Low Levels of IgM Recognizing 4-Hydroxy-2-Nonenal-Modified Apolipoprotein A-I Peptide and Its Association with the Severity of Coronary Artery Disease in Taiwanese Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. In-Gel Digestion and Identification of Novel HNE Modifications Using Nano-LC-MS/MS and PEAKS 7 Software
2.4. Confirmation of HNE-Modified ApoA-I by IP-WB
2.5. Evaluation of ApoA-I Levels by WB
2.6. Quantification of Plasma HNE-Protein Adducts and Autoantibodies Recognizing Unmodified and HNE-ApoA-I Peptide Adducts by an ELISA
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Identification and Validation of Novel HNE Modifications of Plasma ApoA-I
3.3. Determination of Levels of Plasma in ApoA-I and HNE-Protein Adducts
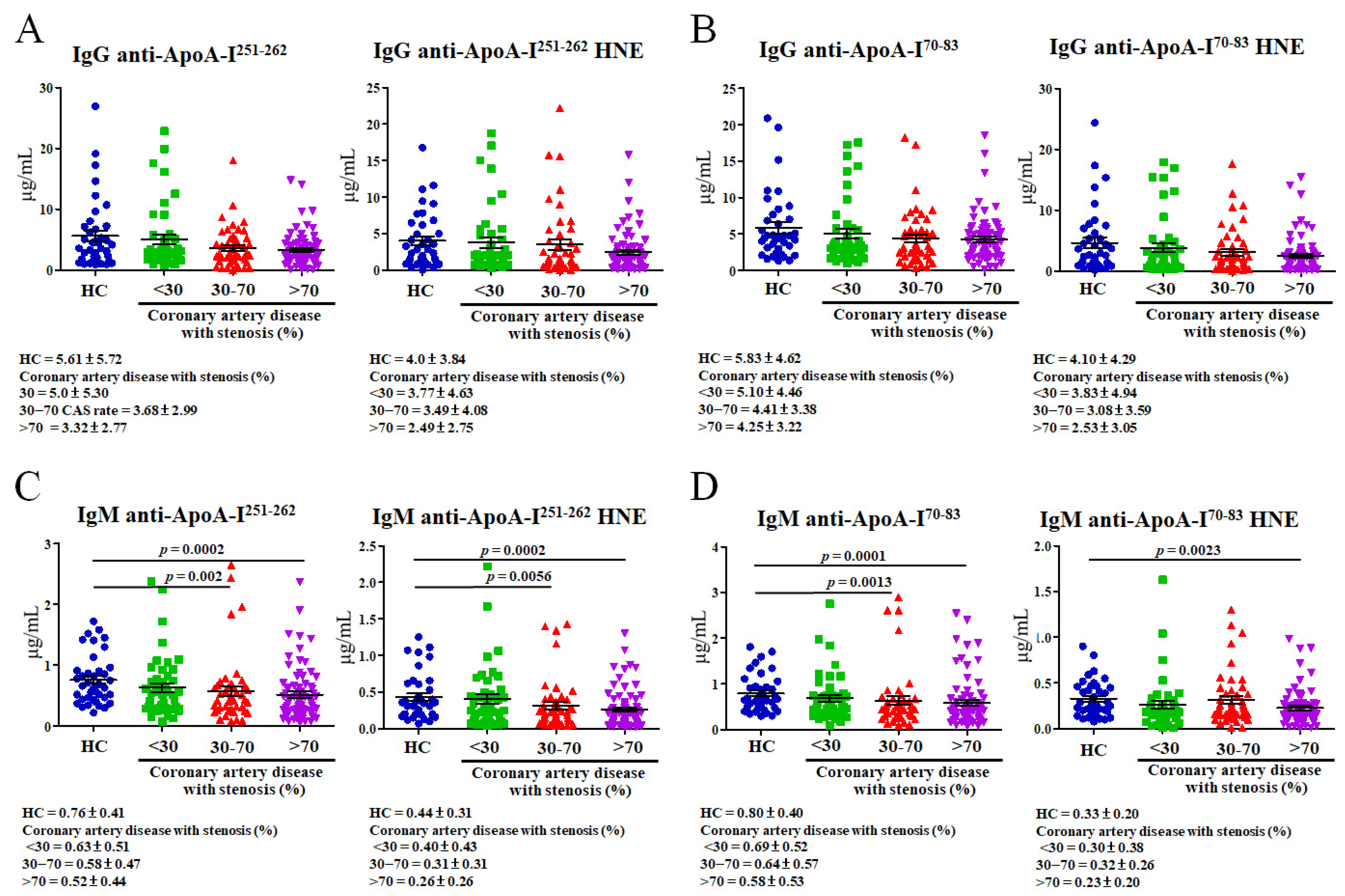
3.4. Determining Levels of Plasma Autoantibodies That Recognize Unmodified and HNE-ApoA-I Peptide Adducts
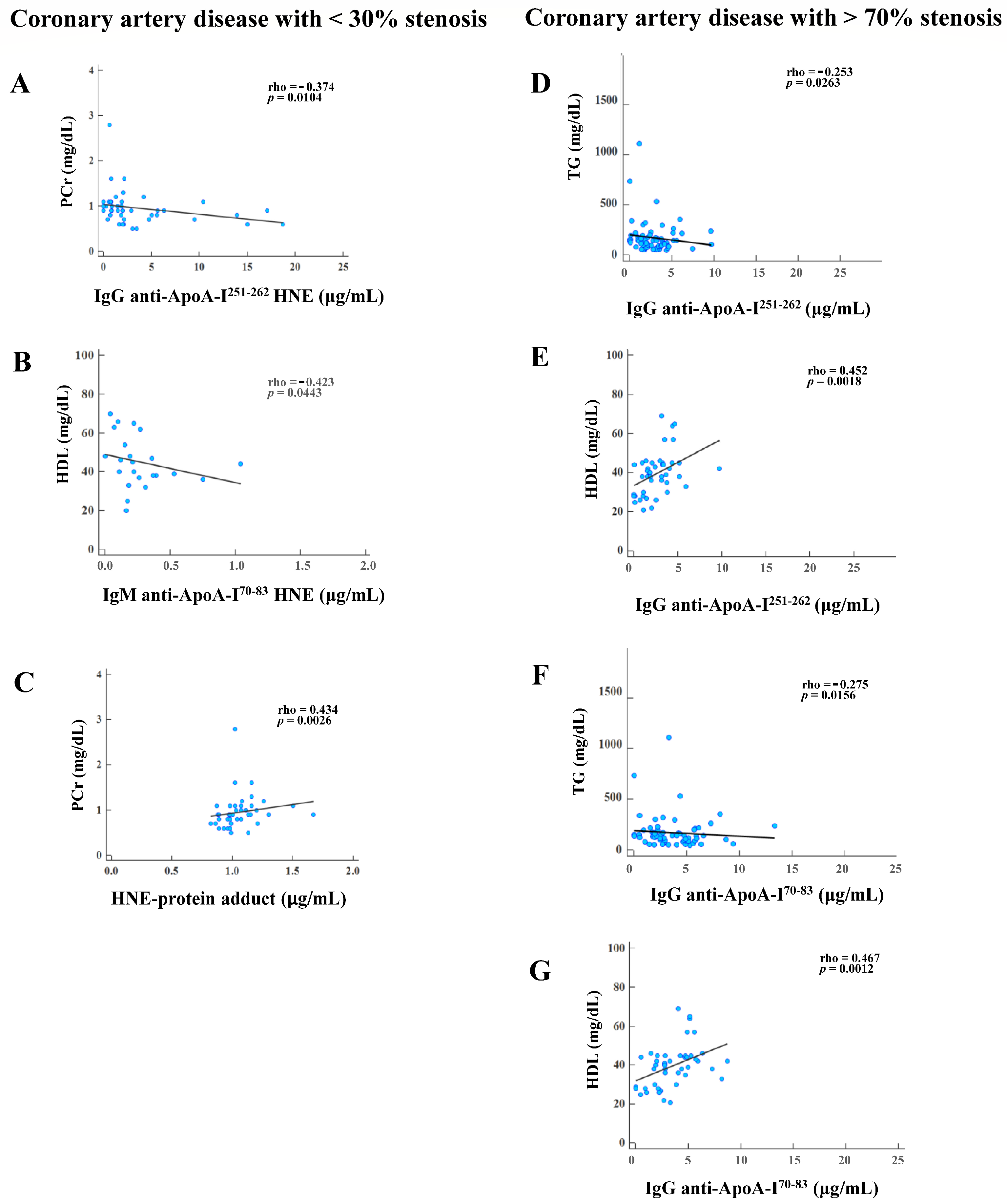
3.5. Correlations of Plasma HNE-Protein Adduct and Anti-Unmodified and Anti-HNE-Peptide Adduct Autoantibodies with Blood Tests in CAD Patients with Various Degrees of Stenosis
3.6. Associations between Plasma Autoantibodies and HNE-Protein Adducts of CAD Patients with >30% Versus <30% Stenosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Faviou, E.; Vourli, G.; Nounopoulos, C.; Zachari, A.; Dionyssiou-Asteriou, A. Circulating oxidized low density lipoprotein, autoantibodies against them and homocysteine serum levels in diagnosis and estimation of severity of coronary artery disease. Free Radic. Res. 2005, 39, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.G.T.; Kwon, E. Risk Factors for Coronary Artery Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ueng, K.C.; Chiang, C.E.; Chao, T.H.; Wu, Y.W.; Lee, W.L.; Li, Y.H.; Ting, K.H.; Su, C.H.; Lin, H.J.; Su, T.C.; et al. 2023 Guidelines of the Taiwan Society of Cardiology on the Diagnosis and Management of Chronic Coronary Syndrome. Acta Cardiol. Sin. 2023, 39, 4–96. [Google Scholar] [PubMed]
- Pieris, R.R.; Al-Sabti, H.A.; Al-Abri, Q.S.; Rizvi, S.G. Prevalence Pattern of Risk Factors for Coronary Artery Disease among Patients Presenting for Coronary Artery Bypass Grafting in Oman. Oman Med. J. 2014, 29, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Gisonno, R.A.; Prieto, E.D.; Gorgojo, J.P.; Curto, L.M.; Rodriguez, M.E.; Rosu, S.A.; Gaddi, G.M.; Finarelli, G.S.; Cortez, M.F.; Schinella, G.R.; et al. Fibrillar conformation of an apolipoprotein A-I variant involved in amyloidosis and atherosclerosis. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129515. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Barnett, J.; Fong, L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta 1990, 1044, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Dayer, J.M. High-density lipoprotein-associated apolipoprotein A-I: The missing link between infection and chronic inflammation? Autoimmun. Rev. 2002, 1, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Munoz, F.; Valle, Y.; Munoz-Valle, J.F.; Martinez-Fernandez, D.E.; Reynoso-Villalpando, G.L.; Flores-Salinas, H.E.; Llamas-Covarrubias, M.A.; Padilla-Gutierrez, J.R. APOA1 and APOB polymorphisms and apolipoprotein concentrations as biomarkers of risk in acute coronary syndrome: Relationship with lipid-lowering therapy effectiveness. Med. Clin. 2018, 151, 1–7. [Google Scholar] [CrossRef]
- Cho, K.H.; Shin, D.G.; Baek, S.H.; Kim, J.R. Myocardial infarction patients show altered lipoprotein properties and functions when compared with stable angina pectoris patients. Exp. Mol. Med. 2009, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Antiochos, P.; Marques-Vidal, P.; Virzi, J.; Pagano, S.; Satta, N.; Bastardot, F.; Hartley, O.; Montecucco, F.; Mach, F.; Waeber, G.; et al. Association between anti-apolipoprotein A-1 antibodies and cardiovascular disease in the general population. Results from the CoLaus study. Thromb. Haemost. 2016, 116, 764–771. [Google Scholar]
- Keller, P.F.; Pagano, S.; Roux-Lombard, P.; Sigaud, P.; Rutschmann, O.T.; Mach, F.; Hochstrasser, D.; Vuilleumier, N. Autoantibodies against apolipoprotein A-1 and phosphorylcholine for diagnosis of non-ST-segment elevation myocardial infarction. J. Intern. Med. 2012, 271, 451–462. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Ducret, A.; Ferber, P.; Gaertner, H.; Hartley, O.; Pagano, S.; Butterfield, M.; Langen, H.; Vuilleumier, N.; Cutler, P. Definition of human apolipoprotein A-I epitopes recognized by autoantibodies present in patients with cardiovascular diseases. J. Biol. Chem. 2014, 289, 28249–28259. [Google Scholar] [CrossRef]
- Batuca, J.R.; Amaral, M.C.; Favas, C.; Paula, F.S.; Ames, P.R.J.; Papoila, A.L.; Delgado Alves, J. Extended-release niacin increases anti-apolipoprotein A-I antibodies that block the antioxidant effect of high-density lipoprotein-cholesterol: The EXPLORE clinical trial. Br. J. Clin. Pharmacol. 2017, 83, 1002–1010. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Status of oxidative stress in rheumatoid arthritis. Int. J. Rheum. Dis. 2009, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hoff, H.F.; O’Neil, J.; Chisolm, G.M., 3rd; Cole, T.B.; Quehenberger, O.; Esterbauer, H.; Jurgens, G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis 1989, 9, 538–549. [Google Scholar] [CrossRef]
- Sayre, L.M.; Lin, D.; Yuan, Q.; Zhu, X.; Tang, X. Protein adducts generated from products of lipid oxidation: Focus on HNE and one. Drug Metab. Rev. 2006, 38, 651–675. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zhu, H.; Xiong, Y.L. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J. Agric. Food Chem. 2012, 60, 9727–9736. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Lin, H.; Li, Z.; Yuan, F.; Gao, Q.; Ma, J. Effect of 4-hydroxy-2-nonenal treatment on the IgE binding capacity and structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chem. 2016, 212, 313–322. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Testa, G.; Gamba, P.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxysterols and 4-hydroxy-2-nonenal contribute to atherosclerotic plaque destabilization. Free Radic. Biol. Med. 2017, 111, 140–150. [Google Scholar] [CrossRef]
- Eggleton, P.; Nissim, A.; Ryan, B.J.; Whiteman, M.; Winyard, P.G. Detection and isolation of human serum autoantibodies that recognize oxidatively modified autoantigens. Free Radic. Biol. Med. 2013, 57, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Shobaili, H.A.; Al Robaee, A.A.; Alzolibani, A.A.; Rasheed, Z. Antibodies against 4-hydroxy-2-nonenal modified epitopes recognized chromatin and its oxidized forms: Role of chromatin, oxidized forms of chromatin and 4-hydroxy-2-nonenal modified epitopes in the etiopathogenesis of SLE. Dis. Markers 2012, 33, 19–34. [Google Scholar] [CrossRef]
- Mottaran, E.; Stewart, S.F.; Rolla, R.; Vay, D.; Cipriani, V.; Moretti, M.; Vidali, M.; Sartori, M.; Rigamonti, C.; Day, C.P.; et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Smith, M.A.; Avila, J.; Nunomura, A.; Siedlak, S.L.; Zhu, X.; Perry, G.; Sayre, L.M. In Alzheimer’s disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J. Neurochem. 2000, 75, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Pozzi, R.; Leonarduzzi, G.; Aroasio, E.; Gamba, P.; Gargiulo, S.; Rabajoli, F.; Ferrari, F.; Greco Lucchina, P.; Poli, G. Lipid peroxidation and inflammatory molecules as markers of coronary artery disease. Redox Rep. 2007, 12, 81–85. [Google Scholar] [CrossRef]
- Wakamatsu, T.H.; Dogru, M.; Matsumoto, Y.; Kojima, T.; Kaido, M.; Ibrahim, O.M.; Sato, E.A.; Igarashi, A.; Ichihashi, Y.; Satake, Y.; et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Catarina, B.; Afonso, C.M.S. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2019, 23, 101066. [Google Scholar]
- Ong, C.T.; Wong, Y.S.; Sung, S.F.; Wu, C.S.; Hsu, Y.C.; Su, Y.H.; Hung, L.C. Progression of Mild to Moderate Stenosis in the Internal Carotid Arteries of Patients With Ischemic Stroke. Front. Neurol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguade-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef]
- Moladoust, H.; Mokhtari-Dizaji, M.; Ojaghi-Haghighi, Z.; Noohi, F. Non-invasive assessment of coronary artery stenosis with estimation of myocardial wall stress. J. Tehran Heart Cent. 2010, 5, 29–35. [Google Scholar]
- Hung, M.J.; Cherng, W.J. Comparison of white blood cell counts in acute myocardial infarction patients with significant versus insignificant coronary artery disease. Am. J. Cardiol. 2003, 91, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Uen, Y.H.; Lin, K.Y.; Sun, D.P.; Liao, C.C.; Hsieh, M.S.; Huang, Y.K.; Chen, Y.W.; Huang, P.H.; Chen, W.J.; Tai, C.C.; et al. Comparative proteomics, network analysis and post-translational modification identification reveal differential profiles of plasma Con A-bound glycoprotein biomarkers in gastric cancer. J. Proteom. 2013, 83, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-protein adducts in human plasma and serum by ELISA-Comparison of two primary antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Chou, P.L.; Cheng, C.W.; Chang, Y.S.; Chi, W.M.; Tsai, K.L.; Chen, W.J.; Kung, T.S.; Tai, C.C.; Lee, K.W.; et al. Comparative analysis of novel autoantibody isotypes against citrullinated-inter-alpha-trypsin inhibitor heavy chain 3 (ITIH3)(542–556) peptide in serum from Taiwanese females with rheumatoid arthritis, primary Sjogren’s syndrome and secondary Sjogren’s syndrome in rheumatoid arthritis. J. Proteom. 2016, 141, 1–11. [Google Scholar]
- Fenaille, F.; Tabet, J.C.; Guy, P.A. Identification of 4-hydroxy-2-nonenal-modified peptides within unfractionated digests using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2004, 76, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Hu, J.; Yin, Z.; Xu, Z.; Zhang, L.; Fan, L.; Zhuo, Y.; Wang, C. Low serum paraoxonase1 activity levels predict coronary artery disease severity. Oncotarget 2017, 8, 19443–19454. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, R.I.; El-Leboudy, M.H.; El-Deeb, H.M. The relation between ApoB/ApoA-1 ratio and the severity of coronary artery disease in patients with acute coronary syndrome. Egypt. Heart. J. 2021, 73, 24. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; Larosa, J.C.; Welch, K.M.; Amarenco, P.; Demicco, D.A.; Tonkin, A.M.; et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: A meta-analysis. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef]
- Tian, M.; Li, R.; Shan, Z.; Wang, D.W.; Jiang, J.; Cui, G. Comparison of Apolipoprotein B/A1 ratio, Framingham risk score and TC/HDL-c for predicting clinical outcomes in patients undergoing percutaneous coronary intervention. Lipids Health Dis. 2019, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, I.D.; Gisonno, R.A.; Gonzalez, M.C.; Garda, H.A.; Ramella, N.A.; Tricerri, M.A. Understanding the role of apolipoproteinA-I in atherosclerosis. Post-translational modifications synergize dysfunction? Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129732. [Google Scholar] [CrossRef] [PubMed]
- Szapacs, M.E.; Kim, H.Y.; Porter, N.A.; Liebler, D.C. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J. Proteome Res. 2008, 7, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Gaertner, H.; Cerini, F.; Mannic, T.; Satta, N.; Teixeira, P.C.; Cutler, P.; Mach, F.; Vuilleumier, N.; Hartley, O. The Human Autoantibody Response to Apolipoprotein A-I Is Focused on the C-Terminal Helix: A New Rationale for Diagnosis and Treatment of Cardiovascular Disease? PLoS ONE 2015, 10, e0132780. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Radmard, N.; Faghani-Makrani, A.; Rasouli, M. Serum Creatinine and Occurrence and Severity of Coronary Artery Disease. Med. Arch. 2019, 73, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Maruhashi, T.; Kishimoto, S.; Matsui, S.; Hashimoto, H.; Takaeko, Y.; Yusoff, F.M.; Kihara, Y.; Chayama, K.; Goto, C.; et al. Target of Triglycerides as Residual Risk for Cardiovascular Events in Patients With Coronary Artery Disease- Post Hoc Analysis of the FMD-J Study A. Circ. J. 2019, 83, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Faergeman, O.; Holme, I.; Fayyad, R.; Bhatia, S.; Grundy, S.M.; Kastelein, J.J.; LaRosa, J.C.; Larsen, M.L.; Lindahl, C.; Olsson, A.G.; et al. Plasma triglycerides and cardiovascular events in the Treating to New Targets and Incremental Decrease in End-Points through Aggressive Lipid Lowering trials of statins in patients with coronary artery disease. Am. J. Cardiol. 2009, 104, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Christodoulidis, G.; Cheng, J.W.; Vittorio, T.J.; Lerakis, S. High-density lipoprotein functionality in coronary artery disease. Am. J. Med. Sci. 2014, 347, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J. Natural IgM antibodies against oxidation-specific epitopes. J. Clin. Immunol. 2010, 30 (Suppl. S1), S56–S60. [Google Scholar] [CrossRef]
- Ogden, C.A.; Kowalewski, R.; Peng, Y.; Montenegro, V.; Elkon, K.B. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity 2005, 38, 259–264. [Google Scholar] [CrossRef]
- Chou, M.Y.; Fogelstrand, L.; Hartvigsen, K.; Hansen, L.F.; Woelkers, D.; Shaw, P.X.; Choi, J.; Perkmann, T.; Backhed, F.; Miller, Y.I.; et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Investig. 2009, 119, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kumano-Kuramochi, M.; Shimozu, Y.; Wakita, C.; Ohnishi-Kameyama, M.; Shibata, T.; Matsunaga, S.; Takano-Ishikawa, Y.; Watanabe, J.; Goto, M.; Xie, Q.; et al. Identification of 4-hydroxy-2-nonenal-histidine adducts that serve as ligands for human lectin-like oxidized LDL receptor-1. Biochem. J. 2012, 442, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.J.; Shen, W.C.; Wu, J.Z.; Chang, Y.S.; Lin, C.Y. Autoantibodies to Oxidatively Modified Peptide: Potential Clinical Application in Coronary Artery Disease. Diagnostics 2022, 12, 2269. [Google Scholar] [CrossRef]
- Verma, A.K.; Chandra, S.; Singh, R.G.; Singh, T.B.; Srivastava, S.; Srivastava, R. Serum prolidase activity and oxidative stress in diabetic nephropathy and end stage renal disease: A correlative study with glucose and creatinine. Biochem. Res. Int. 2014, 2014, 291458. [Google Scholar] [CrossRef]


| Variables a | Discovery Set b | Validation Set b | |||||
|---|---|---|---|---|---|---|---|
| Healthy Controls, n = 20 | Coronary Artery Disease with Stenosis (%) | Healthy Controls, n = 40 | Coronary Artery Disease with Stenosis (%) | ||||
