Employing Bidirectional Two-Sample Mendelian Randomization Analysis to Verify the Potential of Polyunsaturated Fatty Acid Levels in the Prevention of Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
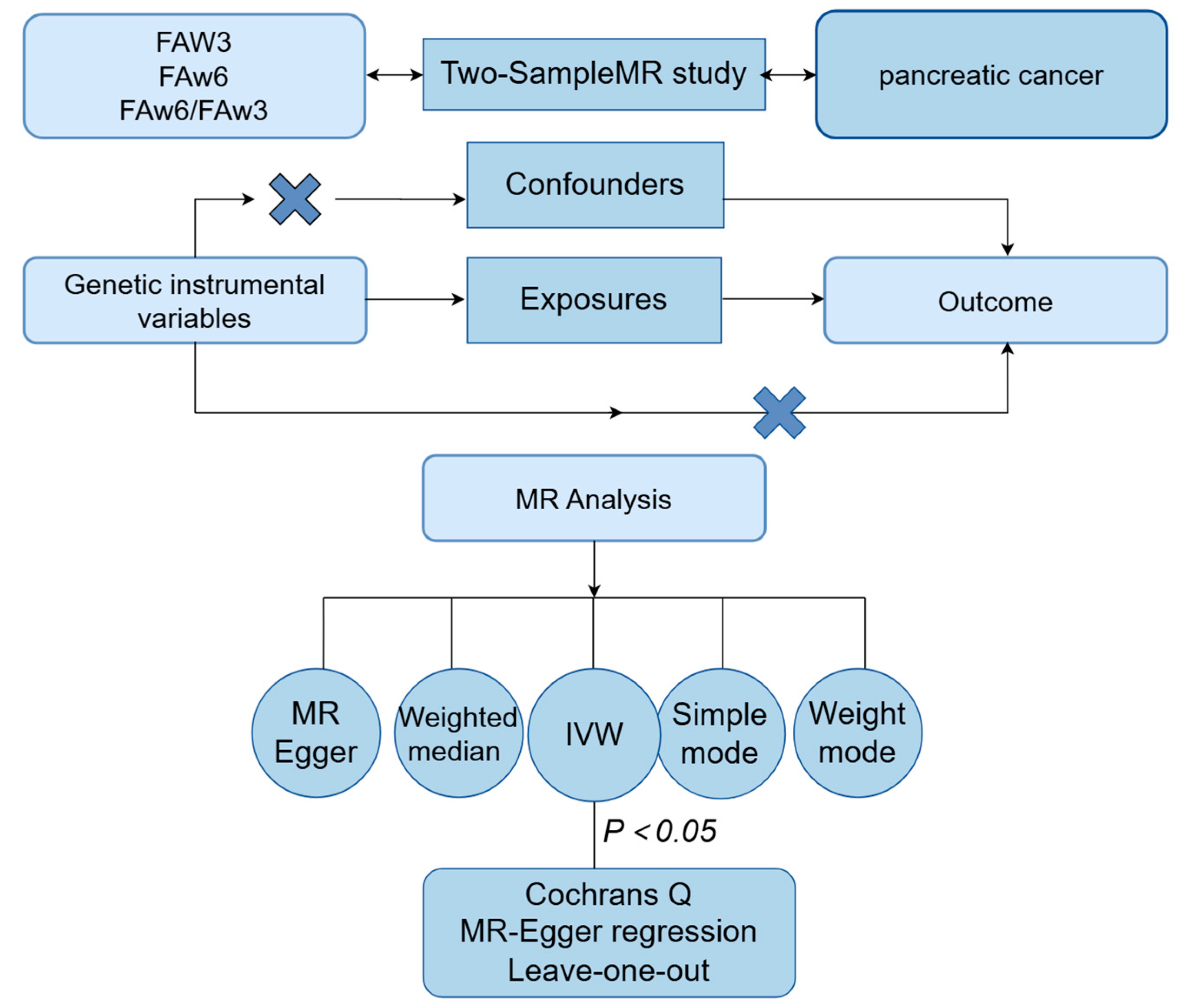
2.1. Study Design
2.2. Data Sources
2.3. Selection of Instrumental Variables
2.4. MR Method
2.5. Statistical Analysis
3. Results
3.1. Selection of Instrumental Variables
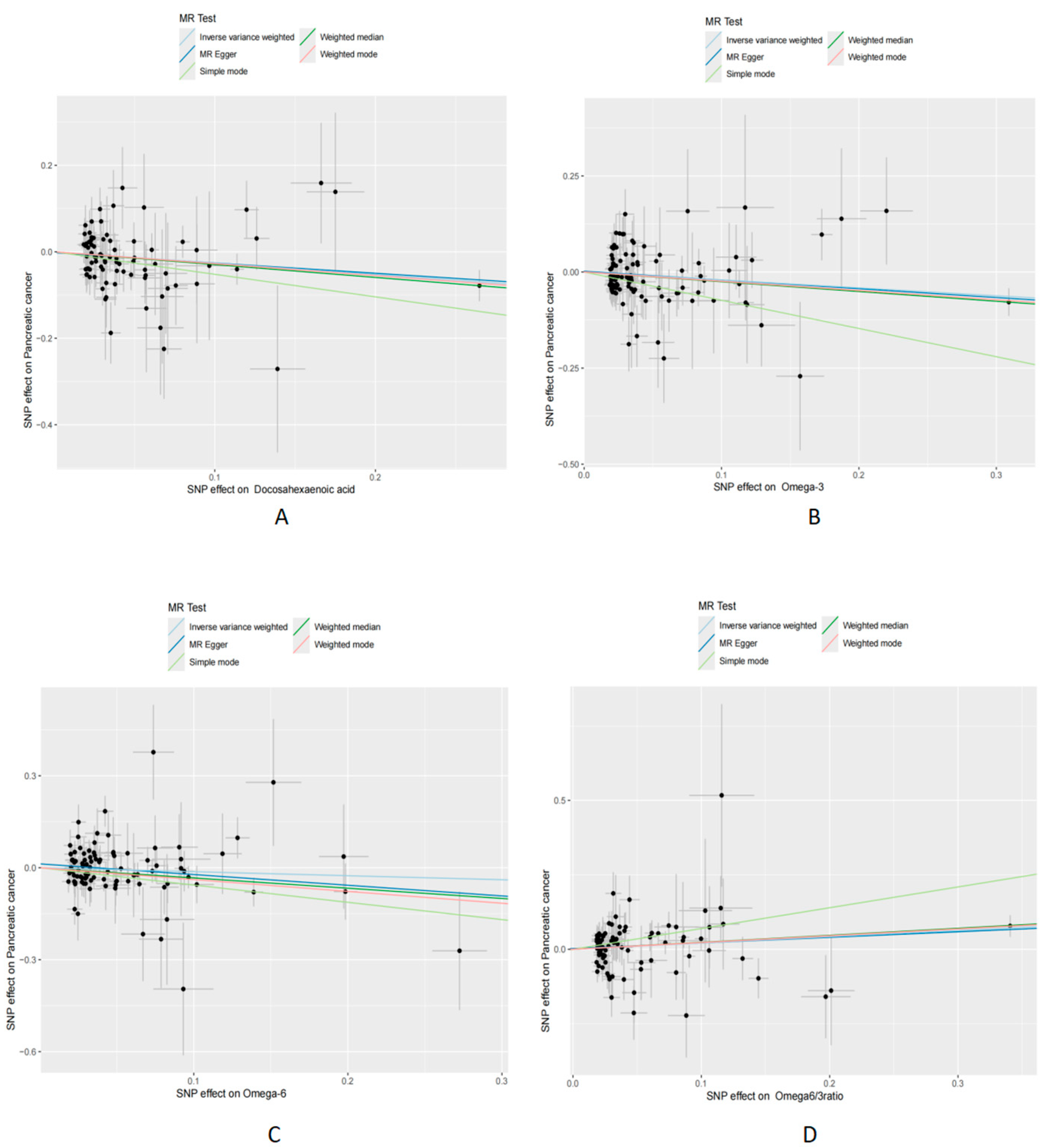
3.2. Mendelian Randomization Analysis
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Ros, E.; Mataix, J. Fatty acid composition of nuts--implications for cardiovascular health. Br. J. Nutr. 2006, 96 (Suppl. 2), S29–S35. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Stürmer, T.; Glynn, R.J.; Rassen, J.; Schneeweiss, S. Confounding Control in Healthcare Database Research: Challenges and Potential Approaches. Med. Care 2010, 48, S114–S120. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Yang, J.; Ren, B.; Wang, H.; Chen, G.; Zhao, F.; You, L.; Wang, W.; Zhao, Y. Metabolism of pancreatic cancer: Paving the way to better anticancer strategies. Mol. Cancer 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.P.C. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar]
- Soreide, K.; Ismail, W.; Roalso, M.; Ghotbi, J.; Zaharia, C. Early Diagnosis of Pancreatic Cancer: Clinical Premonitions, Timely Precursor Detection and Increased Curative-Intent Surgery. Cancer Control 2023, 30, 10732748231154711. [Google Scholar] [CrossRef]
- Falconer, J.S.; Ross, J.A.; Fearon, K.C.H.; Hawkins, R.A.; O’Riordain, M.G.; Carter, D.C. Effect of eicosapentaenoic acid and other fatty acids on the growth in vitro of human pancreatic cancer cell lines. Br. J. Cancer 1994, 69, 826–832. [Google Scholar] [CrossRef]
- Mohammed, A.; Janakiram, N.B.; Brewer, M.; Duff, A.; Lightfoot, S.; Brush, R.S.; Anderson, R.E.; Rao, C.V. Endogenous n-3 Polyunsaturated Fatty Acids Delay Progression of Pancreatic Ductal Adenocarcinoma in Fat-1-p48Cre/+-LSL-KrasG12D/+ Mice. Neoplasia 2012, 14, 1249-IN46. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Yu, J.; Xiao, J.; Cao, B.-W. The Consumption of Omega-3 Polyunsaturated Fatty Acids Improves Clinical Outcomes and Prognosis in Pancreatic Cancer Patients: A Systematic Evaluation. Nutr. Cancer 2015, 67, 112–118. [Google Scholar] [CrossRef]
