Potential New Inflammatory Markers in Bronchiectasis: A Literature Review
Abstract
1. Introduction
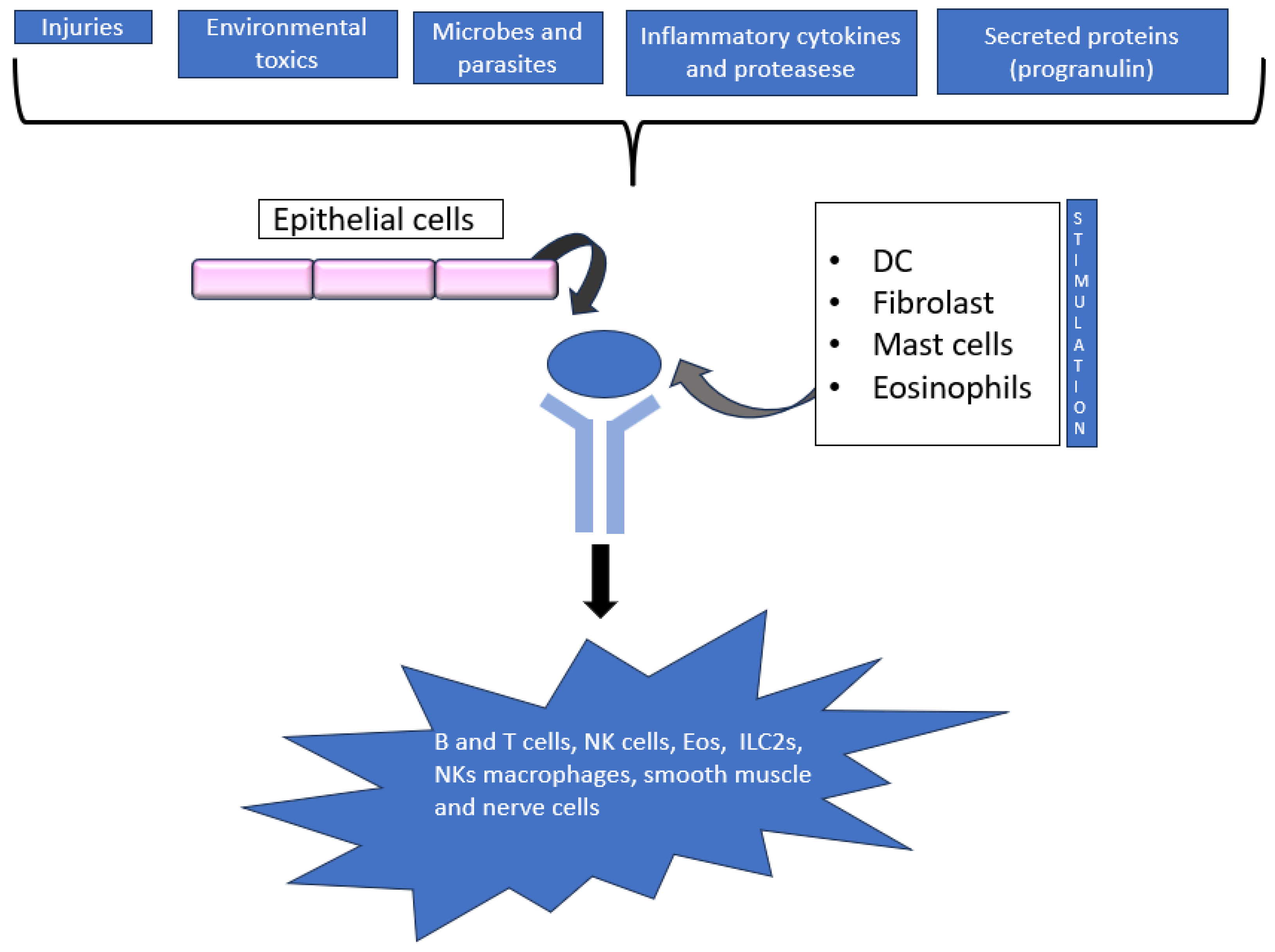
2. Thymic Stromal Lymphopoietin (TSLP)
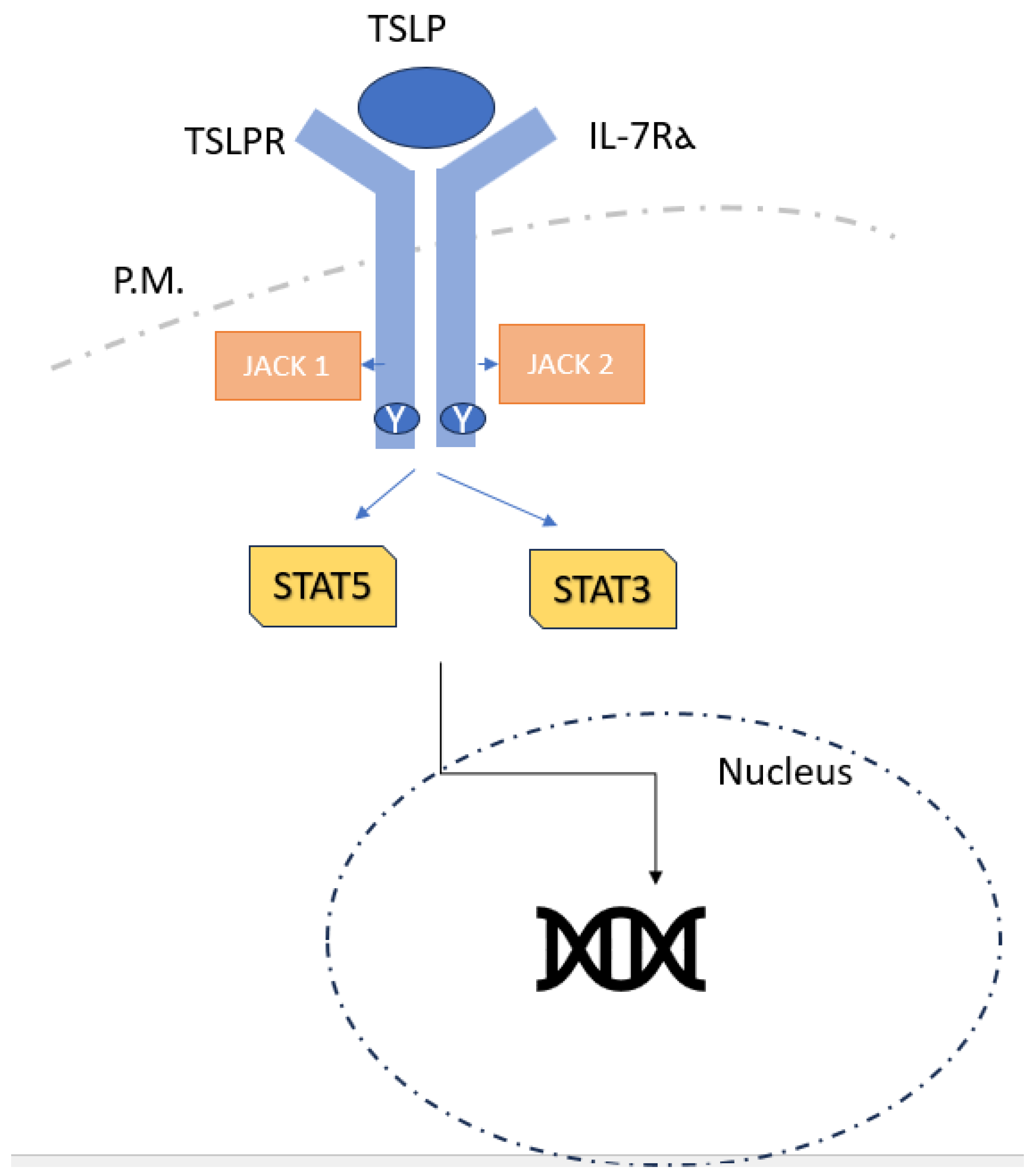
2.1. TSLP Signalling
2.2. TSLP and Respiratory Disease
2.3. TSLP and Blood Neutrophil-to-Lymphocyte Ratio (NLR)
2.4. Measurement of TSLP
3. Mucins
3.1. Mucins and Respiratory Diseases
3.2. Measure of Mucins on Sputum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chalmers, J.D.; Aliberti, S.; Blasi, F. Management of Bronchiectasis in Adults. Eur. Respir. J. 2015, 45, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Lonni, S.; Chalmers, J.D.; Goeminne, P.C.; Mcdonnell, M.J.; Dimakou, K.; De Soyza, A.; Polverino, E.; Van de Kerkhove, C.; Rutherford, R.; Davison, J.; et al. Etiology of non–cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann. Am. Thorac. Soc. 2015, 12, 1764–1770. [Google Scholar] [CrossRef]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Stuart Elborn, J.; Andres Floto, R.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for Bronchiectasis in Adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.F.; Tan, N.S.; Dehghan, R.; Shen, M.; Liew, M.F.; Bee, S.W.L.; Sia, Y.Y.; Liu, J.; Khor, C.C.; Kwok, I.; et al. Shortened Telomere Length in Sputum Cells of Bronchiectasis Patients is Associated with Dysfunctional Inflammatory Pathways. Lung 2022, 200, 401–407. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Aliberti, S.; Goeminne, P.C.; Restrepo, M.I.; Finch, S.; Pesci, A.; Dupont, L.J.; Fardon, T.C.; Wilson, R.; Loebinger, M.R.; et al. Comorbidities and the Risk of Mortality in Patients with Bronchiectasis: An International Multicentre Cohort Study. Lancet Respir. Med. 2016, 4, 969–979. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Aliberti, S.; Goeminne, P.C.; Dimakou, K.; Zucchetti, S.C.; Davidson, J.; Ward, C.; Laffey, J.G.; Finch, S.; Pesci, A.; et al. Multidimensional Severity Assessment in Bronchiectasis: An Analysis of Seven European Cohorts. Thorax 2016, 71, 1110–1118. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The Bronchiectasis Severity Index an International Derivation and Validation Study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Sotgiu, G.; Lapi, F.; Gramegna, A.; Cricelli, C.; Blasi, F. Prevalence and Incidence of Bronchiectasis in Italy. BMC Pulm. Med. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Millett, E.R.C.; Joshi, M.; Navaratnam, V.; Thomas, S.L.; Hurst, J.R.; Smeeth, L.; Brown, J.S. Changes in the Incidence, Prevalence and Mortality of Bronchiectasis in the UK from 2004 to 2013: A Population-Based Cohort Study. Eur. Respir. J. 2016, 47, 186–193. [Google Scholar] [CrossRef]
