Hereditary Gastrointestinal Tumor Syndromes: When Risk Comes with Your Genes
Abstract
1. Introduction
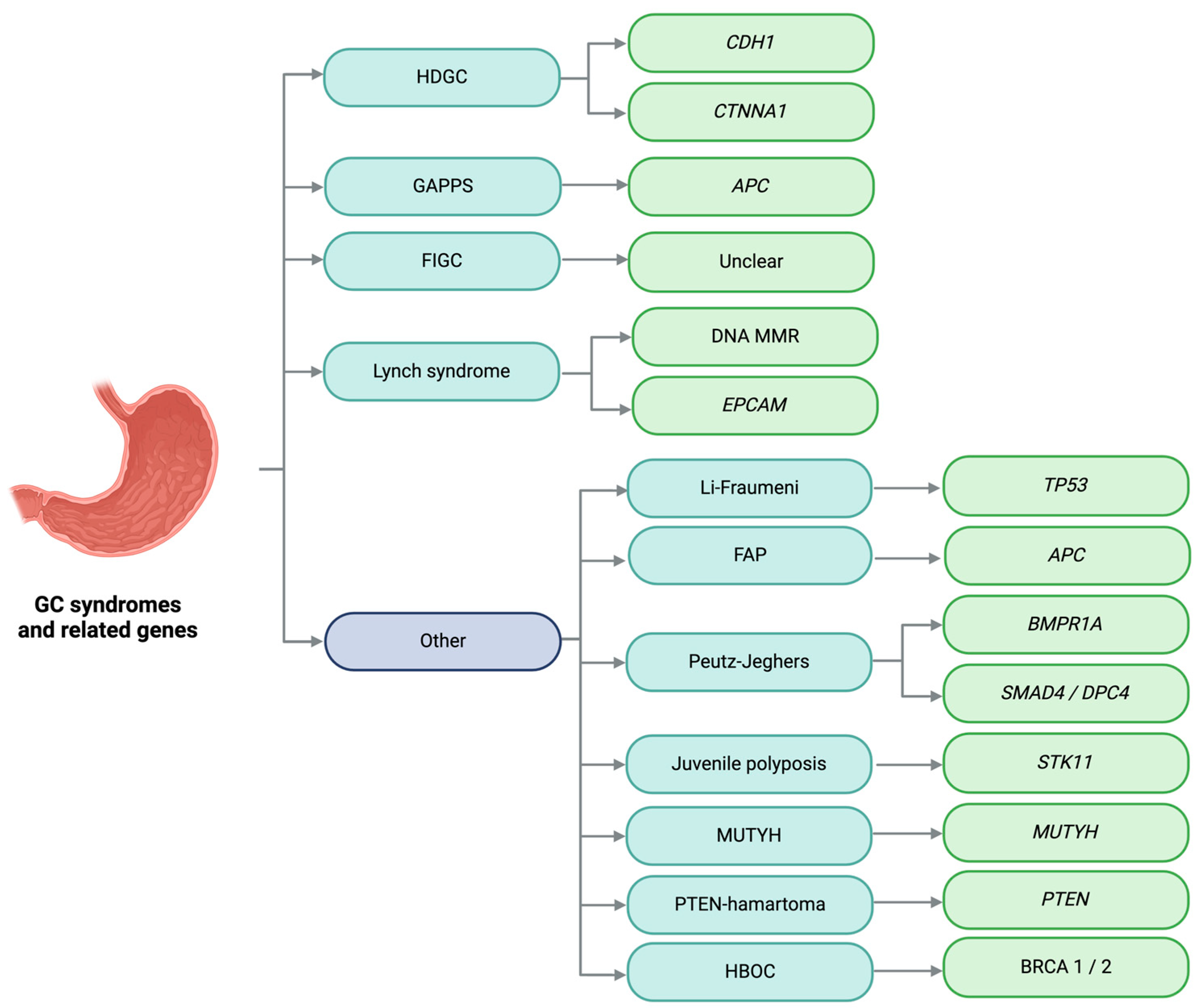
2. Gastric Cancer
Familial and Hereditary Gastric Cancer
3. Hereditary Diffuse Gastric Cancer
3.1. Clinical Presentation
3.2. Gastric Cancer Risk
3.2.1. Individuals with Pathogenic CDH1 Variants
3.2.2. Individuals with Pathogenic CTNNA1 Variants
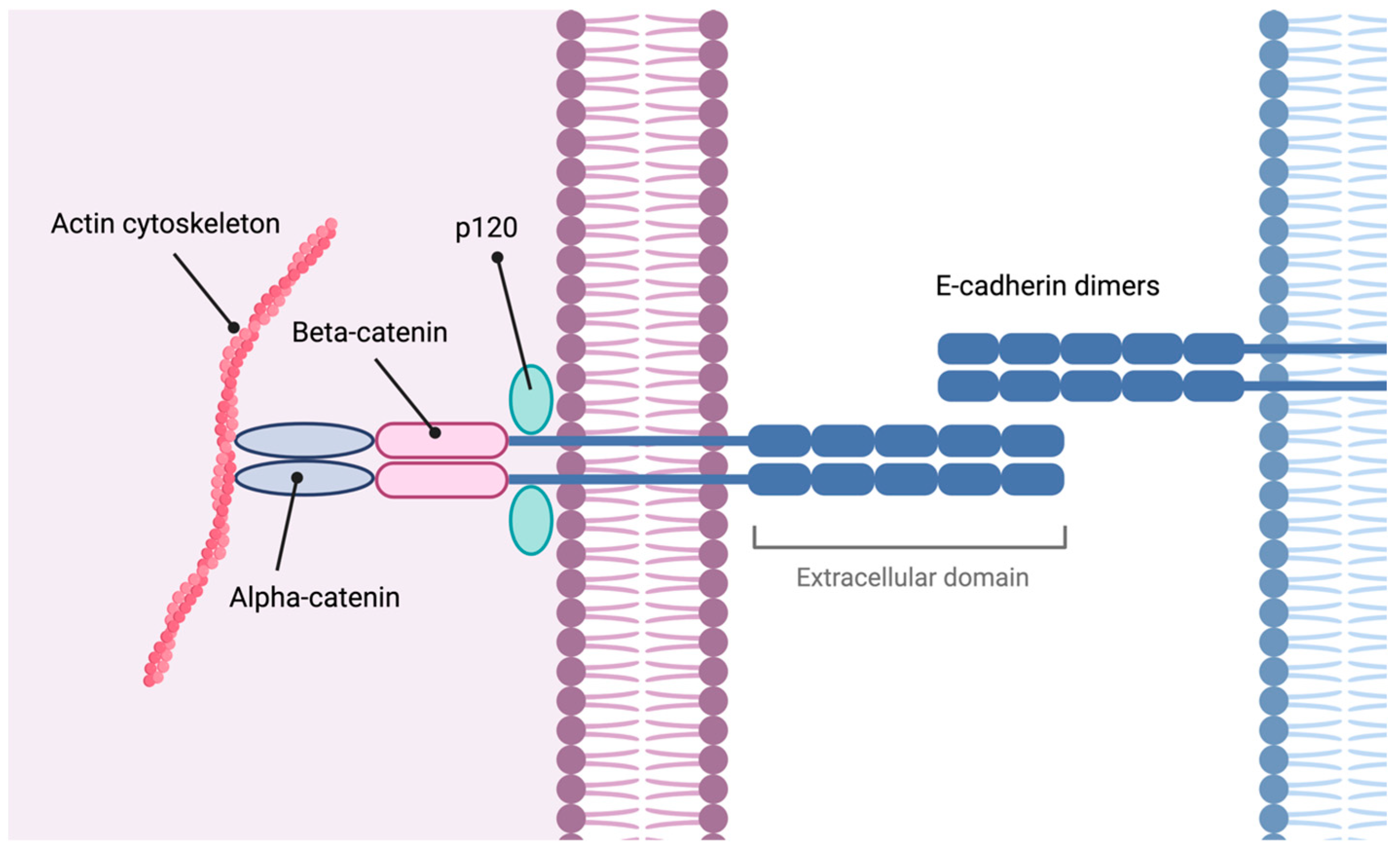
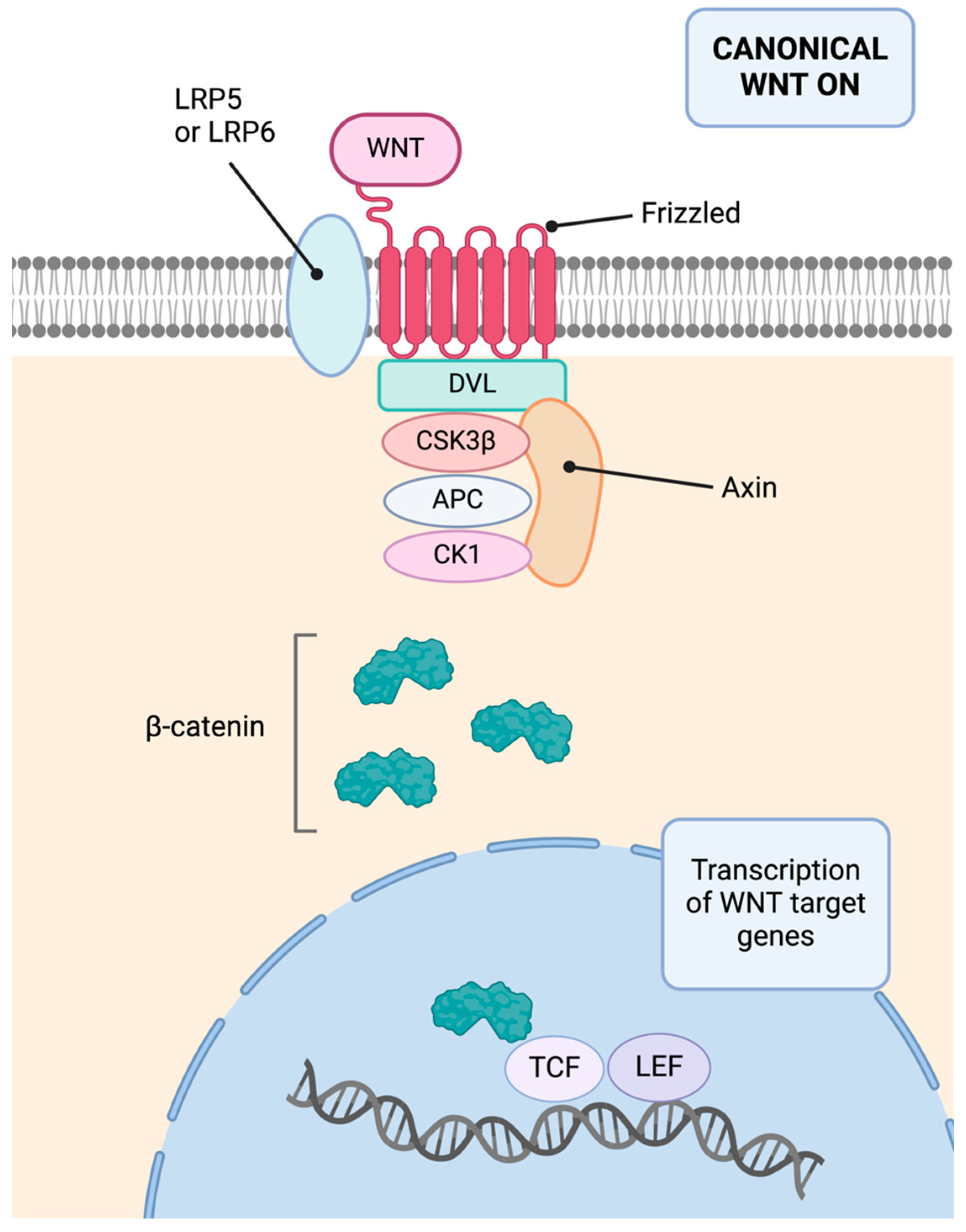
3.3. Molecular Basis
3.3.1. CDH1
3.3.2. CTNNA1
3.3.3. Other Molecular Alterations
3.4. Follow-Up and Treatment
4. Gastric Adenocarcinoma and Proximal Polyposis of the Stomach
4.1. Gastric Cancer Risk
4.2. Molecular Basis
4.3. Follow-Up and Treatment
5. Familial Intestinal Gastric Cancer
5.1. Clinical Presentation
5.2. Gastric Cancer Risk
5.3. Molecular Basis
5.4. Follow-Up and Treatment
6. Li–Fraumeni Syndrome
6.1. Clinical Presentation and Gastric Cancer Risk
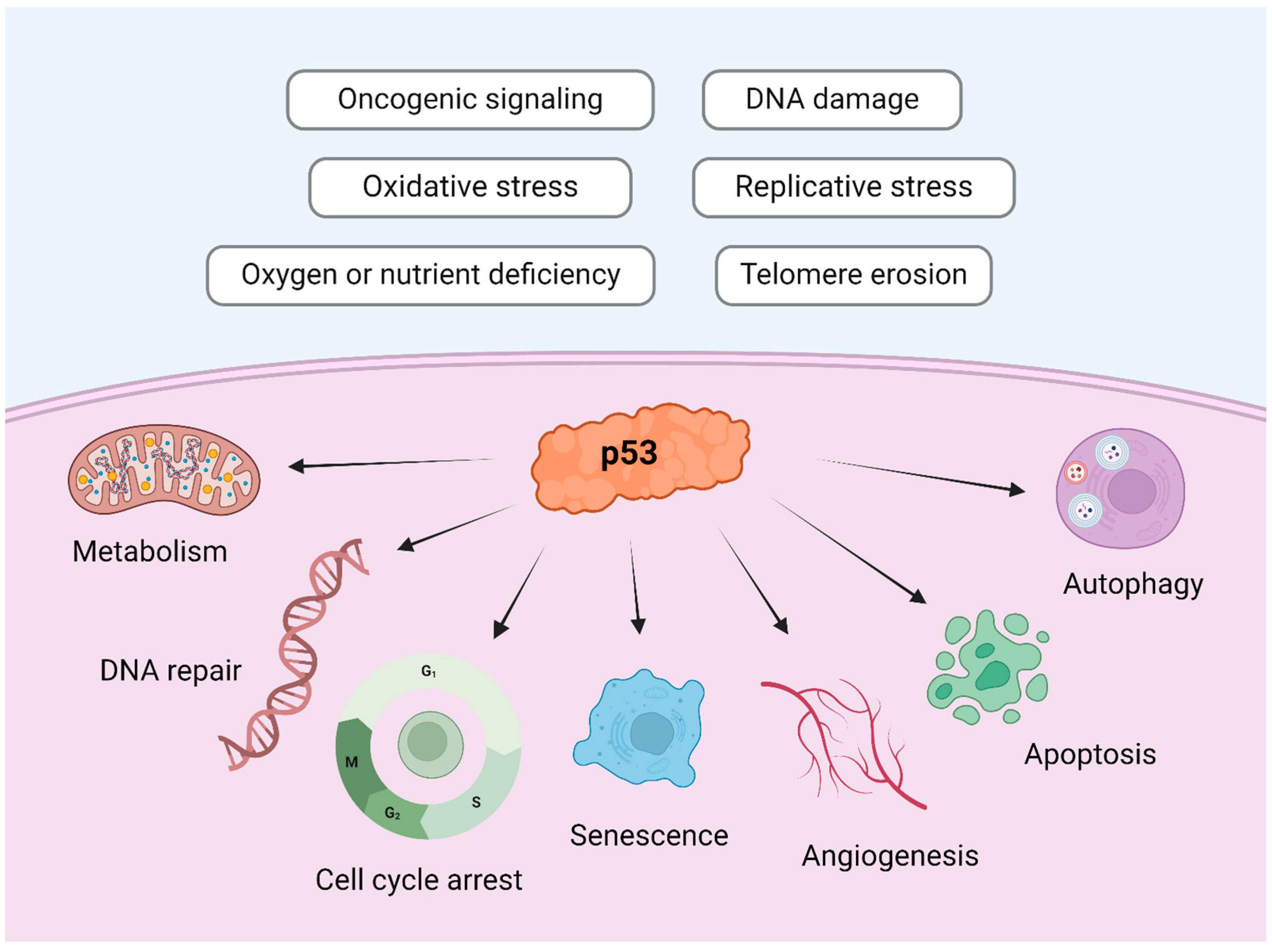
6.2. Molecular Basis
6.3. Follow-Up and Treatment
7. Hereditary Breast and Ovarian Cancer
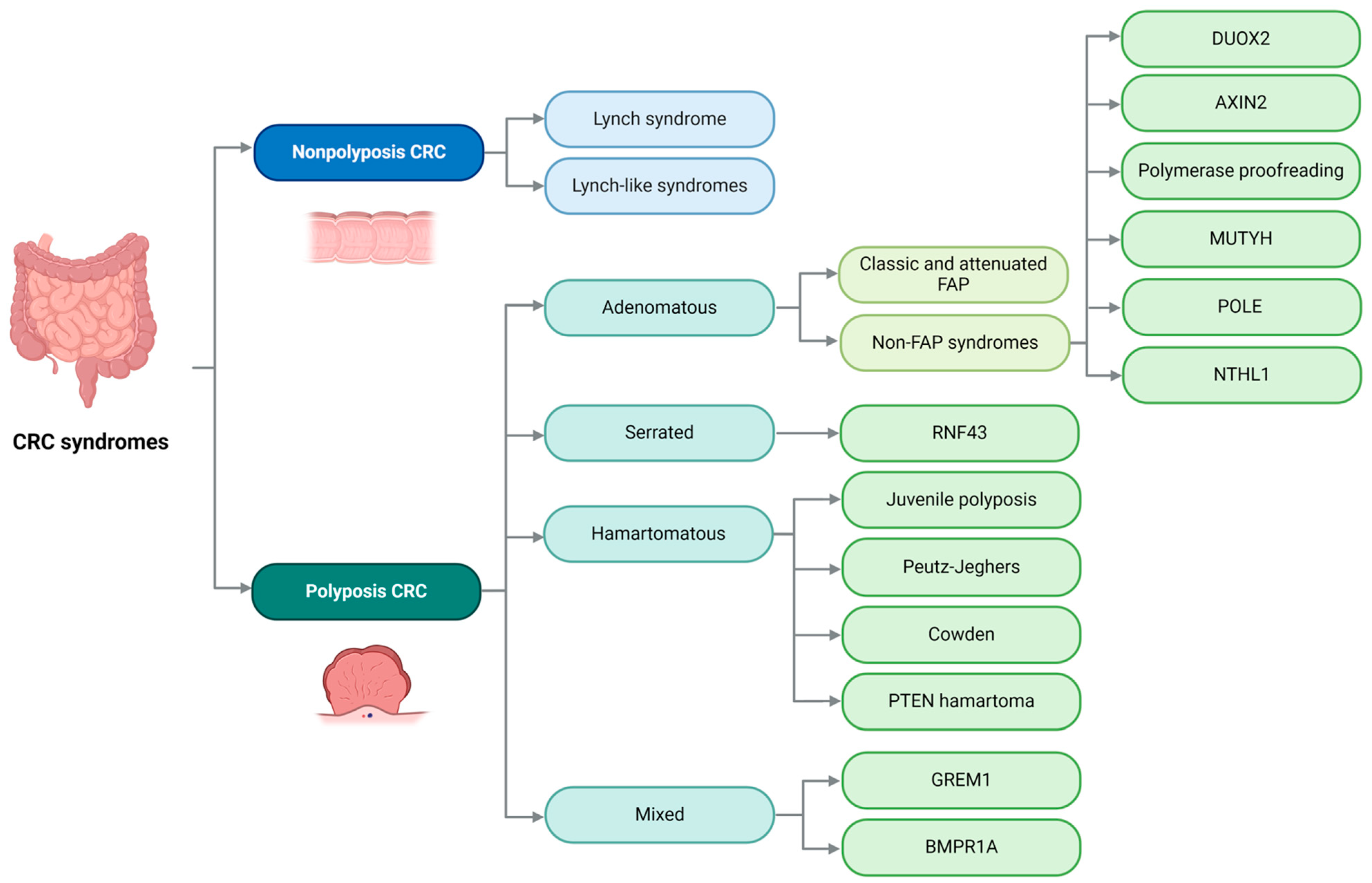
8. Colon
9. Lynch Syndrome
9.1. Clinical Presentation
9.2. Colorectal Cancer Risk
9.3. Gastric Cancer Risk
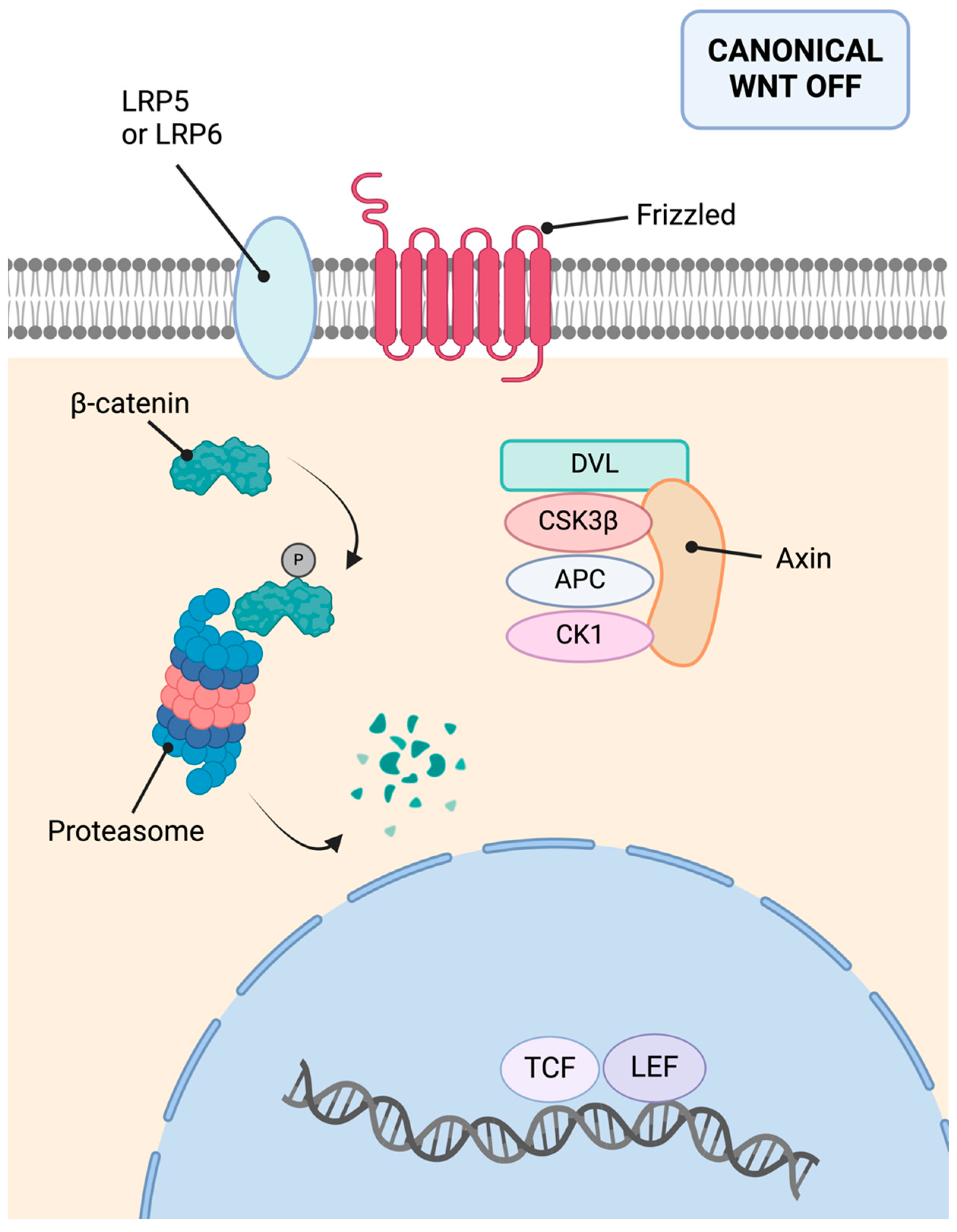
9.4. Molecular Basis
9.5. Follow-Up and Treatment
10. Lynch-like Syndromes
10.1. Constitutional Mismatch Repair Deficiency Syndrome
10.2. Familial Colorectal Cancer Type X Syndrome
11. Classic and Attenuated Familial Adenomatous Polyposis
11.1. Clinical Presentation
11.2. Risk of Colorectal Cancer
11.3. Molecular Basis
11.4. Follow-Up and Treatment
12. MUTYH-Associated Polyposis
Molecular Basis
13. NTHL1-Associated Tumor Syndrome
Molecular Basis
14. Polymerase Proofreading-Associated Polyposis
15. Axis Inhibition Protein 2 (AXIN2)-Associated Polyposis
16. Dual Oxidase 2 (DUOX2)-Associated Polyposis
17. Other Candidate Genes in Adenomatous Polyposis Syndromes
18. Hereditary Polyposis Colorectal Carcinoma with Serrated Polyps
18.1. Clinical Presentation
18.2. Molecular Basis
18.3. Follow-Up and Treatment
18.4. RNF43-Associated Polyposis
19. Juvenile Polyposis Syndrome
Juvenile Polyposis of Infancy
20. Peutz–Jeghers Syndrome
20.1. Clinical Presentation
20.2. Colorectal Cancer Risk
20.3. Molecular Basis
20.4. Follow-Up and Treatment
21. Cowden Syndrome
21.1. Colorectal Cancer Risk
21.2. Molecular Basis
21.3. Follow-Up and Treatment
21.4. PTEN Hamartoma Tumor Syndrome
22. Hereditary Polyposis with Mixed Polyps Syndrome
22.1. GREM1-Associated Mixed Polyposis
22.2. BMPR1A-Associated Mixed Polyposis
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sabour, L.; Sabour, M.; Ghorbian, S. Clinical Applications of Next-Generation Sequencing in Cancer Diagnosis. Pathol. Oncol. Res. 2017, 23, 225–234. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pinheiro, P.S.; Callahan, K.E.; Altekruse, S.F. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer 2017, 20, 573–582. [Google Scholar] [CrossRef] [PubMed]
- El Halabi, M.; Horanieh, R.; Tamim, H.; Mukherji, D.; Jdiaa, S.; Temraz, S.; Shamseddine, A.; Barada, K. The impact of age on prognosis in patients with gastric cancer: Experience in a tertiary care centre. J. Gastrointest. Oncol. 2020, 11, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, C.D.; Medina, L.O.; Muñoz, L.E.; de las Heras, S.G.G.; Aceñero, M.J.F. Is there still a place for conventional histopathology in the age of molecular medicine? Laurén classification, inflammatory infiltration and other current topics in gastric cancer diagnosis and prognosis. Histol. Histopathol. 2021, 36, 587–613. [Google Scholar] [CrossRef]
- Mariette, C.; Carneiro, F.; Grabsch, H.I.; van der Post, R.S.; Allum, W.; de Manzoni, G.; Luca, B.G.; Maria, B.; Jean-Francois, F.; Uberto, F.; et al. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kole, C.; Charalampakis, N.; Sakellariou, S.; Papaxoinis, G.; Apostolou, K.G.; Machairas, N.; Papanikolaou, I.S.; Schizas, D. Hereditary Diffuse Gastric Cancer: A 2022 Update. J. Pers. Med. 2022, 12, 2032. [Google Scholar] [CrossRef]
- Taja-Chayeb, L.; Vidal-Millán, S.; Trejo-Becerril, C.; Pérez-Cárdenas, E.; Chávez-Blanco, A.; Domínguez-Gómez, G.; González-Fierro, A.; Romo-Pérez, A.; Dueñas-González, A. Hereditary diffuse gastric cancer (HDGC). An overview. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101820. [Google Scholar] [CrossRef] [PubMed]
- Lerner, B.A.; Llor, X. Genetic Gastric Cancer Risk Syndromes. Curr. Treat. Options Gastroenterol. 2020, 18, 604–615. [Google Scholar] [CrossRef]
- Carvalho, J.; Oliveira, P.; Senz, J.; São José, C.; Hansford, S.; Teles, S.P.; Ferreira, M.; Corso, G.; Pinheiro, H.; Lemos, D.; et al. Redefinition of familial intestinal gastric cancer: Clinical and genetic perspectives. J. Med. Genet. 2021, 58, 1–11. [Google Scholar] [CrossRef]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet. Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef]
- Jones, E. Familial gastric cancer. N. Z. Med. J. 1964, 63, 287–296. [Google Scholar]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef]
- Gayther, S.; Gorringe, K.; Ramus, S.; Huntsman, D.; Roviello, F.; Grehan, N.; Machado, J.; Pinto, E.; Seruca, R.; Halling, K.; et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998, 15, 4086–4089. [Google Scholar]
- Richards, F.M.; McKee, S.A.; Rajpar, M.H.; Cole, T.R.P.; Evans, D.G.R.; Jankowski, J.A.; McKeown, C.; Sanders, D.S.A.; Maher, E.R. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum. Mol. Genet. 1999, 8, 607–610. [Google Scholar] [CrossRef]
- Gregory, S.N.; Davis, J.L. CDH1 and hereditary diffuse gastric cancer: A narrative review. Chin. Clin. Oncol. 2023, 12, 25. [Google Scholar] [CrossRef]
- Caldas, C.; Carneiro, F.; Lynch, H.T.; Yokota, J.; Wiesner, G.L.; Powell, S.M.; Lewis, F.R.; Huntsman, D.G.; Pharoah, P.D.P.; Jankowski, J.A.; et al. Familial gastric cancer: Overview and guidelines for management. J. Med. Genet. 1999, 36, 873–880. [Google Scholar]
- Lynch, H.T.; Grady, W.; Suriano, G.; Huntsman, D. Gastric cancer: New genetic developments. J. Surg. Oncol. 2005, 90, 114–133. [Google Scholar] [CrossRef]
- Shepard, B.; Yoder, L.; Holmes, C. Prophylactic Total Gastrectomy for Hereditary Diffuse Gastric Cancer. ACG Case Rep. J. 2016, 3, e179. [Google Scholar] [CrossRef]
- Rocha, J.P.; Gullo, I.; Wen, X.; Devezas, V.; Baptista, M.; Oliveira, C.; Carneiro, F. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology 2018, 73, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Tsugeno, Y.; Nakano, K.; Nakajima, T.; Namikawa, K.; Takamatsu, M.; Yamamoto, N.; Fujisaki, J.; Nunobe, S.; Kitagawa, M.; Takeuchi, K.; et al. Histopathologic Analysis of Signet-ring Cell Carcinoma In Situ in Patients with Hereditary Diffuse Gastric Cancer. Am. J. Surg. Pathol. 2020, 44, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Carvalho, J.; Marrelli, D.; Vindigni, C.; Carvalho, B.; Seruca, R.; Roviello, F.; Oliveira, C. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J. Clin. Oncol. 2013, 31, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Ranola, J.M.O.; Marshall, M.L.; Susswein, L.R.; Graceffo, S.; Bohnert, K.; Tsai, G.; Klein, R.T.; Hruska, K.S.; Shirts, B.H. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol. 2019, 5, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Xicola, R.M.; Li, S.; Rodriguez, N.; Reinecke, P.; Karam, R.; Speare, V.; Black, M.H.; Laduca, H.; Llor, X. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J. Med. Genet. 2019, 56, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Cosma, L.S.; Schlosser, S.; Tews, H.C.; Müller, M.; Kandulski, A. Hereditary Diffuse Gastric Cancer: Molecular Genetics, Biological Mechanisms and Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 7281. [Google Scholar] [CrossRef] [PubMed]
- Blair, V.; Martin, I.; Shaw, D.; Winship, I.; Kerr, D.; Arnold, J.; Harawira, P.; McLeod, M.; Parry, S.; Charlton, A.; et al. Hereditary diffuse gastric cancer: Diagnosis and management. Clin. Gastroenterol. Hepatol. 2006, 4, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Benusiglio, P.R.; Malka, D.; Rouleau, E.; de Pauw, A.; Buecher, B.; Noguès, C.; Fourme, E.; Colas, C.; Coulet, F.; Warcoin, M.; et al. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: A multicentre study. J. Med. Genet. 2013, 50, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Jung, I.; Orlowska, J.; Sugimura, H.; Kadar, Z.; Turdean, S.; Bara, T. Hereditary diffuse gastric cancer—An overview. Pathol. Res. Pract. 2015, 211, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.D.P.; Guilford, P.; Caldas, C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001, 121, 1348–1353. [Google Scholar] [CrossRef]
- Cisco, R.M.; Ford, J.M.; Norton, J.A. Hereditary diffuse gastric cancer. Cancer 2008, 113, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Coudert, M.; Drouet, Y.; Delhomelle, H.; Svrcek, M.; Benusiglio, P.R.; Coulet, F.; Clark, D.F.; Katona, B.W.; Van Hest, L.P.; Van Der Kolk, L.E.; et al. First estimates of diffuse gastric cancer risks for carriers of CTNNA1 germline pathogenic variants. J. Med. Genet. 2022, 59, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, T.; Yap, A.S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 2015, 17, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; De Bruin, J.; Nabais, S.; Ligtenberg, M.; Moutinho, C.; Nagengast, F.M.; Seruca, R.; Van Krieken, H.; Carneiro, F. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in an HDGC tumour. Oncogene 2004, 23, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Corso, F.; Bellerba, F.; Carneiro, P.; Seixas, S.; Cioffi, A.; La Vecchia, C.; Magnoni, F.; Bonanni, B.; Veronesi, P.; et al. Geographical Distribution of E-cadherin Germline Mutations in the Context of Diffuse Gastric Cancer: A Systematic Review. Cancers 2021, 13, 1269. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Shin, J.H.; Kee, S.H. E-cadherin expression increases cell proliferation by regulating energy metabolism through nuclear factor-κB in AGS cells. Cancer Sci. 2017, 108, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef] [PubMed]
- Koni, M.; Pinnarò, V.; Brizzi, M.F. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int. J. Mol. Sci. 2020, 21, 7697. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Dual role of E-cadherin in cancer cells. Tissue Barriers 2022, 10, 2005420. [Google Scholar] [CrossRef]
- Majewski, I.J.; Kluijt, I.; Cats, A.; Scerri, T.S.; De Jong, D.; Kluin, R.J.C.; Hansford, S.; Hogervorst, F.B.L.; Bosma, A.J.; Hofland, I.; et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J. Pathol. 2013, 229, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.; Benusiglio, P.R.; Coulet, F.; Boussemart, L.; Golmard, L.; Spier, I.; Hüneburg, R.; Aretz, S.; Colas, C.; Oliveira, C. Cancer predisposition and germline CTNNA1 variants. Eur. J. Med. Genet. 2021, 64, 104316. [Google Scholar] [CrossRef] [PubMed]
- Benusiglio, P.R.; Colas, C.; Guillerm, E.; Canard, A.; Delhomelle, H.; Warcoin, M.; Bellanger, J.; Eyries, M.; Zizi, M.; Netter, J.; et al. Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer 2019, 22, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.F.; Michalski, S.T.; Tondon, R.; Nehoray, B.; Ebrahimzadeh, J.; Hughes, S.K.; Soper, E.R.; Domchek, S.M.; Rustgi, A.K.; Pineda-Alvarez, D.; et al. Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet. Med. 2020, 22, 840–846. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Xu, Y.; Li, C.; Lv, X.; Han, X.; Chen, X.; Chen, Y.; Yu, Z. The Role of CTNNA1 in Malignancies: An Updated Review. J. Cancer 2023, 14, 219–230. [Google Scholar] [CrossRef]
- Pokutta, S.; Choi, H.J.; Ahlsen, G.; Hansen, S.D.; Weis, W.I. Structural and thermodynamic characterization of cadherin·β-catenin·α-catenin complex formation. J. Biol. Chem. 2014, 289, 13589–13601. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Weis, W.I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 2007, 23, 237–261. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Ma, L. α-catenin. A tumor suppressor beyond adherens junctions. Cell Cycle 2014, 13, 2334–2339. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin. Cancer Biol. 2022, 85, 107–122. [Google Scholar] [CrossRef]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci. 2022, 136, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015, 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kidambi, T.; Lin, J.; Idos, G. Genetic Syndromes Associated with Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 147–162. [Google Scholar] [CrossRef] [PubMed]
- van der Post, R.S.; Vogelaar, I.P.; Carneiro, F.; Guilford, P.; Huntsman, D.; Hoogerbrugge, N.; Caldas, C.; Chelcun Schreiber, K.E.; Hardwick, R.H.; Ausems, M.G.E.M.; et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J. Med. Genet. 2015, 52, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F. Familial and hereditary gastric cancer, an overview. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101800. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.C.; Olivier, A.; Honing, J.; Lydon, A.M.; Richardson, S.; O’Donovan, M.; Tischkowitz, M.; Fitzgerald, R.C.; di Pietro, M. Endoscopic surveillance with systematic random biopsy for the early diagnosis of hereditary diffuse gastric cancer: A prospective 16-year longitudinal cohort study. Lancet Oncol. 2023, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Long, J.M.; Ginsberg, G.G.; Katona, B.W. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J. Gastroenterol. 2019, 25, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Ruff, S.; Curtin, B.; Quezado, M.; Heller, T.; Koh, C.; Steinberg, S.M.; Connolly, M.; Hernandez, J.M.; Davis, J.L. Evaluation of confocal endoscopic microscopy for detection of early-stage gastric cancer in hereditary diffuse gastric cancer (HDGC) syndrome. J. Gastrointest. Oncol. 2019, 10, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Muzammil, M.A.; Dahiya, D.S.; Ali, F.; Yasin, S.; Hanif, W.; Gangwani, M.K.; Aziz, M.; Khalaf, M.; Basuli, D.; et al. Artificial intelligence in gastrointestinal endoscopy: A comprehensive review. Ann. Gastroenterol. 2024, 37, 133–141. [Google Scholar] [CrossRef]
- Chen, P.C.; Lu, Y.R.; Kang, Y.N.; Chang, C.C. The Accuracy of Artificial Intelligence in the Endoscopic Diagnosis of Early Gastric Cancer: Pooled Analysis Study. J. Med. Internet Res. 2022, 24, e27694. [Google Scholar] [CrossRef]
- Worthley, D.L.; Phillips, K.D.; Wayte, N.; Schrader, K.A.; Healey, S.; Kaurah, P.; Shulkes, A.; Grimpen, F.; Clouston, A.; Moore, D.; et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut 2012, 61, 774–779. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.B.; Ee, H.; Kumarasinghe, M.P. Neoplastic Lesions of Gastric Adenocarcinoma and Proximal Polyposis Syndrome (GAPPS) Are Gastric Phenotype. Am. J. Surg. Pathol. 2018, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.; Streubel, B.; Asari, R.; Dejaco, C.; Oberhuber, G. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)—A rare recently described gastric polyposis syndrome—Report of a case. Z. Gastroenterol. 2017, 55, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, U. Gastric adenocarcinoma and proximal polyposis of the stomach: Diagnosis and clinical perspectives. Clin. Exp. Gastroenterol. 2018, 11, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J. Gastroenterol. 2008, 14, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Woods, S.L.; Healey, S.; Beesley, J.; Chen, X.; Lee, J.S.; Sivakumaran, H.; Wayte, N.; Nones, K.; Waterfall, J.J.; et al. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am. J. Hum. Genet. 2016, 98, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Foretova, L.; Navratilova, M.; Svoboda, M.; Grell, P.; Nemec, L.; Sirotek, L.; Obermannova, R.; Novotny, I.; Sachlova, M.; Fabian, P.; et al. GAPPS—Gastric Adenocarcinoma and Proximal Polyposis of the Stomach Syndrome in 8 Families Tested at Masaryk Memorial Cancer Institute—Prevention and Prophylactic Gastrectomies. Klin. Onkol. 2019, 32, S109–S117. [Google Scholar] [CrossRef] [PubMed]
- Repak, R.; Kohoutova, D.; Podhola, M.; Rejchrt, S.; Minarik, M.; Benesova, L.; Lesko, M.; Bures, J. The first European family with gastric adenocarcinoma and proximal polyposis of the stomach: Case report and review of the literature. Gastrointest. Endosc. 2016, 84, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Sera, T.; Aoyama, R.; Sawada, A.; Kasashima, H.; Ogisawa, K.; Bamba, H.; Yashiro, M. Two families with gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): Case reports and literature review. J. Gastrointest. Oncol. 2023, 14, 2650–2657. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Matsumoto, C.; Mimori, K.; Baba, H. The comprehensive review of gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) from diagnosis and treatment. Ann. Gastroenterol. Surg. 2023, 7, 725. [Google Scholar] [CrossRef]
- Tacheci, I.; Repak, R.; Podhola, M.; Benesova, L.; Cyrany, J.; Bures, J.; Kohoutova, D. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)—A Helicobacter-opposite point. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101728. [Google Scholar] [CrossRef]
- Salami, A.; Stone, J.; Greenberg, R.; Leighton, J.; Miick, R.; Zavala, S.; Zeitzer, K.; Bakhos, C. Early Prophylactic Gastrectomy for the Management of Gastric Adenomatous Proximal Polyposis Syndrome (GAPPS). ACS Case Rev. Surg. 2022, 3, 62–68. [Google Scholar]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. Familial gastric cancer: Genetic susceptibility, pathology, and implications for management. Lancet. Oncol. 2015, 16, e60–e70. [Google Scholar] [CrossRef]
- Llach, J.; Salces, I.; Guerra, A.; Peñas, B.; Rodriguez-Alcalde, D.; Redondo, P.D.; Cubiella, J.; Murcia, Ó.; Escalante, M.; Gratacós-Ginès, J.; et al. Endoscopic surveillance for familial intestinal gastric cancer in low-incidence areas: An effective strategy. Int. J. Cancer 2024, 154, 124–132. [Google Scholar] [CrossRef]
- Garcia-Pelaez, J.; Barbosa-Matos, R.; São José, C.; Sousa, S.; Gullo, I.; Hoogerbrugge, N.; Carneiro, F.; Oliveira, C. Gastric cancer genetic predisposition and clinical presentations: Established heritable causes and potential candidate genes. Eur. J. Med. Genet. 2022, 65, 104401. [Google Scholar] [CrossRef]
- Vogelaar, I.P.; van der Post, R.S.; van de Vosse, E.; van Krieken, J.H.J.M.; Hoogerbrugge, N.; Ligtenberg, M.J.L.; Gómez García, E. Gastric cancer in three relatives of a patient with a biallelic IL12RB1 mutation. Fam. Cancer 2015, 14, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Zhuang, Y.; Wu, H. Identification of the potential molecular mechanism and driving mutations in the pathogenesis of familial intestinal gastric cancer by whole exome sequencing. Oncol. Rep. 2018, 40, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Roncalli, F.; Marrelli, D.; Carneiro, F.; Roviello, F. History, pathogenesis, and management of familial gastric cancer: Original study of John XXIII’s family. Biomed. Res. Int. 2013, 2013, 385132. [Google Scholar] [CrossRef]
- Sereno, M.; Aguayo, C.; Guillén Ponce, C.; Gómez-Raposo, C.; Zambrana, F.; Gómez-López, M.; Casado, E. Gastric tumours in hereditary cancer syndromes: Clinical features, molecular biology and strategies for prevention. Clin. Transl. Oncol. 2011, 13, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Pedrazzani, C.; Marrelli, D.; Pinto, E.; Roviello, F. Familial gastric cancer and Li-Fraumeni syndrome. Eur. J. Cancer Care 2010, 19, 377–381. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann. Intern. Med. 1969, 71, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fraumeni, J.; Mulvihill, J.; Blattner, W.; Dreyfus, M.; Tucker, M.; Miller, R. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988, 48, 5358–5362. [Google Scholar] [PubMed]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Bougeard, G.; Renaux-Petel, M.; Flaman, J.M.; Charbonnier, C.; Fermey, P.; Belotti, M.; Gauthier-Villars, M.; Stoppa-Lyonnet, D.; Consolino, E.; Brugières, L.; et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J. Clin. Oncol. 2015, 33, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Masciari, S.; Dewanwala, A.; Stoffel, E.M.; Lauwers, G.Y.; Zheng, H.; Achatz, M.I.; Riegert-Johnson, D.; Foretova, L.; Silva, E.M.; Digianni, L.; et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet. Med. 2011, 13, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Powers, J.; McKenna, D.B.; Long, J.M.; Le, A.N.; Hausler, R.; Zelley, K.; Jennings, S.; Domchek, S.M.; Nathanson, K.L.; et al. Upper Gastrointestinal Cancer Risk and Surveillance Outcomes in Li-Fraumeni Syndrome. Am. J. Gastroenterol. 2020, 115, 2095–2097. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Goldgar, D.; Sodha, N.; Ohgaki, H.; Kleihues, P.; Hainaut, P.; Eeles, R. Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003, 63, 6643–6650. [Google Scholar] [PubMed]
- Hamzehloie, T.; Mojarrad, M.; Hasanzadeh-Nazarabadi, M.; Shekouhi, S. The role of tumor protein 53 mutations in common human cancers and targeting the murine double minute 2-p53 interaction for cancer therapy. Iran. J. Med. Sci. 2012, 37, 3–8. [Google Scholar] [PubMed]
- Voskarides, K.; Giannopoulou, N. The Role of TP53 in Adaptation and Evolution. Cells 2023, 12, 512. [Google Scholar] [CrossRef]
- Villani, A.; Shore, A.; Wasserman, J.D.; Stephens, D.; Kim, R.H.; Druker, H.; Gallinger, B.; Naumer, A.; Kohlmann, W.; Novokmet, A.; et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet. Oncol. 2016, 17, 1295–1305. [Google Scholar] [CrossRef]
- Kratz, C.P.; Achatz, M.I.; Brugieres, L.; Frebourg, T.; Garber, J.E.; Greer, M.L.C.; Hansford, J.R.; Janeway, K.A.; Kohlmann, W.K.; McGee, R.; et al. Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome. Clin. Cancer Res. 2017, 23, e38–e45. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Tjandra, D.; Boussioutas, A. Li Fraumeni Syndrome predisposes to gastro-esophageal junction tumours. Fam. Cancer 2024, 23, 29–33. [Google Scholar] [CrossRef]
- Momozawa, Y.; Sasai, R.; Usui, Y.; Shiraishi, K.; Iwasaki, Y.; Taniyama, Y.; Parsons, M.T.; Mizukami, K.; Sekine, Y.; Hirata, M.; et al. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2022, 8, 871–878. [Google Scholar] [CrossRef]
- Buckley, K.H.; Niccum, B.A.; Maxwell, K.N.; Katona, B.W. Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers. Cancers 2022, 14, 5953. [Google Scholar] [CrossRef]
- Uson, P.L.S.; Riegert-Johnson, D.; Boardman, L.; Kisiel, J.; Mountjoy, L.; Patel, N.; Lizaola-Mayo, B.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; et al. Germline Cancer Susceptibility Gene Testing in Unselected Patients with Colorectal Adenocarcinoma: A Multicenter Prospective Study. Clin. Gastroenterol. Hepatol. 2022, 20, e508–e528. [Google Scholar] [CrossRef]
- Armelao, F.; De Pretis, G. Familial colorectal cancer: A review. World J. Gastroenterol. 2014, 20, 9292–9298. [Google Scholar] [CrossRef]
- Stadler, Z.K.; Maio, A.; Chakravarty, D.; Kemel, Y.; Sheehan, M.; Salo-Mullen, E.; Tkachuk, K.; Fong, C.J.; Nguyen, B.; Erakky, A.; et al. Therapeutic Implications of Germline Testing in Patients with Advanced Cancers. J. Clin. Oncol. 2021, 39, 2698–2709. [Google Scholar] [CrossRef]
- You, Y.N.; Moskowitz, J.B.; Chang, G.J.; Mork, M.E.; Rodriguez-Bigas, M.A.; Bednarski, B.K.; Messick, C.A.; Tillman, M.M.; Skibber, J.M.; Nguyen, S.T.; et al. Germline Cancer Risk Profiles of Patients with Young-Onset Colorectal Cancer: Findings from a Prospective Universal Germline Testing and Telegenetics Program. Dis. Colon Rectum 2023, 66, 531–542. [Google Scholar] [CrossRef]
- Rebuzzi, F.; Ulivi, P.; Tedaldi, G. Genetic Predisposition to Colorectal Cancer: How Many and Which Genes to Test? Int. J. Mol. Sci. 2023, 24, 2137. [Google Scholar] [CrossRef]
- Boland, C.R.; Lynch, H.T. The history of Lynch syndrome. Fam. Cancer 2013, 12, 145–157. [Google Scholar] [CrossRef]
- Douglas, J.A.; Gruber, S.B.; Meister, K.A.; Bonner, J.; Watson, P.; Krush, A.J.; Lynch, H.T. History and molecular genetics of Lynch syndrome in family G: A century later. JAMA 2005, 294, 2195–2202. [Google Scholar] [CrossRef]
- Picó, M.D.; Castillejo, A.; Murcia, Ó.; Giner-Calabuig, M.; Alustiza, M.; Sánchez, A.; Moreira, L.; Pellise, M.; Castells, A.; Carrillo-Palau, M.; et al. Clinical and Pathological Characterization of Lynch-Like Syndrome. Clin. Gastroenterol. Hepatol. 2020, 18, 368–374.e1. [Google Scholar] [CrossRef]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009, 302, 1790–1795. [Google Scholar] [CrossRef]
- Edwards, P.; Monahan, K.J. Diagnosis and management of Lynch syndrome. Frontline Gastroenterol. 2022, 13, e80–e87. [Google Scholar] [CrossRef]
- Muller, C.; Matthews, L.; Kupfer, S.S.; Weiss, J.M. Effective Identification of Lynch Syndrome in Gastroenterology Practice. Curr. Treat. Options Gastroenterol. 2019, 17, 666. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261. [Google Scholar] [CrossRef]
- Morris, B.; Hughes, E.; Rosenthal, E.; Gutin, A.; Bowles, K.R. Classification of genetic variants in genes associated with Lynch syndrome using a clinical history weighting algorithm. BMC Genet. 2016, 17, 99. [Google Scholar] [CrossRef][Green Version]
- Boland, C.R.; Yurgelun, M.B.; Mraz, K.A.; Boland, P.M. Managing gastric cancer risk in lynch syndrome: Controversies and recommendations. Fam. Cancer 2022, 21, 75. [Google Scholar] [CrossRef]
- Sobocińska, J.; Kolenda, T.; Teresiak, A.; Badziąg-Leśniak, N.; Kopczyńska, M.; Guglas, K.; Przybyła, A.; Filas, V.; Bogajewska-Ryłko, E.; Lamperska, K.; et al. Diagnostics of Mutations in MMR/ EPCAM Genes and Their Role in the Treatment and Care of Patients with Lynch Syndrome. Diagnostics 2020, 10, 786. [Google Scholar] [CrossRef]
- Maratt, J.K.; Stoffel, E. Identification of Lynch Syndrome. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Hitchins, M.P.; Dámaso, E.; Alvarez, R.; Zhou, L.; Hu, Y.; Diniz, M.A.; Pineda, M.; Capella, G.; Pearlman, R.; Hampel, H. Constitutional MLH1 Methylation Is a Major Contributor to Mismatch Repair-Deficient, MLH1-Methylated Colorectal Cancer in Patients Aged 55 Years and Younger. J. Natl. Compr. Cancer Netw. 2023, 21, 743–752.e11. [Google Scholar] [CrossRef]
- Peltomäki, P. Lynch syndrome genes. Fam. Cancer 2005, 4, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Bhaijee, F.; Brown, A.S. Muir-Torre syndrome. Arch. Pathol. Lab. Med. 2014, 138, 1685–1689. [Google Scholar] [CrossRef]
- Sijmons, R.H.; Greenblatt, M.S.; Genuardi, M. Gene variants of unknown clinical significance in Lynch syndrome. Introd. Clinicians. Fam. Cancer 2013, 12, 181–187. [Google Scholar] [CrossRef]
- Suerink, M.; Rodríguez-Girondo, M.; van der Klift, H.M.; Colas, C.; Brugieres, L.; Lavoine, N.; Jongmans, M.; Munar, G.C.; Evans, D.G.; Farrell, M.P.; et al. An alternative approach to establishing unbiased colorectal cancer risk estimation in Lynch syndrome. Genet. Med. 2019, 21, 2706–2712. [Google Scholar] [CrossRef]
- Jiang, B.; Ofshteyn, A.; Idrees, J.J.; Giglia, M.; Gallego, C.; Stein, S.L.; Steinhagen, E. Total abdominal colectomy is cost-effective in treating colorectal cancer in patients with genetically diagnosed Lynch Syndrome. Am. J. Surg. 2019, 218, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; Burn, J.; et al. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef]
- Chrysafi, P.; Jani, C.T.; Lotz, M.; Al Omari, O.; Singh, H.; Stafford, K.; Agarwal, L.; Rupal, A.; Dar, A.Q.; Dangelo, A.; et al. Prevalence of Variants of Uncertain Significance in Patients Undergoing Genetic Testing for Hereditary Breast and Ovarian Cancer and Lynch Syndrome. Cancers 2023, 15, 5762. [Google Scholar] [CrossRef]
- Clift, K.; Macklin, S.; Halverson, C.; McCormick, J.B.; Abu Dabrh, A.M.; Hines, S. Patients’ views on variants of uncertain significance across indications. J. Community Genet. 2020, 11, 139–145. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Farha, N.; Burke, C.A.; Katona, B.W. Upper Gastrointestinal Cancer Surveillance in Lynch Syndrome. Cancers 2022, 14, 1000. [Google Scholar] [CrossRef] [PubMed]
- Bin Naeem, S.; Ullah, N.; Jhatial, M.A.; Muzaffar, S.; Abbas, M.; Iftikhar, I.; Jameel, A.; Masood Sheikh, R. Constitutional Mismatch Repair Deficiency (CMMRD) Syndrome: A Case Report of a Patient with Multiple Metachronous Malignancies. Cureus 2023, 15, e41870. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.; Colas, C.; Shuen, A.; Hampel, H.; Foulkes, W.D.; Feldman, H.B.; Goldberg, Y.; Muleris, M.; Schneider, K.W.; McGee, R.B.; et al. Diagnostic criteria for constitutional mismatch repair deficiency (CMMRD): Recommendations from the international consensus working group. J. Med. Genet. 2022, 59, 318–327. [Google Scholar] [CrossRef]
- Nejadtaghi, M.; Jafari, H.; Farrokhi, E.; Samani, K.G. Familial Colorectal Cancer Type X (FCCTX) and the correlation with various genes-A systematic review. Curr. Probl. Cancer 2017, 41, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.A.d.O.; de Andrade, E.S.; de Campos Reis Galvão, H.; da Silva Sábato, C.; Campacci, N.; de Paula, A.E.; Evangelista, A.F.; Santana, I.V.V.; Melendez, M.E.; Reis, R.M.; et al. New insights on familial colorectal cancer type X syndrome. Sci. Rep. 2022, 12, 2846. [Google Scholar] [CrossRef]
- Martín-Morales, L.; Garre, P.; Lorca, V.; Cazorla, M.; Llovet, P.; Bando, I.; García-Barberan, V.; González-Morales, M.L.; Esteban-Jurado, C.; De La Hoya, M.; et al. BRIP1, a Gene Potentially Implicated in Familial Colorectal Cancer Type X. Cancer Prev. Res. 2021, 14, 185–193. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med. Sci. 2018, 6, 31. [Google Scholar] [CrossRef]
- Bülow, S.; Berk, T.; Neale, K. The history of familial adenomatous polyposis. Fam. Cancer 2006, 5, 213–220. [Google Scholar] [CrossRef]
- Meera Khan, P.; Tops, C.M.J.; Broek, M.V.D.; Breukel, C.; Wijnen, J.T.; Oldenburg, M.; Bos, J.V.D.; van Leeuwen-Cornelisse, I.S.J.; Vasen, H.F.A.; Griffioen, G.; et al. Close linkage of a highly polymorphic marker (D5S37) to familial adenomatous polyposis (FAP) and confirmation of FAP localization on chromosome 5q21-q22. Hum. Genet. 1988, 79, 183–185. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Potter, D.D.; Moir, C.R.; El-Youssef, M. The natural history of familial adenomatous polyposis syndrome: A 24 year review of a single center experience in screening, diagnosis, and outcomes. J. Pediatr. Surg. 2014, 49, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Y. Application of Molecular Profiling in Colorectal Cancer Surgery: Update on Surgical Management of FAP. Clin. Colon Rectal Surg. 2023, 36, 385. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, J.G.; Bülow, S.; Højen, H.; Jelsig, A.M.; Jespersen, N.; Andersen, K.K.; Wewer, M.D.; Burisch, J.; Pommergaard, H.C. Cancer in Patients with Familial Adenomatous Polyposis: A Nationwide Danish Cohort Study with Matched Controls. Gastroenterology 2023, 165, 573–581.e3. [Google Scholar] [CrossRef] [PubMed]
- Stec, R.; Pławski, A.; Synowiec, A.; Mączewski, M.; Szczylik, C. Colorectal cancer in the course of familial adenomatous polyposis syndrome (“de novo” pathogenic mutation of APC gene): Case report, review of the literature and genetic commentary. Arch. Med. Sci. 2010, 6, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanoghli, Z.; Nieuwenhuis, M.H.; Houwing-Duistermaat, J.J.; Jagmohan-Changur, S.; Hes, F.J.; Tops, C.M.; Wagner, A.; Aalfs, C.M.; Verhoef, S.; Gómez García, E.B.; et al. Colorectal cancer risk variants at 8q23.3 and 11q23.1 are associated with disease phenotype in APC mutation carriers. Fam. Cancer 2016, 15, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; McGinty, P.; Cuthill, V.; Hawkins, M.; Moorghen, M.; Clark, S.K.; Latchford, A. MUTYH-associated polyposis-colorectal phenotype and management. Color. Dis. 2020, 22, 1271–1278. [Google Scholar] [CrossRef]
- Weiss, J.M.; Gupta, S.; Burke, C.A.; Axell, L.; Chen, L.M.; Chung, D.C.; Clayback, K.M.; Dallas, S.; Felder, S.; Gbolahan, O.; et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 1122–1132. [Google Scholar] [CrossRef]
- Colas, C.; Bonadona, V.; Baert-Desurmont, S.; Bonnet, D.; Coulet, F.; Dhooge, M.; Saurin, J.C.; Remenieras, A.; Bignon, Y.J.; Caron, O.; et al. MUTYH-associated polyposis: Review and update of the French recommendations established in 2012 under the auspices of the National Cancer institute (INCa). Eur. J. Med. Genet. 2020, 63, 104078. [Google Scholar] [CrossRef] [PubMed]
- Magrin, L.; Fanale, D.; Brando, C.; Corsini, L.R.; Randazzo, U.; Di Piazza, M.; Gurrera, V.; Pedone, E.; Bazan Russo, T.D.; Vieni, S.; et al. MUTYH-associated tumor syndrome: The other face of MAP. Oncogene 2022, 41, 2531–2539. [Google Scholar] [CrossRef]
- Tieu, A.H.; Edelstein, D.; Axilbund, J.; Romans, K.E.; Brosens, L.A.; Wiley, E.; Hylind, L.; Giardiello, F.M. Clinical Characteristics of Multiple Colorectal Adenoma Patients without Germline APC or MYH Mutations. J. Clin. Gastroenterol. 2016, 50, 584. [Google Scholar] [CrossRef]
- Gohil, D.; Sarker, A.H.; Roy, R. Base Excision Repair: Mechanisms and Impact in Biology, Disease, and Medicine. Int. J. Mol. Sci. 2023, 24, 14186. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.B.; Klungland, A.; Rognes, T.; Leiros, I. DNA repair in mammalian cells: Base excision repair: The long and short of it. Cell. Mol. Life Sci. 2009, 66, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Quintana, V.G.; Sweasy, J.B. NTHL1 in genomic integrity, aging and cancer. DNA Repair. 2020, 93, 102920. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Quintana, I.; Mur, P.; Munoz-Torres, P.M.; Alonso, M.H.; Navarro, M.; Terradas, M.; Piñol, V.; Brunet, J.; Moreno, V.; et al. NTHL1 biallelic mutations seldom cause colorectal cancer, serrated polyposis or a multi-tumor phenotype, in absence of colorectal adenomas. Sci. Rep. 2019, 9, 9020. [Google Scholar] [CrossRef] [PubMed]
- Grot, N.; Kaczmarek-Ryś, M.; Lis-Tanaś, E.; Kryszczyńska, A.; Nowakowska, D.; Jakubiuk-Tomaszuk, A.; Paszkowski, J.; Banasiewicz, T.; Hryhorowicz, S.; Pławski, A. NTHL1 Gene Mutations in Polish Polyposis Patients-Weighty Player or Vague Background? Int. J. Mol. Sci. 2023, 24, 14548. [Google Scholar] [CrossRef] [PubMed]
- Weren, R.D.A.; Ligtenberg, M.J.L.; Geurts van Kessel, A.; De Voer, R.M.; Hoogerbrugge, N.; Kuiper, R.P. NTHL1 and MUTYH polyposis syndromes: Two sides of the same coin? J. Pathol. 2018, 244, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.H.; Jelsig, A.M.; Yassin, H.M.; Lindberg, L.J.; Wadt, K.A.W.; Karstensen, J.G. Intestinal and extraintestinal neoplasms in patients with NTHL1 tumor syndrome: A systematic review. Fam. Cancer 2022, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Weatherill, C.B.; Burke, S.A.; Haskins, C.G.; Berry, D.K.; Homer, J.P.; Demeure, M.J.; Darabi, S. Six case reports of NTHL1-associated tumor syndrome further support it as a multi-tumor predisposition syndrome. Clin. Genet. 2023, 103, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Boulouard, F.; Kasper, E.; Buisine, M.P.; Lienard, G.; Vasseur, S.; Manase, S.; Bahuau, M.; Barouk Simonet, E.; Bubien, V.; Coulet, F.; et al. Further delineation of the NTHL1 associated syndrome: A report from the French Oncogenetic Consortium. Clin. Genet. 2021, 99, 662–672. [Google Scholar] [CrossRef]
- Palles, C.; Martin, L.; Domingo, E.; Chegwidden, L.; McGuire, J.; Cuthill, V.; Heitzer, E.; Kerr, R.; Kerr, D.; Kearsey, S.; et al. The clinical features of polymerase proof-reading associated polyposis (PPAP) and recommendations for patient management. Fam. Cancer 2022, 21, 197–209. [Google Scholar] [CrossRef]
- Magrin, L.; Fanale, D.; Brando, C.; Fiorino, A.; Corsini, L.R.; Sciacchitano, R.; Filorizzo, C.; Dimino, A.; Russo, A.; Bazan, V. POLE, POLD1, and NTHL1: The last but not the least hereditary cancer-predisposing genes. Oncogene 2021, 40, 5893–5901. [Google Scholar] [CrossRef]
- Leclerc, J.; Beaumont, M.; Vibert, R.; Pinson, S.; Vermaut, C.; Flament, C.; Lovecchio, T.; Delattre, L.; Demay, C.; Coulet, F.; et al. AXIN2 germline testing in a French cohort validates pathogenic variants as a rare cause of predisposition to colorectal polyposis and cancer. Genes. Chromosomes Cancer 2023, 62, 210–222. [Google Scholar] [CrossRef]
- Miete, C.; Solis, G.P.; Koval, A.; Brückner, M.; Katanaev, V.L.; Behrens, J.; Bernkopf, D.B. Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth. Nat. Commun. 2022, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Chen, H.; Wong, C.C.; Peng, Y.; Gou, H.; Zhang, J.; Pan, Y.; Chen, D.; Lin, Y.; Wang, S.; et al. ALKBH5 Drives Immune Suppression Via Targeting AXIN2 to Promote Colorectal Cancer and Is a Target for Boosting Immunotherapy. Gastroenterology 2023, 165, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Reynoso, M.A.; Rosas-Enríquez, V.; Saucedo-Sariñana, A.M.; Pérez-Coria, M.; Gallegos-Arreola, M.P.; Salas-González, E.; Barros-Núñez, P.; Juárez-Vázquez, C.I.; Flores-Martínez, S.E.; Sánchez-Corona, J. Genotypes and Haplotypes in the AXIN2 and TCF7L2 Genes are Associated with Susceptibility and with Clinicopathological Characteristics in Breast Cancer Patients. Br. J. Biomed. Sci. 2022, 79, 10211. [Google Scholar] [CrossRef]
- Kizys, M.M.L.; Louzada, R.A.; Mitne-Neto, M.; Jara, J.R.; Furuzawa, G.K.; De Carvalho, D.P.; Dias-Da-Silva, M.R.; Nesi-França, S.; Dupuy, C.; Maciel, R.M.B. DUOX2 Mutations Are Associated with Congenital Hypothyroidism with Ectopic Thyroid Gland. J. Clin. Endocrinol. Metab. 2017, 102, 4060–4071. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Fritsch, J.; González, E.E.; Landau, K.S.; Santander, A.M.; Fernández, I.; Hazime, H.; Davies, J.M.; Santaolalla, R.; Phillips, M.C.; et al. Epithelial TLR4 Signaling Activates DUOX2 to Induce Microbiota-Driven Tumorigenesis. Gastroenterology 2021, 160, 797–808.e6. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Feng, L.; Zhi, L.; Jiang, D.; Yu, B.; Zhang, Z.; Gao, B.; Zhang, C.; Li, M.; et al. DUOX2 promotes the progression of colorectal cancer cells by regulating the AKT pathway and interacting with RPL3. Carcinogenesis 2021, 42, 105–117. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, Y.; Ding, Y.; Wang, J.; Tan, Y.; Xu, D.; Yuan, Y. A truncated protein product of the germline variant of the DUOX2 gene leads to adenomatous polyposis. Cancer Biol. Med. 2021, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Te Paske, I.B.A.W.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; de Voer, R.M. Candidate Gene Discovery in Hereditary Colorectal Cancer and Polyposis Syndromes–Considerations for Future Studies. Int. J. Mol. Sci. 2020, 21, 8757. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Jelsig, A.M.; Byrjalsen, A.; Madsen, M.B.; Kuhlmann, T.P.; Hansen, T.v.O.; Wadt, K.A.W.; Karstensen, J.G. Novel Genetic Causes of Gastrointestinal Polyposis Syndromes. Appl. Clin. Genet. 2021, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Win, A.K.; Walters, R.J.; Buchanan, D.D.; Jenkins, M.A.; Sweet, K.; Frankel, W.L.; De La Chapelle, A.; McKeone, D.M.; Walsh, M.D.; Clendenning, M.; et al. Cancer risks for relatives of patients with serrated polyposis. Am. J. Gastroenterol. 2012, 107, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Guarinos, C.; Sánchez-Fortún, C.; Rodríguez-Soler, M.; Alenda, C.; Payá, A.; Jover, R. Serrated polyposis syndrome: Molecular, pathological and clinical aspects. World J. Gastroenterol. 2012, 18, 2452. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Yamada, A.; Ikegami, S.; Haider, H.; Komaki, Y.; Komaki, F.; Micic, D.; Sakuraba, A. Risk of Colorectal Cancer in Serrated Polyposis Syndrome: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 622–630.e7. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Solomons, J.; Risby, P.; Gabriel, J.; Bedenham, T.; Johnson, M.; Atkinson, N.; Bailey, A.A.; Bird-Lieberman, E.; Leedham, S.J.; et al. Germline variant testing in serrated polyposis syndrome. J. Gastroenterol. Hepatol. 2022, 37, 861–869. [Google Scholar] [CrossRef]
- Carballal, S.; Balaguer, F.; IJspeert, J.E.G. Serrated polyposis syndrome; epidemiology and management. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101791. [Google Scholar] [CrossRef]
- East, J.E.; Atkin, W.S.; Bateman, A.C.; Clark, S.K.; Dolwani, S.; Ket, S.N.; Leedham, S.J.; Phull, P.S.; Rutter, M.D.; Shepherd, N.A.; et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017, 66, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Van Leerdam, M.E.; Roos, V.H.; Van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.C.; et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Lai, J.C.W.; Ho, S.L.; Leung, W.K.; Law, W.L.; Lee, J.F.Y.; Chan, A.K.W.A.S.Y.; Tsui, W.Y.; Chan, A.K.W.A.S.Y.; Lee, B.C.H.; et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut 2017, 66, 1645–1656. [Google Scholar] [CrossRef]
- Chan, J.M.; Clendenning, M.; Joseland, S.; Georgeson, P.; Mahmood, K.; Joo, J.E.; Walker, R.; Como, J.; Preston, S.; Chai, S.M.; et al. Inherited BRCA1 and RNF43 pathogenic variants in a familial colorectal cancer type X family. Fam. Cancer 2024, 23, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mikaeel, R.R.; Young, J.P.; Li, Y.; Poplawski, N.K.; Smith, E.; Horsnell, M.; Uylaki, W.; Tomita, Y.; Townsend, A.R.; Feng, J.; et al. RNF43 pathogenic Germline variant in a family with colorectal cancer. Clin. Genet. 2022, 101, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, L.; Shi, X.; Gao, S.; Yue, C.; Zhang, L.; Bai, Y.; Wang, Q.; Okada, A.; Yasui, T.; et al. RNF43 is a novel tumor-suppressor and prognostic indicator in clear cell renal cell carcinoma. Oncol. Res. 2021, 29, 159. [Google Scholar] [CrossRef] [PubMed]
- Radaszkiewicz, T.; Nosková, M.; Gömöryová, K.; Blanářová, O.V.; Radaszkiewicz, K.A.; Picková, M.; Víchová, R.; Gybeľ, T.; Kaiser, K.; Demková, L.; et al. RNF43 inhibits WNT5A-driven signaling and suppresses melanoma invasion and resistance to the targeted therapy. Elife 2021, 10, e65759. [Google Scholar] [CrossRef] [PubMed]
- Dal Buono, A.; Gaiani, F.; Poliani, L.; Laghi, L. Juvenile polyposis syndrome: An overview. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101799. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Li, J.; Zhao, Z.Y.; Xu, X.D.; Du, Y.Q.; Yan, H.L.; Liu, L.J.; Bai, C.G.; Zhang, W. Juvenile polyposis syndrome might be misdiagnosed as familial adenomatous polyposis: A case report and literature review. BMC Gastroenterol. 2020, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Latchford, A.R.; Neale, K.; Phillips, R.K.S.; Clark, S.K. Juvenile polyposis syndrome: A study of genotype, phenotype, and long-term outcome. Dis. Colon Rectum 2012, 55, 1038–1043. [Google Scholar] [CrossRef]
- MacFarland, S.P.; Ebrahimzadeh, J.E.; Zelley, K.; Begum, L.; Bass, L.M.; Brand, R.E.; Dudley, B.; Fishman, D.S.; Ganzak, A.; Karloski, E.; et al. Phenotypic Differences in Juvenile Polyposis Syndrome with or without a Disease-causing SMAD4/ BMPR1A Variant. Cancer Prev. Res. 2021, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.G.; Ahmed, A.F.; Ringold, J.R.; Anderson, M.E.; Bair, J.L.; Mitros, F.A.; Lynch, H.T.; Tinley, S.T.; Petersen, G.M.; Giardiello, F.M.; et al. Germline SMAD4 or BMPR1A mutations and phenotype of juvenile polyposis. Ann. Surg. Oncol. 2002, 9, 901–906. [Google Scholar] [CrossRef]
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111. [Google Scholar] [CrossRef]
- Katz, L.H.; Gingold-Belfer, R.; Vainer, E.; Hegger, S.; Laish, I.; Derazne, E.; Weintraub, I.; Reznick-Levi, G.; Goldberg, Y.; Levi, Z.; et al. Phenotypic diversity among juvenile polyposis syndrome patients from different ethnic background. Hered. Cancer Clin. Pract. 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Micolonghi, C.; Piane, M.; Germani, A.; Sadeghi, S.; Libi, F.; Savio, C.; Fabiani, M.; Mancini, R.; Ranieri, D.; Pizzuti, A.; et al. A New SMAD4 Splice Site Variant in a Three-Generation Italian Family with Juvenile Polyposis Syndrome. Diagnostics 2022, 12, 2684. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Umeno, J.; Jimbo, K.; Arai, M.; Iwama, I.; Kashida, H.; Kudo, T.; Koizumi, K.; Sato, Y.; Sekine, S.; et al. Clinical Guidelines for Diagnosis and Management of Juvenile Polyposis Syndrome in Children and Adults-Secondary Publication. J. Anus Rectum Colon 2023, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Busoni, V.B.; Orsi, M.; Lobos, P.A.; D’Agostino, D.; Wagener, M.; De La Iglesia, P.; Fox, V.L. Successful Treatment of Juvenile Polyposis of Infancy with Sirolimus. Pediatrics 2019, 144, e20182922. [Google Scholar] [CrossRef] [PubMed]
- Dahdaleh, F.S.; Carr, J.C.; Calva, D.; Howe, J.R. Juvenile polyposis and other intestinal polyposis syndromes with microdeletions of chromosome 10q22-23. Clin. Genet. 2012, 81, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Beggs, A.D.; Latchford, A.R.; Vasen, H.F.A.; Moslein, G.; Alonso, A.; Aretz, S.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; et al. Peutz-Jeghers syndrome: A systematic review and recommendations for management. Gut 2010, 59, 975–986. [Google Scholar] [CrossRef] [PubMed]
- McGarrity, T.J.; Amos, C.I.; Baker, M.J. Peutz-Jeghers Syndrome. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993; 1993–2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1266/ (accessed on 1 May 2024).
- Tavusbay, C.; Acar, T.; Kar, H.; Atahan, K.; Kamer, E. The patients with Peutz-Jeghers syndrome have a high risk of developing cancer. Turk. J. Surg. 2018, 34, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Klimkowski, S.; Ibrahim, M.; Rovira, J.J.I.; Elshikh, M.; Javadi, S.; Klekers, A.R.; Abusaif, A.A.; Moawad, A.W.; Ali, K.; Elsayes, K.M. Peutz-Jeghers Syndrome and the Role of Imaging: Pathophysiology, Diagnosis, and Associated Cancers. Cancers 2021, 13, 5121. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, A.; Amos, C.I.; Bale, S.J.; Zhu, D.; Arold, S.; Berrueta, J.; Nabon, N.; McGarrity, T. Findings from the Peutz-Jeghers syndrome registry of uruguay. PLoS ONE 2013, 8, e79639. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, E.R.; Park, J.J.; Kim, E.S.; Goong, H.J.; Kim, K.O.; Nam, J.H.; Park, Y.; Lee, S.P.; Jang, H.J. Cancer risk in patients with Peutz-Jeghers syndrome in Korea: A retrospective multi-center study. Korean J. Intern. Med. 2023, 38, 176–185. [Google Scholar] [CrossRef]
- Shukla, R.; Tiwari, P.; Dariya, S.; Jain, S.; Sharma, S.; Laddha, A.; Lahoti, B.; Thanna, H. Peutz-Jeghers Syndrome: Lessons to be Learned in the Clinical Diagnosis. J. Indian.. Assoc. Pediatr. Surg. 2023, 28, 218. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.Y.; Wu, S.; Shinagare, S.A.; Lauwers, G.Y.; Yilmaz, O.; Wu, C.L.; Deshpande, V. Peutz-Jeghers syndrome: A critical look at colonic Peutz-Jeghers polyps. Mod. Pathol. 2013, 26, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, N.; Yamaguchi, H.; Kaminishi, M. Differential diagnosis of solitary gastric Peutz-Jeghers-type polyp with stomach cancer: A case report. Int. J. Surg. Case Rep. 2018, 51, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Shakil, S.; Aldaher, Z.; DiValentin, L. Peutz-Jeghers Syndrome Presenting with Anemia: A Case Report. Cureus 2022, 14, e26481. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jin, X.W.; Li, B.R.; Zhu, M.; Li, J.; Mao, G.P.; Zhang, Y.F.; Ning, S. Bin Cancer risk in patients with Peutz-Jeghers syndrome: A retrospective cohort study of 336 cases. Tumour Biol. 2017, 39, 1010428317705131. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, J.M. Peutz-Jeghers Syndrome. In Small Intestine Disease: A Comprehensive Guide to Diagnosis and Management; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 231–233. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Tacheci, I.; Kopacova, M.; Bures, J. Peutz-Jeghers syndrome. Curr. Opin. Gastroenterol. 2021, 37, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Altamish, M.; Dahiya, R.; Singh, A.K.; Mishra, A.; Aljabali, A.A.A.; Satija, S.; Mehta, M.; Dureja, H.; Prasher, P.; Negi, P.; et al. Role of the Serine/Threonine Kinase 11 (STK11) or Liver Kinase B1 (LKB1) Gene in Peutz-Jeghers Syndrome. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Inukai, K.; Ikegami, Y.; Awata, T.; Katayama, S. LKB1, an upstream AMPK kinase, regulates glucose and lipid metabolism in cultured liver and muscle cells. Biochem. Biophys. Res. Commun. 2006, 351, 595–601. [Google Scholar] [CrossRef]
- Konen, J.; Wilkinson, S.; Lee, B.; Fu, H.; Zhou, W.; Jiang, Y.; Marcus, A.I. LKB1 kinase-dependent and -independent defects disrupt polarity and adhesion signaling to drive collagen remodeling during invasion. Mol. Biol. Cell 2016, 27, 1069–1084. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sakamoto, H.; Kumagai, H.; Abe, T.; Ishiguro, S.; Uchida, K.; Kawasaki, Y.; Saida, Y.; Sano, Y.; Takeuchi, Y.; et al. Clinical Guidelines for Diagnosis and Management of Peutz-Jeghers Syndrome in Children and Adults. Digestion 2023, 104, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Marcus, A.I.; Vertino, P.M. Dysregulation of mTOR activity through LKB1 inactivation. Chin. J. Cancer 2013, 32, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Ryan, B.; Patel, M.; Chan, N.; Guo, Y.; Aisner, J.; Jabbour, S.K.; Pine, S. Clinical outcomes and immune phenotypes associated with STK11 co-occurring mutations in non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Laderian, B.; Mundi, P.; Fojo, T.; Bates, S.E. Emerging Therapeutic Implications of STK11 Mutation: Case Series. Oncologist 2020, 25, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Bardeesy, N. LKB1; linking cell structure and tumor suppression. Oncogene 2008, 27, 6908–6919. [Google Scholar] [CrossRef] [PubMed]
- Karuman, P.; Gozani, O.; Odze, R.D.; Zhou, X.C.; Zhu, H.; Shaw, R.; Brien, T.P.; Bozzuto, C.D.; Ooi, D.; Cantley, L.C.; et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 2001, 7, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, Y.; Tian, H.; Yu, K.Z.; He, J.X.; Shen, W.Y. The tumor suppressor LKB1 antagonizes WNT signaling pathway through modulating GSK3β activity in cell growth of esophageal carcinoma. Tumour Biol. 2014, 35, 995–1002. [Google Scholar] [CrossRef]
- Kuburich, N.A.; Sabapathy, T.; Demestichas, B.R.; Maddela, J.J.; den Hollander, P.; Mani, S.A. Proactive and reactive roles of TGF-β in cancer. Semin. Cancer Biol. 2023, 95, 120–139. [Google Scholar] [CrossRef]
- Syed, V. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Wagner, A.; Aretz, S.; Auranen, A.; Bruno, M.J.; Cavestro, G.M.; Crosbie, E.J.; Goverde, A.; Jelsig, A.M.; Latchford, A.; van Leerdam, M.E.; et al. The Management of Peutz-Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J. Clin. Med. 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Mester, J.; Eng, C. Cowden syndrome: Recognizing and managing a not-so-rare hereditary cancer syndrome. J. Surg. Oncol. 2015, 111, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Gammon, A.; Jasperson, K.; Champine, M. Genetic basis of Cowden syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2016, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Patraquim, C.; Fernandes, V.; Martins, S.; Antunes, A.; Marques, O.; Carvalho, J.L.; Correia-Pinto, J.; Meireles, C.; Ferreira, A.M. A Pediatric Case of Cowden Syndrome with Graves’ Disease. Case Rep. Pediatr. 2017, 2017, 2750523. [Google Scholar] [CrossRef]
- Stanich, P.P.; Owens, V.L.; Sweetser, S.; Khambatta, S.; Smyrk, T.C.; Richardson, R.L.; Goetz, M.P.; Patnaik, M.M. Colonic Polyposis and Neoplasia in Cowden Syndrome. Mayo Clin. Proc. 2011, 86, 489. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, U.; Huntoon, K.; Smith-Cohn, M.; Shaw, A.; Elder, J.B. Bilateral Recurrent Dysplastic Cerebellar Gangliocytoma (Lhermitte-Duclos Disease) in Cowden Syndrome: A Case Report and Literature Review. World Neurosurg. 2019, 127, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Perriard, J.; Saurat, J.H.; Harms, M. An overlap of Cowden’s disease and Bannayan-Riley-Ruvalcaba syndrome in the same family. J. Am. Acad. Dermatol. 2000, 42, 348–350. [Google Scholar] [CrossRef]
- Heald, B.; Mester, J.; Rybicki, L.; Orloff, M.S.; Burke, C.A.; Eng, C. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology 2010, 139, 1927–1933. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Colonna, F.; Calapà, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.J.; Zori, R.T. Genetic insights into familial cancers—Update and recent discoveries. Cancer Lett. 2002, 181, 125–164. [Google Scholar] [CrossRef] [PubMed]
- Smerdel, M.P.; Skytte, A.B.; Jelsig, A.M.; Ebbehøj, E.; Stochholm, K. Revised Danish guidelines for the cancer surveillance of patients with Cowden Syndrome. Eur. J. Med. Genet. 2020, 63, 103873. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Dennis, P.A. PTEN hamartoma tumor syndromes. Eur. J. Hum. Genet. 2008, 16, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, D.D.; Taher, A.; Wong, V.K.; Elsaiey, A.; Consul, N.; Mahmoud, H.S.; Mujtaba, B.; Stanietzky, N.; Elsayes, K.M. PTEN Hamartoma Tumor Syndrome/Cowden Syndrome: Genomics, Oncogenesis, and Imaging Review for Associated Lesions and Malignancy. Cancers 2021, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Eu, K.W.; Kumarasinghe, M.P.; Li, H.H.; Loi, C.; Cheah, P.Y. Mapping of hereditary mixed polyposis syndrome (HMPS) to chromosome 10q23 by genomewide high-density single nucleotide polymorphism (SNP) scan and identification of BMPR1A loss of function. J. Med. Genet. 2006, 43, e13. [Google Scholar] [CrossRef]
- Gao, Z.; Houthuijzen, J.M.; ten Dijke, P.; Brazil, D.P. GREM1 signaling in cancer: Tumor promotor and suppressor? J. Cell Commun. Signal. 2023, 17, 1517–1526. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Alkuraya, F.S. Cenani-Lenz syndrome and other related syndactyly disorders due to variants in LRP4, GREM1/FMN1, and APC: Insight into the pathogenesis and the relationship to polyposis through the WNT and BMP antagonistic pathways. Am. J. Med. Genet. A 2019, 179, 266–279. [Google Scholar] [CrossRef]
- Aglago, E.K.; Kim, A.; Lin, Y.; Qu, C.; Evangelou, M.; Ren, Y.; Morrison, J.; Albanes, D.; Arndt, V.; Barry, E.L.; et al. A Genetic Locus within the FMN1/GREM1 Gene Region Interacts with Body Mass Index in Colorectal Cancer Risk. Cancer Res. 2023, 83, 2572–2583. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Lei, Y.; Wang, Z.M.; Han, H.; Xing, J.J.; Xu, X.D.; Gao, X.H.; Zhang, W.; Yu, E. Da Re-recognition of BMPR1A-related polyposis: Beyond juvenile polyposis and hereditary mixed polyposis syndrome. Gastroenterol. Rep. 2023, 11, goac082. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Aceñero, M.J.; Díaz del Arco, C. Hereditary Gastrointestinal Tumor Syndromes: When Risk Comes with Your Genes. Curr. Issues Mol. Biol. 2024, 46, 6440-6471. https://doi.org/10.3390/cimb46070385
Fernández Aceñero MJ, Díaz del Arco C. Hereditary Gastrointestinal Tumor Syndromes: When Risk Comes with Your Genes. Current Issues in Molecular Biology. 2024; 46(7):6440-6471. https://doi.org/10.3390/cimb46070385
Chicago/Turabian StyleFernández Aceñero, María Jesús, and Cristina Díaz del Arco. 2024. "Hereditary Gastrointestinal Tumor Syndromes: When Risk Comes with Your Genes" Current Issues in Molecular Biology 46, no. 7: 6440-6471. https://doi.org/10.3390/cimb46070385
APA StyleFernández Aceñero, M. J., & Díaz del Arco, C. (2024). Hereditary Gastrointestinal Tumor Syndromes: When Risk Comes with Your Genes. Current Issues in Molecular Biology, 46(7), 6440-6471. https://doi.org/10.3390/cimb46070385