- Yao, X.; Tian, Z. Saturated, Monounsaturated and Polyunsaturated Fatty Acids Intake and Risk of Pancreatic Cancer: Evidence from Observational Studies. PLoS ONE 2015, 10, e0130870. [Google Scholar] [CrossRef]
- Rasooly, D.; Patel, C.J. Conducting a Reproducible Mendelian Randomization Analysis Using the R Analytic Statistical Environment. Curr. Protoc. Hum. Genet. 2019, 101, e82. [Google Scholar] [CrossRef]
- Smith, G.D.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. Genome-wide genetic data on ~500,000 UK Biobank participants. BioRxiv 2017. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Gkatzionis, A.; Burgess, S.; Newcombe, P.J. Statistical methods for cis-Mendelian randomization with two-sample summary-level data. Genet. Epidemiol. 2023, 47, 3–25. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA-J. Am. Med. Assoc. 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Frank, R.D.; Anderson, K.E.; Bamlet, W.R.; Oberg, A.L.; Rabe, K.G.; Olson, J.E.; Sinha, R.; Petersen, G.M.; et al. Fatty acids found in dairy, protein and unsaturated fatty acids are associated with risk of pancreatic cancer in a case-control study. Int. J. Cancer 2014, 134, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Haqq, J.; Howells, L.M.; Garcea, G.; Dennison, A.R. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem™. Mol. Nutr. Food Res. 2016, 60, 1437–1447. [Google Scholar] [CrossRef]
- Song, K.-S.; Jing, K.; Kim, J.-S.; Yun, E.-J.; Shin, S.; Seo, K.-S.; Park, J.-H.; Heo, J.-Y.; Kang, J.X.; Suh, K.-S.; et al. Omega-3-Polyunsaturated Fatty Acids Suppress Pancreatic Cancer Cell Growth in vitro and in vivo via Downregulation of Wnt/Beta-Catenin Signaling. Pancreatology 2011, 11, 574–584. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczynski, A.; Ogluszka, M.; Nawrocka, A.; Polawska, E.; Grzesiak, A.; Slaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchala, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macri, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zheng, W.; Blot, W.J.; Steinwandel, M.D.; Wen, W.; Xiang, Y.-B.; Gao, Y.-T.; Li, H.