| ≤50 n = 20 | >50 n = 20 | <30 n = 46 | 30~70 n = 47 | >70 n = 79 | |||
| Age (years) | 34 ± 3.39 | 63.6 ± 9.36 ** | 63.4 ± 8.38 ** | 38.3 ± 10.19 | 62.93 ± 10.22 ** | 63.06 ± 9.86 ** | 62.43 ± 9.07 ** |
| Gender | |||||||
| Male, n (%) | 14 (70.0) | 14 (70.0) | 14 (70.0) | 24 (60.0) | 31 (67.4) | 33 (70.2) | 60 (76.0) |
| Drinker, n (%) | 6 (30.0) | 6 (30.0) | 6 (30.0) | 16 (40.0) | 15 (32.6) | 14 (29.8) | 19 (24.0) |
| Used to smoke, n (%) | 0 (0) | 9 (45.0) ** | 5 (25.0) | 1 (2.5) | 15 (32.6) ** | 8 (17.0) | 18 (22.8) ** |
| Current smoker, n (%) | 6 (30.0) | 1 (5.0) | 5 (25.0) | 13 (32.5) | 4 (8.6) ** | 7 (14.8) | 27 (34.1) |
| ESRD, n (%) | 0 (0) | 1 (5) | 7 (35) * | 0 (0) | 1 (2.1) | 2 (4.2) | 13 (16.4) * |
| Hypertension, n (%) | 1 (5.0) | 14 (70.0) ** | 14 (70.0) ** | 2 (5.0) | 30 (65.2) ** | 38 (80.8) ** | 49 (62.0) ** |
| Lipid-lowering agents, n (%) | 0 (0) | 6 (30.0) ** | 11 (55.0) ** | 0 (0) | 11 (23.9) ** | 15 (31.9) ** | 56 (70.8) ** |
| Blood tests | |||||||
| T-CHOL (mg/dL) | 143.06 ± 30.56 | 143.23 ± 32.25 | 119.86 ± 43.53 | 158.19 ± 34.7 | 145.49 ± 35.92 | 139.42 ± 28.6 * | 140.98 ± 45.98 |
| HDL (mg/dL) | 45.62 ± 13.06 | 48.08 ± 12.99 | 31.42 ± 9.57 * | 50.25 ± 13.65 | 45.33 ± 12.88 | 48.81 ± 17.82 | 39.09 ± 12.26 ** |
| LDL (mg/dL) | 80.25 ± 37.04 | 81.29 ± 28.67 | 77.57 ± 34.21 | 92.97 ± 36.24 | 86.33 ± 30.95 | 79.04 ± 30.95 | 89.29 ± 38.69 |
| TGs (mg/dL) | 86.37 ± 30.5 | 82.55 ± 33 | 89.4 ± 34.8 | 76.97 ± 26.62 | 116.58 ± 118.17 | 103.88 ± 114.25 | 141.63 ± 162.55 * |
| PCr (mg/dL) | 0.735 ± 0.122 | 0.935 ± 0.480 | 1.09 ± 0.453 * | 0.753 ± 0.302 | 0.946 ± 0.365 * | 0.917 ± 0.28 | 1.171 ± 0.884 * |
| HNE-protein adduct (μg/mL) | - | - | - | 1.012 ± 0.079 | 1.054 ± 0.157 | 1.052 ± 0.105 | 1.116 ± 0.126 ** |
| Protein a | Modified Peptide | Position b | Obs. c | Calc. d | dM e | dM f | Sequence Found with Modification | ||
|---|---|---|---|---|---|---|---|---|---|
| HC | Coronary Artery Disease with Stenosis (%) | ||||||||
| ≤50 | >50 | ||||||||
| ApoA-I | VSFLSALEEYTK(+138.10) | 251~262 | 1523.812 | 1523.815 | 0.003 | −7.5 | - | + | + |
| ApoA-I | L(+138.10)L(+138.10)DNWDSVTSTFSK | 70~83 | 1887.986 | 1887.985 | −0.001 | −0.34 | - | + | - |
| ApoA-I | DYVSQ(+156.12)FEGSALGK | 52~64 | 1555.770 | 1555.789 | 0.019 | −8.6 | + | + | + |
| Coronary Artery Disease with Stenosis (%) | Multivariate Logistic Regression Model b | Power | |||||
|---|---|---|---|---|---|---|---|
| <30 a | >30 | ||||||
| Cutoff | n = 86 | n = 126 | ORs (95% CI) | p-Value | |||
| HNE-protein adduct | ≤ | 1.02 | 47 | 36 | Ref. | 0.020 | 0.938 |
| > | 1.02 | 39 | 90 | 2.208 (1.131, 4.311) | |||
| IgG anti-ApoA-I251–262 | > | 4.49 | 24 | 22 | Ref. | 0.106 | 0.891 |
| ≤ | 4.49 | 62 | 104 | 1.912 (0.870, 4.201) | |||
| IgG anti-ApoA-I251–262 HNE | > | 3.27 | 30 | 28 | Ref. | 0.202 | 0.542 |
| ≤ | 3.27 | 56 | 98 | 1.612 (0.774, 3.357) | |||
| IgG anti-ApoA-I70–83 | > | 2.91 | 58 | 65 | Ref. | 0.018 | 0.623 |
| ≤ | 2.91 | 28 | 61 | 2.277 (1.149, 4.511) | |||
| IgG anti-ApoA-I70–83 HNE | > | 1.06 | 61 | 70 | Ref. | 0.026 | 0.665 |
| ≤ | 1.06 | 25 | 56 | 2.190 (1.096, 4.374) | |||
| IgM anti-ApoA-I251–262 | > | 0.50 | 51 | 50 | Ref. | 0.054 | 0.715 |
| ≤ | 0.50 | 35 | 76 | 1.888 (0.988, 3.608) | |||
| IgM anti-ApoA-I251–262 HNE | > | 0.30 | 41 | 34 | Ref. | 0.035 | 0.881 |
| ≤ | 0.30 | 45 | 92 | 2.046 (1.051, 3.984) | |||
| IgM anti-ApoA-I70–83 | > | 0.59 | 45 | 38 | Ref. | 0.016 | 0.551 |
| ≤ | 0.59 | 41 | 88 | 2.316 (1.172, 4.579) | |||
| IgM anti-ApoA-I70–83 HNE | > | 0.18 | 57 | 60 | Ref. | 0.316 | 0.372 |
| ≤ | 0.18 | 29 | 66 | 1.427 (0.712, 2.860) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.-H.; Hsu, P.-W.; Tsai, Y.-T.; Chang, C.-C.; Tsai, I.-J.; Hsu, H.; Cheng, M.-H.; Huang, Y.-L.; Lin, H.-T.; Hsu, Y.-C.; et al. Low Levels of IgM Recognizing 4-Hydroxy-2-Nonenal-Modified Apolipoprotein A-I Peptide and Its Association with the Severity of Coronary Artery Disease in Taiwanese Patients. Curr. Issues Mol. Biol. 2024, 46, 6267-6283. https://doi.org/10.3390/cimb46060374
Lei M-H, Hsu P-W, Tsai Y-T, Chang C-C, Tsai I-J, Hsu H, Cheng M-H, Huang Y-L, Lin H-T, Hsu Y-C, et al. Low Levels of IgM Recognizing 4-Hydroxy-2-Nonenal-Modified Apolipoprotein A-I Peptide and Its Association with the Severity of Coronary Artery Disease in Taiwanese Patients. Current Issues in Molecular Biology. 2024; 46(6):6267-6283. https://doi.org/10.3390/cimb46060374
Chicago/Turabian StyleLei, Meng-Huan, Po-Wen Hsu, Yin-Tai Tsai, Chen-Chi Chang, I-Jung Tsai, Hung Hsu, Ming-Hui Cheng, Ying-Li Huang, Hung-Tse Lin, Yu-Cheng Hsu, and et al. 2024. "Low Levels of IgM Recognizing 4-Hydroxy-2-Nonenal-Modified Apolipoprotein A-I Peptide and Its Association with the Severity of Coronary Artery Disease in Taiwanese Patients" Current Issues in Molecular Biology 46, no. 6: 6267-6283. https://doi.org/10.3390/cimb46060374





