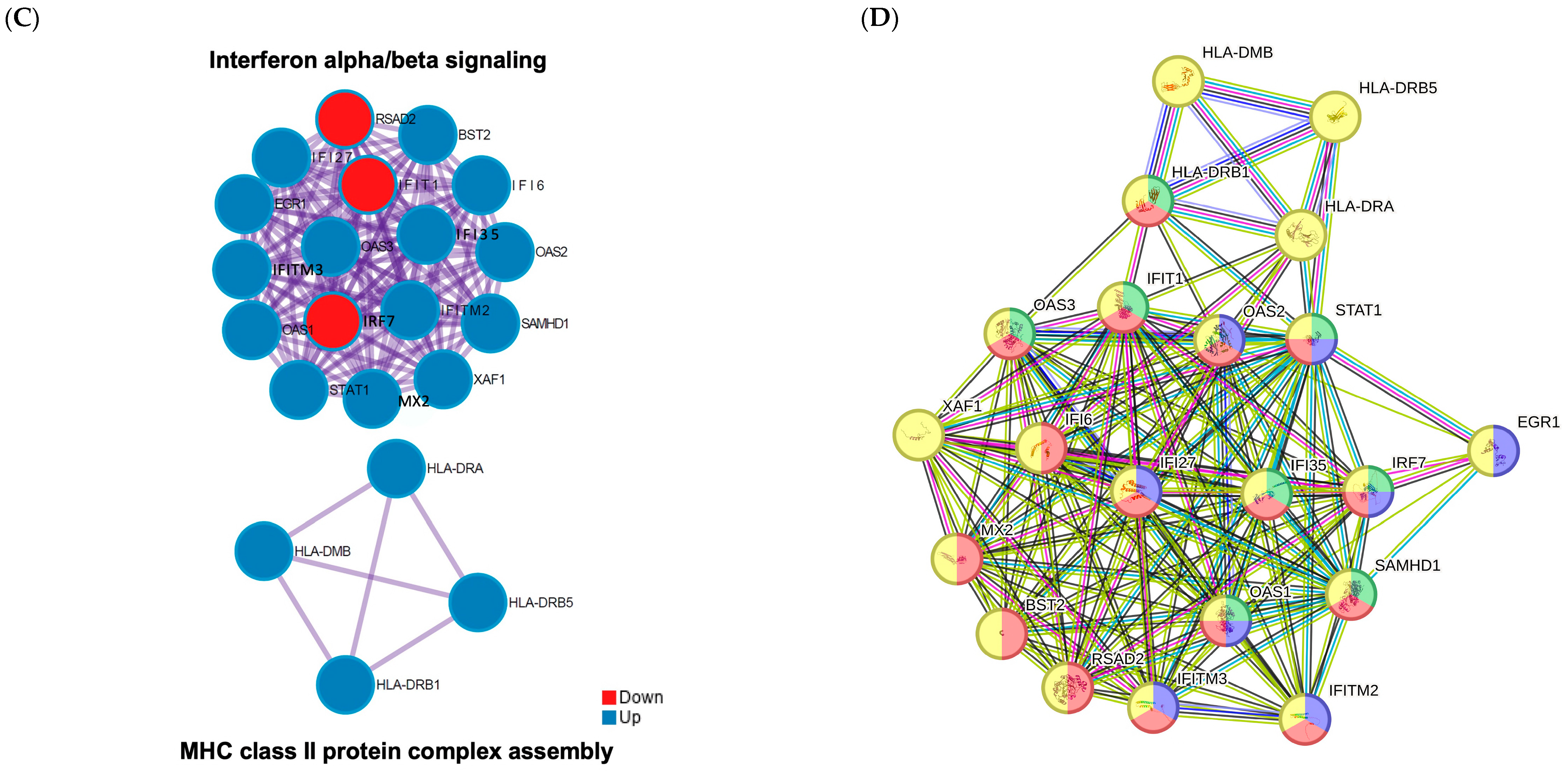
3.4. Functional Enrichment Analysis of the Significant Genes in the Co-Culture
3.5. Candidate Gene Selection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblast |
| CRC | Colorectal cancer |
| CIITA | Class II transactivator |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| DEG | Differentially expressed gene |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| FDR | False discovery rate |
| GO | Gene ontology |
| HCC | Hepatocellular carcinoma |
| HNSCC | Head and neck squamous-cell carcinoma |
| IBC | Inflammatory breast cancer |
| IFNα | Interferon alpha |
| MHC | Major histocompatibility complex |
| NGS | Next-generation sequencing |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDO | Patient-derived organoid |
| PPI | Protein–protein interaction |
| RIN | RNA integrity number |
| TME | Tumor microenvironment |
| TPM | Transcripts per million |
| 5-FU | 5-Fluorouracil |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Wong, M.C.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e961. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Rothenberg, M.L.; Van Cutsem, E.; Benson, A.B., III; Blanke, C.D.; Diasio, R.B.; Grothey, A.; Lenz, H.-J.; Meropol, N.J.; Ramanathan, R.K. The continuum of care: A paradigm for the management of metastatic colorectal cancer. Oncologist 2007, 12, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Maughan, T.S.; James, R.D.; Kerr, D.J.; Ledermann, J.; McArdle, C.; Seymour, M.; Cohen, D.; Hopwood, P.; Johnston, C.; Stephens, R.J. Comparison of survival, palliation, and quality of life with three chemotherapy regimens in metastatic colorectal cancer: A multicentre randomised trial. Lancet 2002, 359, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Govender, K.; van Wyk, J.; Reddy, S.; Ning, Y.; Wexner, S.; Stopforth, L.; Bhadree, S.; Naidoo, V.; Kader, S. Predictors of treatment refusal in patients with colorectal cancer: A systematic review. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2023. [Google Scholar]
- Demissie, K.; Oluwole, O.O.; Balasubramanian, B.A.; Osinubi, O.O.; August, D.; Rhoads, G.G. Racial differences in the treatment of colorectal cancer: A comparison of surgical and radiation therapy between Whites and Blacks. Ann. Epidemiol. 2004, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Malik, J.; Yazdani, H.; Geller, D.A.; Medich, D.; Zureikat, A.; Tohme, S. Refusal of cancer-directed treatment by colon cancer patients: Risk factors and survival outcomes. Am. J. Surg. 2020, 220, 1605–1612. [Google Scholar] [CrossRef]
- Shin, Y.M.; Han, H.S.; Lim, S.W.; Kim, B.C.; Cheoi, K.S.; Eum, Y.O.; Kim, S.T.; Lee, K.H. Combination chemotherapy of oxaliplatin, 5-fluorouracil and low dose leucovorin in patients with advanced colorectal cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2005, 37, 284–289. [Google Scholar] [CrossRef]
- Yaffee, P.; Osipov, A.; Tan, C.; Tuli, R.; Hendifar, A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J. Gastrointest. Oncol. 2015, 6, 185. [Google Scholar]
- Kim, H.K.; Choi, I.J.; Kim, C.G.; Kim, H.S.; Oshima, A.; Michalowski, A.; Green, J.E. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS ONE 2011, 6, e16694. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Sousa E Melo, F.; Van Der Heijden, M.; Cameron, K.; De Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Malanchi, I.; Santamaria-Martínez, A.; Susanto, E.; Peng, H.; Lehr, H.-A.; Delaloye, J.-F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2012, 481, 85–89. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.T.; Sugimoto, H.; Cooke, V.G.; MacDonald, B.A.; Mehta, A.I.; LeBleu, V.S.; Dewar, R.; Rocha, R.M.; Brentani, R.R.; Resnick, M.B. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA 2011, 108, 16002–16007. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.L.; Puré, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife 2020, 9, e57243. [Google Scholar] [CrossRef]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J.; Yokota, S.J.; Bankert, R.B. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef]
- Tauriello, D.V.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the tumour microenvironment: Cancer associated fibroblast gene expression patterns identify both common and unique features of tumour-stroma crosstalk across cancer types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef]
- Peng, S.; Chen, D.; Cai, J.; Yuan, Z.; Huang, B.; Li, Y.; Wang, H.; Luo, Q.; Kuang, Y.; Liang, W. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol. Oncol. 2021, 15, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fong, E.L.S.; Zhu, C.; Lin, Q.X.X.; Xiong, M.; Li, A.; Li, T.; Benoukraf, T.; Yu, H.; Liu, S. Hydrogel-based colorectal cancer organoid co-culture models. Acta Biomater. 2021, 132, 461–472. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Wang, L.; Li, M.; Ge, Z.; Noordam, L.; Lieshout, R.; Verstegen, M.M.; Ma, B.; Su, J. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 407–431. [Google Scholar] [CrossRef]
- Xiao, W.; Pahlavanneshan, M.; Eun, C.-Y.; Zhang, X.; DeKalb, C.; Mahgoub, B.; Knaneh-Monem, H.; Shah, S.; Sohrabi, A.; Seidlits, S.K. Matrix stiffness mediates pancreatic cancer chemoresistance through induction of exosome hypersecretion in a cancer associated fibroblasts-tumor organoid biomimetic model. Matrix Biol. Plus 2022, 14, 100111. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Al Mazeedi, M.A.; Almazyadi, H.A.; Kallmeyer, K.; Dandara, C.; Pepper, M.S. The role of tumor microenvironment in chemoresistance: To survive, keep your enemies closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef]
- Devarasetty, M.; Mazzocchi, A.R.; Skardal, A. Applications of bioengineered 3D tissue and tumor organoids in drug development and precision medicine: Current and future. BioDrugs 2018, 32, 53–68. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R. Organoid modeling of the tumor immune microenvironment. Cell 2018, 175, 1972–1988.e1916. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Rodman, C.; Atala, A.; Soker, S. Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann. Biomed. Eng. 2015, 43, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hong, S.N.; Kim, E.R.; Chang, D.K.; Kim, Y.H. Epithelial regeneration ability of Crohn’s disease assessed using patient-derived intestinal organoids. Int. J. Mol. Sci. 2021, 22, 6013. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Song, J.H.; Cha, Y.E.; Chang, D.K.; Kim, Y.H.; Hong, S.N. Intestinal epithelial responses to IL-17 in adult stem cell-derived human intestinal organoids. J. Crohn’s Colitis 2022, 16, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Ryu, K.B.; Medina Jiménez, B.I.; Kim, J.; Lee, H.Y.; Cho, S.J. Muscular development in urechis unicinctus (Echiura, Annelida). Int. J. Mol. Sci. 2020, 21, 2306. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Li, T.; Wernersson, R.; Hansen, R.B.; Horn, H.; Mercer, J.; Slodkowicz, G.; Workman, C.T.; Rigina, O.; Rapacki, K.; Stærfeldt, H.H. A scored human protein–protein interaction network to catalyze genomic interpretation. Nat. Methods 2017, 14, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Türei, D.; Korcsmáros, T.; Saez-Rodriguez, J. OmniPath: Guidelines and gateway for literature-curated signaling pathway resources. Nat. Methods 2016, 13, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Chatr-Aryamontri, A.; Oughtred, R.; Boucher, L.; Rust, J.; Chang, C.; Kolas, N.K.; O’Donnell, L.; Oster, S.; Theesfeld, C.; Sellam, A. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 2017, 45, D369–D379. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Kamali Zonouzi, S.; Pezeshki, P.; Razi, S.; Rezaei, N. Cancer-associated fibroblasts in colorectal cancer. Clin. Transl. Oncol. 2021, 24, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar]
- Bu, L.; Baba, H.; Yasuda, T.; Uchihara, T.; Ishimoto, T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020, 111, 3468–3477. [Google Scholar] [CrossRef]
- Chiavarina, B.; Turtoi, A. Collaborative and defensive fibroblasts in tumor progression and therapy resistance. Curr. Med. Chem. 2017, 24, 2846–2859. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Steeg, P.S. Endocytosis: A pivotal pathway for regulating metastasis. Br. J. Cancer 2021, 124, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kajiho, H.; Kajiho, Y.; Frittoli, E.; Confalonieri, S.; Bertalot, G.; Viale, G.; Di Fiore, P.P.; Oldani, A.; Garre, M.; Beznoussenko, G.V. RAB2A controls MT1-MMP endocytic and E-cadherin polarized Golgi trafficking to promote invasive breast cancer programs. EMBO Rep. 2016, 17, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.F.; Ferreira, W.A.S.; Cordeiro de Lima, V.C.; Medina, T.D.S. Antiviral responses in cancer: Boosting antitumor immunity through activation of interferon pathway in the tumor microenvironment. Front. Immunol. 2021, 12, 782852. [Google Scholar] [CrossRef]
- Rizza, P.; Moretti, F.; Belardelli, F. Recent advances on the immunomodulatory effects of IFN-α: Implications for cancer immunotherapy and autoimmunity. Autoimmunity 2010, 43, 204–209. [Google Scholar] [CrossRef]
- Hosein, A.N.; Livingstone, J.; Buchanan, M.; Reid, J.F.; Hallett, M.; Basik, M. A functional in vitro model of heterotypic interactions reveals a role for interferon-positive carcinoma associated fibroblasts in breast cancer. BMC Cancer 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, W.; Zhang, L.; Liu, S.; Zhao, M.; Zhou, G.; Wang, L.; Jin, S.; Zhang, Z.; Hu, J. Interferon-alpha promotes immunosuppression through IFNAR1/STAT1 signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Trowsdale, J. Genomic structure and function in the MHC. Trends Genet. 1993, 9, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Unanue, E.R.; Turk, V.; Neefjes, J. Variations in MHC class II antigen processing and presentation in health and disease. Annu. Rev. Immunol. 2016, 34, 265–297. [Google Scholar] [CrossRef]
- Thibodeau, J.; Bourgeois-Daigneault, M.-C.; Lapointe, R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology 2012, 1, 908–916. [Google Scholar] [CrossRef]
- Van Den Elsen, P.J. Expression regulation of major histocompatibility complex class I and class II encoding genes. Front. Immunol. 2011, 2, 48. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Chung, C.; Mou, H.; Bauer-Rowe, K.E.; Xifaras, M.E.; Ergin, I.; Dohnalova, L.; Biton, M.; Shekhar, K.; Eskiocak, O. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell 2021, 28, 1922–1935.e1925. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Lopatin, U.; Chieppa, M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: Implications for immune tolerance. Curr. Opin. Immunol. 2008, 20, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.-W.; Smillie, C.; Shekhar, K. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 2018, 175, 1307–1320.e1322. [Google Scholar] [CrossRef] [PubMed]
- Vellano, C.P.; White, M.G.; Andrews, M.C.; Chelvanambi, M.; Witt, R.G.; Daniele, J.R.; Titus, M.; McQuade, J.L.; Conforti, F.; Burton, E.M. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy. Nature 2022, 606, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Nadafi, R.; de Graça, C.G.; Keuning, E.D.; Koning, J.J.; de Kivit, S.; Konijn, T.; Henri, S.; Borst, J.; Reijmers, R.M.; van Baarsen, L.G. Lymph node stromal cells generate antigen-specific regulatory T cells and control autoreactive T and B cell responses. Cell Rep. 2020, 30, 4110–4123.e4114. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.-J.; Pereira, E.R.; Andersson, P.; Amoozgar, Z.; Van Wijnbergen, J.W.; O’Melia, M.J.; Zhou, H.; Chatterjee, S.; Ho, W.W.; Posada, J.M. Cancer cell plasticity and MHC-II–mediated immune tolerance promote breast cancer metastasis to lymph nodes. J. Exp. Med. 2023, 220, e20221847. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Gonçalves-Ribeiro, S.; Díaz-Maroto, N.; Berdiel-Acer, M.; Soriano, A.; Guardiola, J.; Martínez-Villacampa, M.; Salazar, R.; Capella, G.; Villanueva, A.; Martínez-Balibrea, E. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget 2016, 7, 59766–59780. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Zhou, J.; Sun, Z.; Bai, C. Response prediction to oxaliplatin plus 5-fluorouracil chemotherapy in patients with colorectal cancer using a four-protein immunohistochemical model. Oncol. Lett. 2019, 18, 2091–2101. [Google Scholar] [CrossRef] [PubMed]



| Contents | Setup of Analysis | Methods | Objectives |
|---|---|---|---|
| Morphological analysis and characteristics of organoids and CAFs | Preparation of CRC organoids and CAFs; indirect co-culture using transwell system | Bright-field and immunofluorescence staining analysis | Analysis of organoid and CAF morphology; assessment of cell viability |
| Co-culture and transcriptome profiling | RNA extraction from co-cultured samples | RNA-seq analysis to determine gene expression profiles | Identification of differentially expressed genes (DEGs) in co-culture vs. monoculture |
| DEG profiling in CRC organoids in a co-culture | RNA-seq data processing using bioinformatics tools | DESeq2 used for differential gene expression analysis | DEGs related to drug resistance identified; comparison of gene expression between different treatment groups |
| Functional enrichment analysis of the significant genes in the co-culture and candidate gene selection | Filtering of significant genes based on expression levels (DEG) and functional relevance | Pathway analysis tools (e.g., GSEA, Metascape) used to determine functional relevance | Identification of key pathways (e.g., JAK/STAT signaling); functional categorization of DEGs and potential biomarkers for drug resistance identified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, K.-B.; Seo, J.-a.; Lee, K.; Choi, J.; Yoo, G.; Ha, J.-h.; Ahn, M.R. Drug-Resistance Biomarkers in Patient-Derived Colorectal Cancer Organoid and Fibroblast Co-Culture System. Curr. Issues Mol. Biol. 2024, 46, 5794-5811. https://doi.org/10.3390/cimb46060346
Ryu K-B, Seo J-a, Lee K, Choi J, Yoo G, Ha J-h, Ahn MR. Drug-Resistance Biomarkers in Patient-Derived Colorectal Cancer Organoid and Fibroblast Co-Culture System. Current Issues in Molecular Biology. 2024; 46(6):5794-5811. https://doi.org/10.3390/cimb46060346
Chicago/Turabian StyleRyu, Kyoung-Bin, Jeong-ah Seo, Kyerim Lee, Juhyun Choi, Geon Yoo, Ji-hye Ha, and Mee Ryung Ahn. 2024. "Drug-Resistance Biomarkers in Patient-Derived Colorectal Cancer Organoid and Fibroblast Co-Culture System" Current Issues in Molecular Biology 46, no. 6: 5794-5811. https://doi.org/10.3390/cimb46060346
APA StyleRyu, K.-B., Seo, J.-a., Lee, K., Choi, J., Yoo, G., Ha, J.-h., & Ahn, M. R. (2024). Drug-Resistance Biomarkers in Patient-Derived Colorectal Cancer Organoid and Fibroblast Co-Culture System. Current Issues in Molecular Biology, 46(6), 5794-5811. https://doi.org/10.3390/cimb46060346