-L.; Stolzenberg-Solomon, R.; Murff, H.J.; et al. Abstract 639: Dietary polyunsaturated fatty acids intake and pancreatic cancer risk in understudied populations. Cancer Res. 2019, 79 (Suppl. 13), 639. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Urzì, A.G.; Tropea, E.; Gattuso, G.; Spoto, G.; Marsala, G.; Calina, D.; Libra, M.; Falzone, L. Ketogenic Diet and Breast Cancer: Recent Findings and Therapeutic Approaches. Nutrients 2023, 15, 4357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Yang, W.; Chen, H.; Geng, X.; Li, G.; Chen, H.; Wang, Y.; Li, L.; Sun, B. Beneficial Diets and Pancreatic Cancer: Molecular Mechanisms and Clinical Practice. Front. Oncol. 2021, 11, 630972. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, J.; Jia, G.; Yang, Z. Dietary Factors and Pancreatic Cancer Risk: An Umbrella Review of Meta-Analyses of Prospective Observational Studies. Adv. Nutr. 2023, 14, 451–464. [Google Scholar] [CrossRef]
- Fujii, R.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Mizuno, G.; Ando, Y.; Maeda, K.; Tsuboi, Y.; Ohashi, K.; Ishikawa, H.; et al. Dietary fish and ω-3 polyunsaturated fatty acids are associated with leukocyte ABCA1 DNA methylation levels. Nutrition 2021, 81, 110951. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, J.; Hatia, R.; Hassan, M.; Daniel, C.R. Dietary Intake of Fatty Acids and Risk of Pancreatic Cancer: A Case-Control Study. J. Nutr. 2022, 152, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kang, K.S.; Okada, K.; Zhu, B.T. EPA, an omega-3 fatty acid, induces apoptosis in human pancreatic cancer cells: Role of ROS accumulation, caspase-8 activation, and autophagy induction. J. Cell. Biochem. 2013, 114, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lim, J.W.; Kim, H. Docoxahexaenoic Acid Induces Apoptosis of Pancreatic Cancer Cells by Suppressing Activation of STAT3 and NF-κB. Nutrients 2018, 10, 1621. [Google Scholar] [CrossRef]
- Samanta, C.; Tewari, S.; Chakraborty, D.; Vaishnav, S. Omega-3 Fatty Acid and Its Protective Effect against Cancer and Cancer-related Complication. J. Pharm. Res. Int. 2022, 34, 51–62. [Google Scholar] [CrossRef]
- Chacinska, M.; Zabielski, P.; Ksiazek, M.; Szalaj, P.; Jarzabek, K.; Kojta, I.; Chabowski, A.; Blachnio-Zabielska, A.U. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.; Delakas, D.; Nakopoulou, L.; Kassimatis, T. Statins and prostate cancer: Molecular and clinical aspects. Eur. J. Cancer 2011, 47, 819–830. [Google Scholar] [CrossRef]
- Graaf, M.R.; Richel, D.J.; van Noorden, C.J.F.; Guchelaar, H.-J. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat. Rev. 2004, 30, 609–641. [Google Scholar] [CrossRef]
- Wang, D.; Rodriguez, E.A.; Barkin, J.S.; Donath, E.M.; Pakravan, A.S. Statin Use Shows Increased Overall Survival in Patients Diagnosed with Pancreatic Cancer: A Meta-Analysis. Gastroenterology 2019, 48, e22–e23. [Google Scholar] [CrossRef] [PubMed]
- Rubanovich, A.V.; Khromov-Borisov, N.N. Theoretical analysis of the predictability indices of the binary genetic tests. Russ. J. Genet. Appl. Res. 2014, 4, 146–158. [Google Scholar] [CrossRef]
- Bouch, R.J.; Zhang, J.; Miller, B.C.; Robbins, C.J.; Mosher, T.H.; Li, W.; Krupenko, S.A.; Nagpal, R.; Zhao, J.; Bloomfeld, R.S.; et al. Distinct inflammatory Th17 subsets emerge in autoimmunity and infection. J. Exp.Med. 2023, 220, e20221911. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ruiz, J.; Xing, F.; Lo, H.-W.; Craddock, L.; Pullikuth, A.K.; Miller, L.D.; Soike, M.H.; O’Neill, S.S.; Watabe, K.; et al. Single-cell sequencing reveals the landscape of the human brain metastatic microenvironment. Commun. Biol. 2023, 6, 760. [Google Scholar] [CrossRef] [PubMed]

| Exposure | Outcome | Method | nsnp | pval | or | or_lci 95 | or_uci 95 |
|---|---|---|---|---|---|---|---|
| Docosahexaenoic acid | Pancreatic cancer | MR Egger | 83 | 0.074 | 0.79 | 0.61 | 1.02 |
| Docosahexaenoic acid | Pancreatic cancer | Weighted median | 83 | 0.03 | 0.74 | 0.57 | 0.96 |
| Docosahexaenoic acid | Pancreatic cancer | Ivw | 83 | 0.004 | 0.77 | 0.64 | 0.92 |
| Docosahexaenoic acid | Pancreatic cancer | Simple mode | 83 | 0.13 | 0.59 | 0.30 | 1.16 |
| Docosahexaenoic acid | Pancreatic cancer | Weighted mode | 83 | 0.03 | 0.76 | 0.60 | 0.97 |
| Omega-3 levels | Pancreatic cancer | MR Egger | 103 | 0.06 | 0.80 | 0.64 | 0.99 |
| Omega-3 levels | Pancreatic cancer | Weighted median | 103 | 0.02 | 0.78 | 0.63 | 0.96 |
| Omega-3 levels | Pancreatic cancer | Ivw | 103 | 0.01 | 0.81 | 0.70 | 0.95 |
| Omega-3 levels | Pancreatic cancer | Simple mode | 103 | 0.005 | 0.48 | 0.29 | 0.79 |
| Omega-3 levels | Pancreatic cancer | Weighted mode | 103 | 0.01 | 0.79 | 0.66 | 0.94 |
| Omega-6 levels | Pancreatic cancer | MR Egger | 96 | 0.09 | 0.71 | 0.47 | 1.05 |
| Omega-6 levels | Pancreatic cancer | Weighted median | 96 | 0.04 | 0.72 | 0.52 | 0.98 |
| Omega-6 levels | Pancreatic cancer | Ivw | 96 | 0.25 | 0.88 | 0.70 | 1.09 |
| Omega-6 levels | Pancreatic cancer | Simple mode | 96 | 0.02 | 0.57 | 0.35 | 0.91 |
| Omega-6 levels | Pancreatic cancer | Weighted mode | 96 | 0.02 | 0.68 | 0.50 | 0.93 |
| Omega-6/-3 ratio | Pancreatic cancer | MR Egger | 85 | 0.10 | 1.21 | 0.97 | 1.51 |
| Omega-6/-3 ratio | Pancreatic cancer | Weighted median | 85 | 0.02 | 1.27 | 1.04 | 1.54 |
| Omega-6/-3 ratio | Pancreatic cancer | Ivw | 85 | 0.02 | 1.24 | 1.04 | 1.47 |
| Omega-6/-3 ratio | Pancreatic cancer | Simple mode | 85 | 0.03 | 2.01 | 1.08 | 3.75 |
| Omega-6/-3 ratio | Pancreatic cancer | Weighted mode | 85 | 0.02 | 1.26 | 1.04 | 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, H.; Zhu, W. Employing Bidirectional Two-Sample Mendelian Randomization Analysis to Verify the Potential of Polyunsaturated Fatty Acid Levels in the Prevention of Pancreatic Cancer. Curr. Issues Mol. Biol. 2024, 46, 6041-6051. https://doi.org/10.3390/cimb46060360
Sha H, Zhu W. Employing Bidirectional Two-Sample Mendelian Randomization Analysis to Verify the Potential of Polyunsaturated Fatty Acid Levels in the Prevention of Pancreatic Cancer. Current Issues in Molecular Biology. 2024; 46(6):6041-6051. https://doi.org/10.3390/cimb46060360
Chicago/Turabian StyleSha, Hao, and Weifeng Zhu. 2024. "Employing Bidirectional Two-Sample Mendelian Randomization Analysis to Verify the Potential of Polyunsaturated Fatty Acid Levels in the Prevention of Pancreatic Cancer" Current Issues in Molecular Biology 46, no. 6: 6041-6051. https://doi.org/10.3390/cimb46060360
APA StyleSha, H., & Zhu, W. (2024). Employing Bidirectional Two-Sample Mendelian Randomization Analysis to Verify the Potential of Polyunsaturated Fatty Acid Levels in the Prevention of Pancreatic Cancer. Current Issues in Molecular Biology, 46(6), 6041-6051. https://doi.org/10.3390/cimb46060360