- Fraser, C.S.; José, R.J. Insights into Personalised Medicine in Bronchiectasis. J. Pers. Med. 2023, 13, 133. [Google Scholar] [CrossRef]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of Tezepelumab on Airway Inflammatory Cells, Remodelling, and Hyperresponsiveness in Patients with Moderate-to-Severe Uncontrolled Asthma (CASCADE): A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial Cell-Derived Cytokines: More than Just Signaling the Alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Friend, S.L.; Hosier, S.; Nelson, A.; Foxworthe, D.; Williams, D.E.; Farr, A. A Thymic Stromal Cell Line Supports in Vitro Development of Surface IgM+ B Cells and Produces a Novel Growth Factor Affecting B and T Lineage Cells. Exp. Hematol. 1994, 22, 321–328. [Google Scholar]
- Sims, J.E.; Williams, D.E.; Morrissey, P.J.; Garka, K.; Foxworthe, D.; Price, V.; Friend, S.L.; Farr, A.; Bedell, M.A.; Jenkins, N.A.; et al. Molecular Cloning and Biological Characterization of a Novel Murine Lymphoid Growth Factor. J. Exp. Med. 2000, 192, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Fry, T.; Schwartzberg, P.L.; Pandey, A.; Mackall, C.L.; Leonard, W.J. A Role for Thymic Stromal Lymphopoietin in CD4(+) T Cell Development. J. Exp. Med. 2004, 200, 159–168. [Google Scholar] [CrossRef]
- Corren, J.; Ziegler, S.F. TSLP: From Allergy to Cancer. Nat. Immunol. 2019, 20, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Leonard, W.J. The Role of Thymic Stromal Lymphopoietin in CD8+ T Cell Homeostasis. J. Immunol. 2008, 181, 7699–7705. [Google Scholar] [CrossRef]
- Lv, J.; Yu, Q.; Lv, J.; Di, C.; Lin, X.; Su, W.; Wu, M.; Xia, Z. Airway Epithelial TSLP Production of TLR2 Drives Type 2 Immunity in Allergic Airway Inflammation. Eur. J. Immunol. 2018, 48, 1838–1850. [Google Scholar] [CrossRef]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.-R.P.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic Stromal Lymphopoietin Is Released by Human Epithelial Cells in Response to Microbes, Trauma, or Inflammation and Potently Activates Mast Cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef]
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.J.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic Stromal Lymphopoietin Is Induced by Respiratory Syncytial Virus-Infected Airway Epithelial Cells and Promotes a Type 2 Response to Infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef] [PubMed]
- Ebina-Shibuya, R.; West, E.E.; Spolski, R.; Li, P.; Oh, J.; Kazemian, M.; Gromer, D.; Swanson, P.; Du, N.; McGavern, D.B.; et al. Thymic Stromal Lymphopoietin Limits Primary and Recall CD8(+) T-Cell Anti-Viral Responses. Elife 2021, 10, e61912. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Hitomi, Y.; Sakashita, M.; Tsunoda, T.; Miyagawa, T.; Doi, S.; Kameda, M.; Fujita, K.; et al. Thymic Stromal Lymphopoietin Gene Promoter Polymorphisms Are Associated with Susceptibility to Bronchial Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 787–793. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human Epithelial Cells Trigger Dendritic Cell Mediated Allergic Inflammation by Producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H.; Ziegler, S.F. The Multiple Facets of Thymic Stromal Lymphopoietin (TSLP) during Allergic Inflammation and Beyond. J. Leukoc. Biol. 2012, 91, 877–886. [Google Scholar] [CrossRef]
- Takai, T. TSLP Expression: Cellular Sources, Triggers, and Regulatory Mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J.; et al. Distinct Roles of Short and Long Thymic Stromal Lymphopoietin Isoforms in House Dust Mite-Induced Asthmatic Airway Epithelial Barrier Disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S.; et al. Functional Analysis of the Thymic Stromal Lymphopoietin Variants in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef]
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a Receptor Subunit Required for Signaling by Thymic Stromal Lymphopoietin. Nat. Immunol. 2000, 1, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Park, L.S.; Martin, U.; Garka, K.; Gliniak, B.; Di Santo, J.P.; Muller, W.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A.; Farr, A.G.; et al. Cloning of the Murine Thymic Stromal Lymphopoietin (TSLP) Receptor: Formation of a Functional Heteromeric Complex Requires Interleukin 7 Receptor. J. Exp. Med. 2000, 192, 659–670. [Google Scholar] [CrossRef]
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N.; et al. Structure and Antagonism of the Receptor Complex Mediated by Human TSLP in Allergy and Asthma. Nat. Commun. 2017, 8, 14937. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP Transcript Expression and Release of TSLP Induced by TLR Ligands and Cytokines in Human Keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef]
- Datta, A.; Alexander, R.; Sulikowski, M.G.; Nicholson, A.G.; Maher, T.M.; Scotton, C.J.; Chambers, R.C. Evidence for a Functional Thymic Stromal Lymphopoietin Signaling Axis in Fibrotic Lung Disease. J. Immunol. 2013, 191, 4867–4879. [Google Scholar] [CrossRef] [PubMed]
- Martin Mena, A.; Langlois, A.; Speca, S.; Schneider, L.; Desreumaux, P.; Dubuquoy, L.; Bertin, B. The Expression of the Short Isoform of Thymic Stromal Lymphopoietin in the Colon Is Regulated by the Nuclear Receptor Peroxisome Proliferator Activated Receptor-Gamma and Is Impaired during Ulcerative Colitis. Front. Immunol. 2017, 8, 1052. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.-H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.-F.; Yao, Z.; Cao, W.; Liu, Y.-J. TSLP-Activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response through OX40 Ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Bjerkan, L.; Schreurs, O.; Engen, S.A.; Jahnsen, F.L.; Baekkevold, E.S.; Blix, I.J.S.; Schenck, K. The Short Form of TSLP Is Constitutively Translated in Human Keratinocytes and Has Characteristics of an Antimicrobial Peptide. Mucosal Immunol. 2015, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, M.C.; Saenz, S.A.; Hill, D.A.; Kim, B.S.; Headley, M.B.; Doering, T.A.; Wherry, E.J.; Jessup, H.K.; Siegel, L.A.; Kambayashi, T.; et al. TSLP Promotes Interleukin-3-Independent Basophil Haematopoiesis and Type 2 Inflammation. Nature 2011, 477, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Salabert-Le Guen, N.; Hémont, C.; Delbove, A.; Poli, C.; Braudeau, C.; Fantou, A.; Amouriaux, K.; Bériou, G.; Martin, J.C.; Colas, L.; et al. Thymic Stromal Lymphopoietin Does Not Activate Human Basophils. J. Allergy Clin. Immunol. 2018, 141, 1476–1479.e6. [Google Scholar]
- Leyva-Castillo, J.M.; Hener, P.; Michea, P.; Karasuyama, H.; Chan, S.; Soumelis, V.; Li, M. Skin Thymic Stromal Lymphopoietin Initiates Th2 Responses through an Orchestrated Immune Cascade. Nat. Commun. 2013, 4, 2847. [Google Scholar] [CrossRef]
- Gandolfo, S.; Bulfoni, M.; Fabro, C.; Russi, S.; Sansonno, D.; Di Loreto, C.; Cesselli, D.; De Vita, S. Thymic Stromal Lymphopoietin Expression from Benign Lymphoproliferation to Malignant B-Cell Lymphoma in Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S1), 55–64. [Google Scholar]
- Wang, Q.; Du, J.; Zhu, J.; Yang, X.; Zhou, B. Thymic Stromal Lymphopoietin Signaling in CD4(+) T Cells Is Required for TH2 Memory. J. Allergy Clin. Immunol. 2015, 135, 781–791.e3. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, T.H.; Kang, S.Y.; Park, H.J.; Lim, S.Y.; Kim, S.H.; Jung, K.S.; Yoo, K.H.; Yoon, H.K.; Rhee, C.K. Association between Serum Levels of Interleukin-25/Thymic Stromal Lymphopoietin and the Risk of Exacerbation of Chronic Obstructive Pulmonary Disease. Biomolecules 2023, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.K.Y.; Lau, T.S.; Chung, K.Y.; Tam, C.; Cheung, T.H.; Yim, S.F.; Lee, J.H.S.; Leung, R.W.T.; Qin, J.; Or, Y.Y.Y.; et al. Short-Form Thymic Stromal Lymphopoietin (SfTSLP) Is the Predominant Isoform Expressed by Gynaecologic Cancers and Promotes Tumour Growth. Cancers 2021, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Martinez-García, M.Á.; Olveira, C.; Girón, R.; García-Clemente, M.; Máiz-Carro, L.; Sibila, O.; Golpe, R.; Méndez, R.; Rodríguez Hermosa, J.L.; Barreiro, E.; et al. Peripheral Neutrophil-to-Lymphocyte Ratio in Bronchiectasis: A Marker of Disease Severity. Biomolecules 2022, 12, 1399. [Google Scholar] [CrossRef] [PubMed]
- Cataudella, E.; Giraffa, C.M.; Di Marca, S.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Di Quattro, R.; et al. Neutrophil-To-Lymphocyte Ratio: An Emerging Marker Predicting Prognosis in Elderly Adults with Community-Acquired Pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Rochman, Y.; Kashyap, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.-U.; Leonard, W.J. Thymic Stromal Lymphopoietin-Mediated STAT5 Phosphorylation via Kinases JAK1 and JAK2 Reveals a Key Difference from IL-7-Induced Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef]
- Kadariya, Y.; Menges, C.W.; Talarchek, J.; Cai, K.Q.; Klein-Szanto, A.J.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Cheung, M.; Mossman, B.T.; Shukla, A.; et al. Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev. Res. 2016, 9, 406–414. [Google Scholar] [CrossRef]
- Lu, N.; Wang, Y.-H.; Wang, Y.-H.; Arima, K.; Hanabuchi, S.; Liu, Y.-J. TSLP and IL-7 Use Two Different Mechanisms to Regulate Human CD4+ T Cell Homeostasis. J. Exp. Med. 2009, 206, 2111–2119. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.M.; Mohammadi, B.; Saghafi, M.R.; Mohammadi, S.; Ghaffari, S.; Mirsadraee, M.; Khakzad, M.R. The Association between MUC5AC and MUC5B Genes Expression and Remodeling Progression in Severe Neutrophilic Asthma: A Direct Relationship. Respir. Med. 2023, 213, 107260. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Lee, H.-C.; Nakayama, T.; Ziegler, S.F. TSLP Enhances the Function of Helper Type 2 Cells. Eur. J. Immunol. 2011, 41, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Astrakhan, A.; Omori, M.; Nguyen, T.; Becker-Herman, S.; Iseki, M.; Aye, T.; Hudkins, K.; Dooley, J.; Farr, A.; Alpers, C.E.; et al. Local Increase in Thymic Stromal Lymphopoietin Induces Systemic Alterations in B Cell Development. Nat. Immunol. 2007, 8, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.O.; John, G.; Germann, M.; Lindemann, H.; Rubin, B.K. MUC5AC and MUC5B Mucins Increase in Cystic Fibrosis Airway Secretions during Pulmonary Exacerbation. Am. J. Respir. Crit. Care Med. 2007, 175, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b Is Required for Airway Defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Sibila, O.; Suarez-Cuartin, G.; Rodrigo-Troyano, A.; Fardon, T.C.; Finch, S.; Mateus, E.F.; Garcia-Bellmunt, L.; Castillo, D.; Vidal, S.; Sanchez-Reus, F.; et al. Secreted Mucins and Airway Bacterial Colonization in Non-CF Bronchiectasis. Respirology 2015, 20, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Chang, M.M.-J.; