Significance of CD10 for Mucosal Immunomodulation by β-Casomorphin-7 in Exacerbation of Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
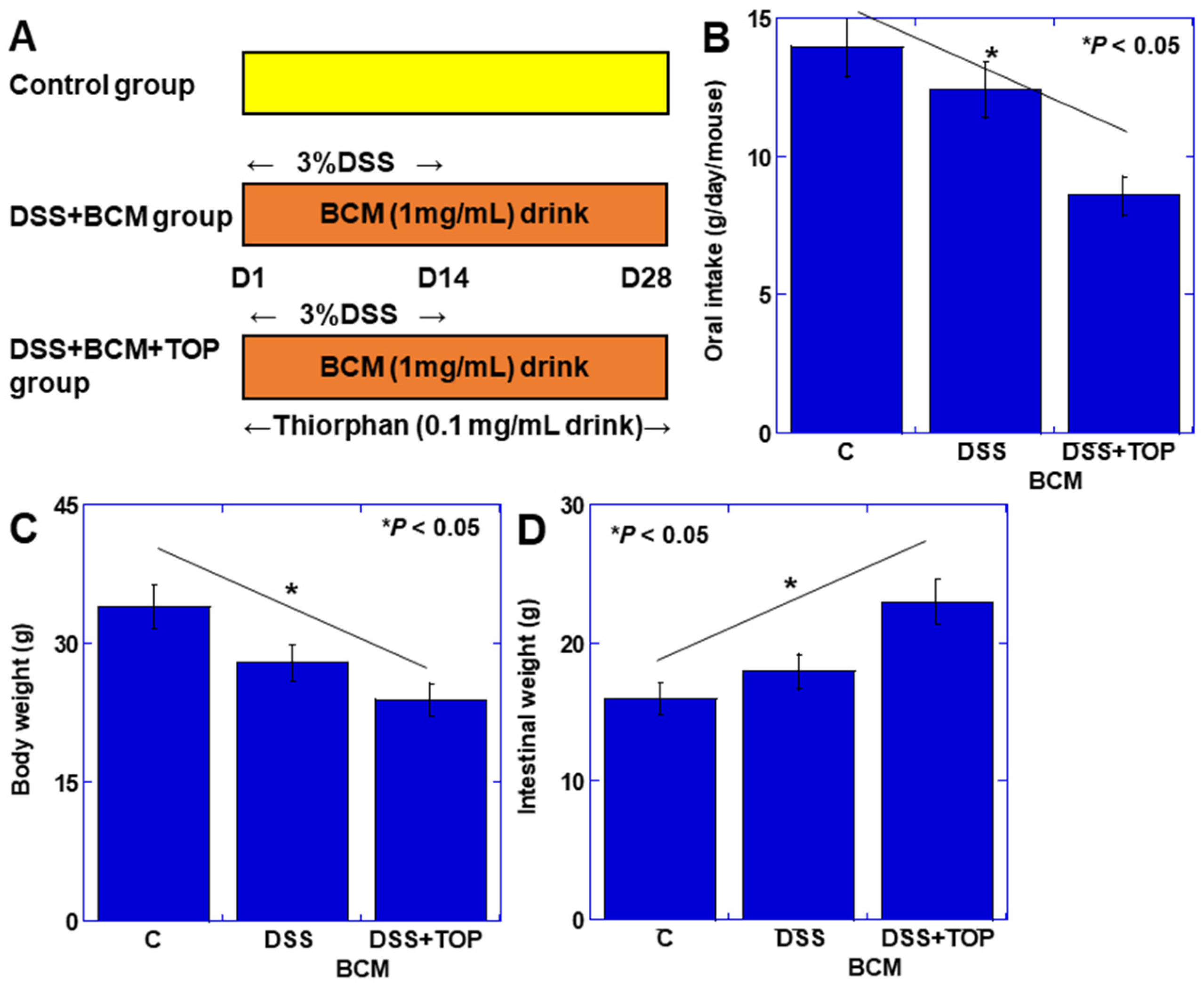
2.2. Mouse Non-Cancer Model
2.3. Mouse DSS Colitis Model
2.4. Protein Extraction
2.5. Spleen Cell Isolation
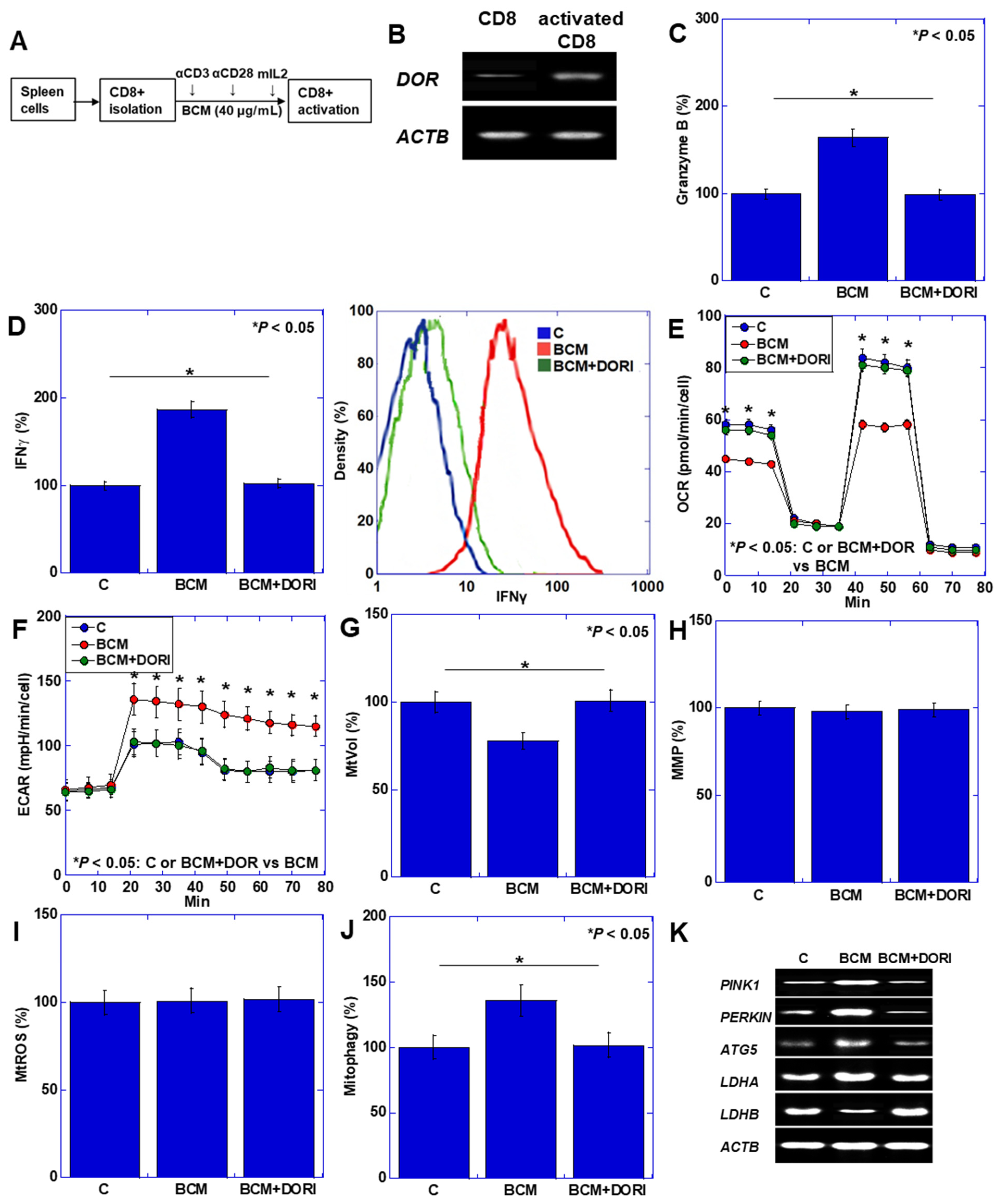
2.6. In Vitro Activation of T Cells
2.7. ELISA
2.8. Mitochondrial Imaging
2.9. Cell Lines
2.10. Flow Cytometry
2.11. Patients
2.12. Methylation-Specific PCR
2.13. Statistical Analysis
3. Results
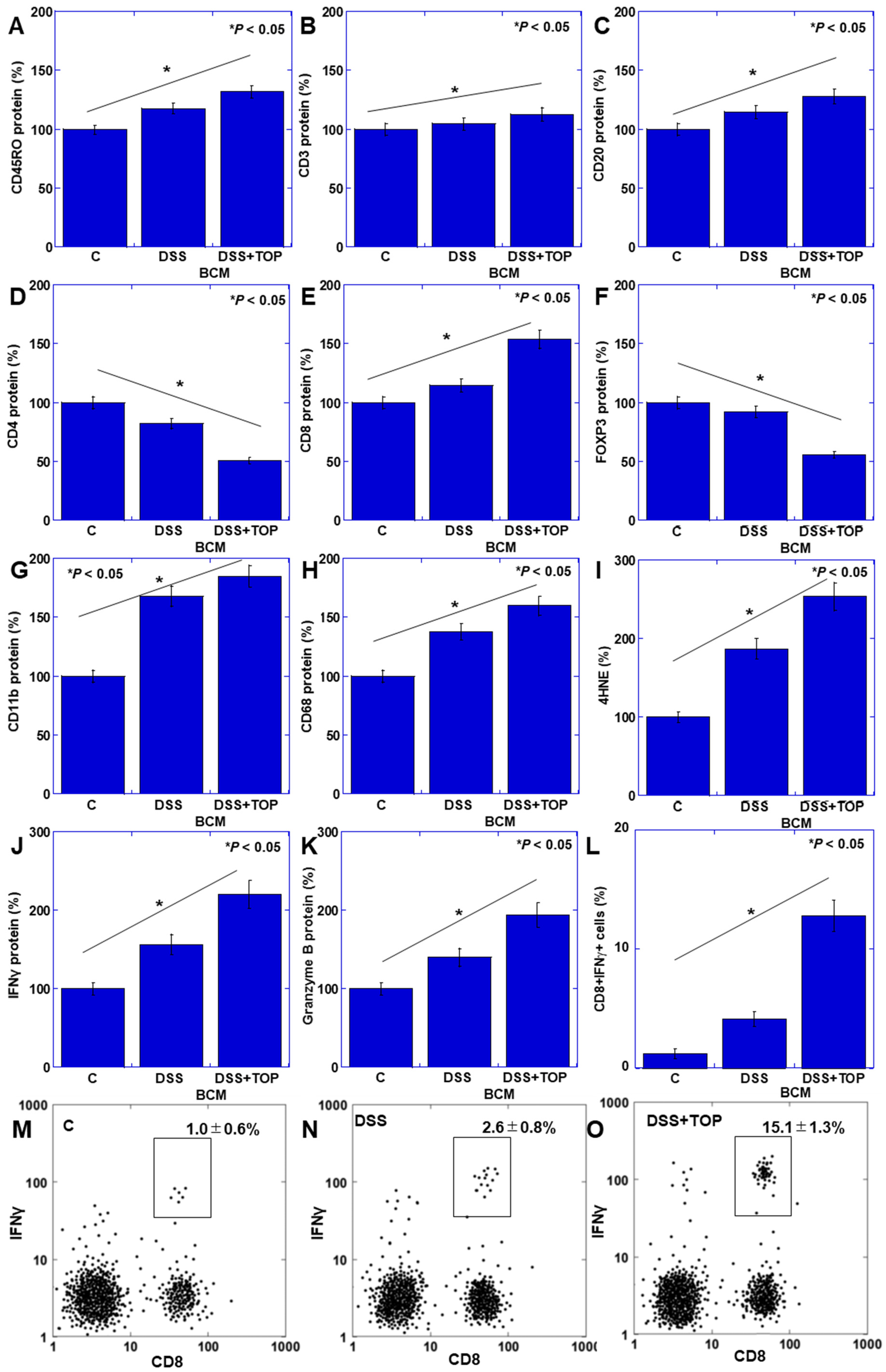
3.1. Effect of BCM in the Mouse Colitis Model
3.2. Activation of CD8+ T Cells by BCM
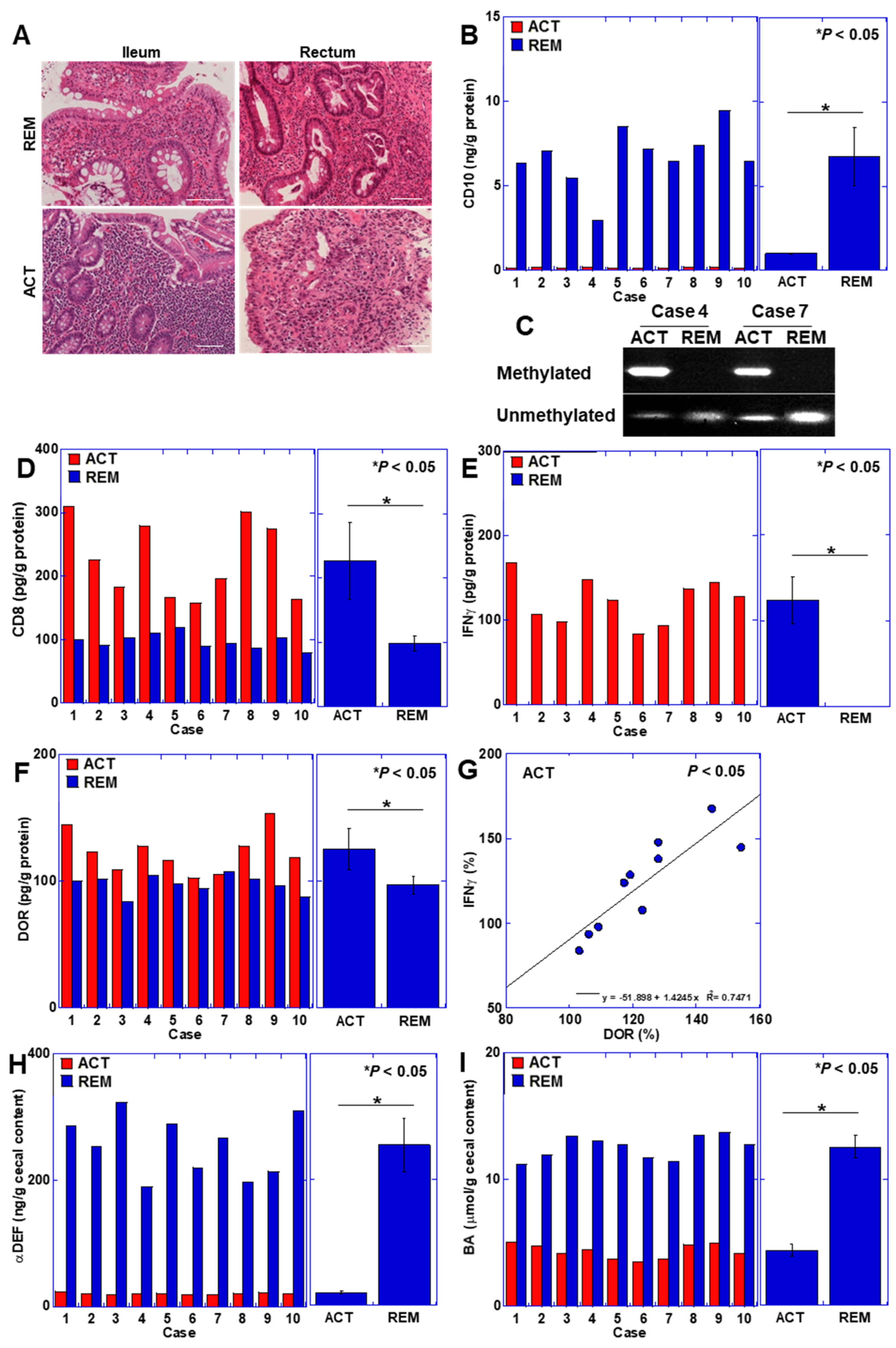
3.3. Effects of BCM on Intestinal Epithelium
3.4. CD10 Status in Human UC Cases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCM | β-casomorphin-7 |
| TOP | thiorphan |
| OXPHOS | oxidative phosphorylation |
| UC | ulcerative colitis |
| Treg | regulatory T cells |
| DSS | dextran sulfate sodium |
| 4HNE | 4-hydroxynonenal |
| IFN | interferon |
| ALV | alvimopan |
| ROS | reactive oxygen species |
| PINK1 | PTEN-induced kinase 1 |
| ATG5 | autophagy-related 5 |
| LDH | lactate dehydrogenase |
| DOR | δ-opioid receptor |
| αDEF | α-defensin |
| BA | butyric acid |
References
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Knowles, S.R.; Graff, L.A.; Wilding, H.; Hewitt, C.; Keefer, L.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef]
- Rahman, S.; Patel, R.K.; Boden, E.; Tsikitis, V.L. Medical Management of Inflammatory Bowel Disease. Surg. Clin. N. Am. 2024, 104, 657–671. [Google Scholar] [CrossRef]
- Kurumi, H.; Yokoyama, Y.; Hirano, T.; Akita, K.; Hayashi, Y.; Kazama, T.; Isomoto, H.; Nakase, H. Cytokine Profile in Predicting the Effectiveness of Advanced Therapy for Ulcerative Colitis: A Narrative Review. Biomedicines 2024, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose Kuzhiyanjal, A.J.; Limdi, J.K. Management of acute severe ulcerative colitis-an update for generalist and specialist clinicians. Br. Med. Bull. 2024, in press. [CrossRef]
- Nagaraj, T.; Shinn, J.; De Felice, K. A practical guide to selecting and using new ulcerative colitis therapies. Curr. Opin. Gastroenterol. 2024, 40, 235–242. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Virta, L.J.; Ashorn, M.; Kolho, K.L. Cow’s milk allergy, asthma, and pediatric IBD. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Czarnywojtek, A.; Wawrzyniak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Dobrowolska, A.; Eder, P. Milk and dairy product consumption in patients with inflammatory bowel disease: Helpful or harmful to bone mineral density? Nutrition 2020, 79–80, 110830. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, Cd012839. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Giannetti, E.; Martinelli, M.; Sciorio, E.; Staiano, A.; Miele, E. Does cow’s milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr. 2013, 102, e273–e278. [Google Scholar] [CrossRef] [PubMed]
- Elitsur, Y.; Luk, G.D. Beta-casomorphin (BCM) and human colonic lamina propria lymphocyte proliferation. Clin. Exp. Immunol. 1991, 85, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Bakogeorgou, E.; Hatzoglou, A.; Damianaki, A.; Martin, P.M.; Castanas, E. Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur. J. Pharmacol. 1997, 335, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Thirupathi, P.; Chung, I.M.; Subramanian, U. β-Casomorphin: A complete health perspective. Food Chem. 2021, 337, 127765. [Google Scholar] [CrossRef] [PubMed]
- De Noni, I.; Fitz Gerald, R.J.; Korhonean, H.J.T.; Le Roux, Y.; Livesey, C.T.; Thorsdottir, I.; Tome, D. Review of the potential health impact of -casomorphins and related peptides. European Food Safety Agency. Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Asledottir, T.; Picariello, G.; Mamone, G.; Ferranti, P.; Røseth, A.; Devold, T.G.; Vegarud, G.E. Degradation of β-casomorphin-7 through in vitro gastrointestinal and jejunal brush border membrane digestion. J. Dairy Sci. 2019, 102, 8622–8629. [Google Scholar] [CrossRef]
- Mori, S.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; Ohmori, H.; Goto, K.; Nakashima, C.; Nishiguchi, Y.; Kawahara, I.; Luo, Y.; et al. Enhancement of Anti-Tumoral Immunity by β-Casomorphin-7 Inhibits Cancer Development and Metastasis of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 8232. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ramasundara, M.; Leach, S.T.; Lemberg, D.A.; Day, A.S. Defensins and inflammation: The role of defensins in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009, 24, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Arnett, H.A.; Viney, J.L. Gatekeepers of intestinal inflammation. Inflamm. Res. 2010, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liu, Z.; Wang, H.; Zhang, L.; Li, M.; Li, G.; Li, C.; Wang, B.; Zhao, C.; Liu, L. Alterations in metabolome and microbiome: New clues on cathelicidin-related antimicrobial peptide alleviates acute ulcerative colitis. Front. Microbiol. 2024, 15, 1306068. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.U.; Koh, A.; Peris, E.; Bergentall, M.; Johansson, M.E.V.; Hansson, G.C.; Drucker, D.J.; Bäckhed, F. GLP-1R signaling modulates colonic energy metabolism, goblet cell number and survival in the absence of gut microbiota. Mol. Metab. 2024, 83, 101924. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Cevallos, S.A.; Byndloss, M.X.; Tiffany, C.R.; Olsan, E.E.; Butler, B.P.; Young, B.M.; Rogers, A.W.L.; Nguyen, H.; Kim, K.; et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis that Exacerbates Pre-inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 273–284.e6. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Chalangal, J.; Mazid, S.; Windisch, K.; Milner, T.A. Sex differences in the rodent hippocampal opioid system following stress and oxycodone associated learning processes. Pharmacol. Biochem. Behav. 2022, 212, 173294. [Google Scholar] [CrossRef]
- Gubatan, J.; Holman, D.R.; Puntasecca, C.J.; Polevoi, D.; Rubin, S.J.; Rogalla, S. Antimicrobial peptides and the gut microbiome in inflammatory bowel disease. World J. Gastroenterol. 2021, 27, 7402–7422. [Google Scholar] [CrossRef]
- Wang, J.W.; Xue, Z.Y.; Wu, A.S. Mechanistic insights into δ-opioid-induced cardioprotection: Involvement of caveolin translocation to the mitochondria. Life Sci. 2020, 247, 116942. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, F.; Mao, J.; Peng, Y.; Shao, N.; Balboni, G.; Yang, Y.; Xia, Y. δ-opioid receptor activation protects against Parkinson’s disease-related mitochondrial dysfunction by enhancing PINK1/Parkin-dependent mitophagy. Aging 2020, 12, 25035–25059. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bian, X.; Zhao, H.; Sun, Y.; Tian, Y.; Li, X.; Tian, W. Morphine Prevents Ischemia/Reperfusion-Induced Myocardial Mitochondrial Damage by Activating δ-opioid Receptor/EGFR/ROS Pathway. Cardiovasc. Drugs Ther. 2022, 36, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Sun, D.; Ling, S.; Tian, Y.; Yang, X.; Sui, J.; Tang, B.; Wang, L. Activated δ-opioid receptors inhibit hydrogen peroxide-induced apoptosis in liver cancer cells through the PKC/ERK signaling pathway. Mol. Med. Rep. 2014, 10, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, Y.; Liang, R.; Yuan, P.; Du, J.; Wang, H.; Wang, L. Activation of the δ-opioid receptor inhibits serum deprivation-induced apoptosis of human liver cells via the activation of PKC and the mitochondrial pathway. Int. J. Mol. Med. 2011, 28, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, M.; Liu, J.; Ji, Y.; Subramanian, M.; Crompton, J.G.; Yu, Z.; Roychoudhuri, R.; Palmer, D.C.; Muranski, P.; Karoly, E.D.; et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig. 2013, 123, 4479–4488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, H.; Lin, H.; Li, R. Somatostatin-SSTR3-GSK3 modulates human T-cell responses by inhibiting OXPHOS. Front. Immunol. 2024, 15, 1322670. [Google Scholar] [CrossRef] [PubMed]
- Xuekai, L.; Yan, S.; Jian, C.; Yifei, S.; Xinyue, W.; Wenyuan, Z.; Shuwen, H.; Xi, Y. Advances in reprogramming of energy metabolism in tumor T cells. Front. Immunol. 2024, 15, 1347181. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Luo, Y.; Fujii, K.; Sasahira, T.; Moriwaka, Y.; Tatsumoto, N.; Sasaki, T.; Yamashita, Y.; Ohmori, H. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut 2010, 59, 348–356. [Google Scholar] [CrossRef]
- Wielscher, M.; Mandaviya, P.R.; Kuehnel, B.; Joehanes, R.; Mustafa, R.; Robinson, O.; Zhang, Y.; Bodinier, B.; Walton, E.; Mishra, P.P.; et al. DNA methylation signature of chronic low-grade inflammation and its role in cardio-respiratory diseases. Nat. Commun. 2022, 13, 2408. [Google Scholar] [CrossRef]
- Lloyd, J.M.; Owens, S.R. CD10 immunohistochemistry stains enteric mucosa, but negative staining is unreliable in the setting of active enteritis. Mod. Pathol. 2011, 24, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Sargın, Z.G.; Erin, N.; Tazegul, G.; Elpek, G.; Yıldırım, B. Profound loss of neprilysin accompanied by decreased levels of neuropeptides and increased CRP in ulcerative colitis. PLoS ONE 2017, 12, e0189526. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Valaiyaduppu Subas, S.; Ghani, M.R.; Busa, V.; Dardeir, A.; Marudhai, S.; Cancarevic, I. Role of Substance P in the Pathophysiology of Inflammatory Bowel Disease and Its Correlation With the Degree of Inflammation. Cureus 2020, 12, e11027. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085. [Google Scholar] [CrossRef] [PubMed]
- Gülpinar, M.A.; Ozbeyli, D.; Arbak, S.; Yeğen, B.C. Anti-inflammatory effect of acute stress on experimental colitis is mediated by cholecystokinin-B receptors. Life Sci. 2004, 75, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Selva, E.; Le Negrate, G.; d’Andrea, L.; Guérin, S.; Rossi, B.; Auberger, P. CD10 inhibitors increase f-Met-Leu-Phe-induced neutrophil transmigration. J. Leukoc. Biol. 1998, 63, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Li, X.; Gao, Y.; Chen, Z.; Zhang, Q.; Wang, Z.; Tian, X. Central administration of human opiorphin alleviates dextran sodium sulfate-induced colitis in mice through activation of the endogenous opioid system. Front. Pharmacol. 2022, 13, 904926. [Google Scholar] [CrossRef] [PubMed]
- Wilenska, B.; Tymecka, D.; Włodarczyk, M.; Sobolewska-Włodarczyk, A.; Wiśniewska-Jarosińska, M.; Dyniewicz, J.; Somogyi, Á.; Fichna, J.; Misicka, A. Enkephalin degradation in serum of patients with inflammatory bowel diseases. Pharmacol. Rep. 2019, 71, 42–47. [Google Scholar] [CrossRef]
- Smarr, B.; Kriegsfeld, L.J. Female mice exhibit less overall variance, with a higher proportion of structured variance, than males at multiple timescales of continuous body temperature and locomotive activity records. Biol. Sex Differ. 2022, 13, 41. [Google Scholar] [CrossRef]
- Lalji, H.M.; Bailey, C.P.; Husbands, S.M.; Bailey, S.J. Effects of sex and hydration status on kappa opioid receptor-mediated diuresis in rats. Basic Clin. Pharmacol. Toxicol. 2024, 134, 792–804. [Google Scholar] [CrossRef]

| RT-PCR Primers | ||||
| Gene | Species | ID | Upper | Lower |
| PINK1 | mouse | NM_026880.2 | ccatcgggatctcaagtccg | agagccaggcgatcatcttg |
| Parkin | mouse | AB019558.1 | cctgcaaacaagcaaccctc | gtcccggcagaaaacaaacc |
| ATG5 | mouse | MG656078.1 | tgtgcttcgagatgtgtggt | ttctggatgaaaggccgctc |
| LDHA | mouse | BC094019.1 | gcatggcagcctcttcctta | ccaagtctgccacagagagg |
| LDHB | mouse | BC046755.1 | gactccgaaaattgtggccg | tccgggagaggtttttcagc |
| MSP Primers | ||||
| Gene | Species | ID | Upper | Lower |
| CD10 (methylated) | human | NG_051105.1 | agaaaacggagcatccgaca | ctgcaagagcagagaaaaca |
| CD10 (unmethylated) | human | NG_051105.1 | agaaaatggagcatctgaca | ctacaagaacagagaaaaca |
| ELISA | ||||
| Target | Species | Cat# | Company | |
| CD45RO | mouse | EM14RB | Thermo Fisher, Tokyo, Japan | |
| CD45RO | human | 65150-1 | Proteintech, Rosemont, IL, USA | |
| CD20 | mouse | LS-F49371 | LSbio, Shirley, MA, USA | |
| CD20 | human | ab285273 | Abcam, Waltham, MA, USA | |
| CD3 | mouse | NBP2-75137 | Novus Biologicals, LLC, Centennial, CO, USA | |
| CD3 | human | CRS015-C02 | Acro Biosystems, Tokyo, Japan | |
| CD4 | mouse | NBP2-75148 | Novus Biologicals, LLC, Centennial, CO, USA | |
| CD4 | human | EH90RB | Thermo Fisher, Tokyo, Japan | |
| CD8 | mouse | NBP2-78729 | Novus Biologicals, LLC, Centennial, CO, USA | |
| CD8 | human | LS-F26719 | Thermo Fisher, Tokyo, Japan | |
| FoxP3 | mouse | ab289645 | Abcam, Waltham, MA, USA | |
| FoxP3 | human | 22228-1-AP | Proteintech, Rosemont, IL, USA | |
| IFNγ | mouse | KMC4021 | Thermo Fisher, Tokyo, Japan | |
| IFNγ | human | EHIFNG | Thermo Fisher, Tokyo, Japan | |
| Granzyme B | mouse | BMS6029 | Thermo Fisher, Tokyo, Japan | |
| Granzyme B | human | ab46142 | Abcam, Waltham, MA, USA | |
| αDefensin | mouse | EK11275 | Signalway Antibody, Greenbelt, MA, USA | |
| αDefensin | human | EL006657HU | Cusabio, Houston, TX, USA | |
| 4HNE | - | ab238538 | Abcam, Waltham, MA, USA | |
| Butyric acid | - | abx258338 | Abbexa Ltd., Cambridge, UK | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagawa, Y.; Fujiwara-Tani, R.; Ikemoto, A.; Sasaki, R.; Ogata, R.; Nishiguchi, Y.; Goto, K.; Kawahara, I.; Sasaki, T.; Kuniyasu, H. Significance of CD10 for Mucosal Immunomodulation by β-Casomorphin-7 in Exacerbation of Ulcerative Colitis. Curr. Issues Mol. Biol. 2024, 46, 6472-6488. https://doi.org/10.3390/cimb46070386
Miyagawa Y, Fujiwara-Tani R, Ikemoto A, Sasaki R, Ogata R, Nishiguchi Y, Goto K, Kawahara I, Sasaki T, Kuniyasu H. Significance of CD10 for Mucosal Immunomodulation by β-Casomorphin-7 in Exacerbation of Ulcerative Colitis. Current Issues in Molecular Biology. 2024; 46(7):6472-6488. https://doi.org/10.3390/cimb46070386
Chicago/Turabian StyleMiyagawa, Yoshihiro, Rina Fujiwara-Tani, Ayaka Ikemoto, Rika Sasaki, Ruiko Ogata, Yukiko Nishiguchi, Kei Goto, Isao Kawahara, Takamitsu Sasaki, and Hiroki Kuniyasu. 2024. "Significance of CD10 for Mucosal Immunomodulation by β-Casomorphin-7 in Exacerbation of Ulcerative Colitis" Current Issues in Molecular Biology 46, no. 7: 6472-6488. https://doi.org/10.3390/cimb46070386
APA StyleMiyagawa, Y., Fujiwara-Tani, R., Ikemoto, A., Sasaki, R., Ogata, R., Nishiguchi, Y., Goto, K., Kawahara, I., Sasaki, T., & Kuniyasu, H. (2024). Significance of CD10 for Mucosal Immunomodulation by β-Casomorphin-7 in Exacerbation of Ulcerative Colitis. Current Issues in Molecular Biology, 46(7), 6472-6488. https://doi.org/10.3390/cimb46070386






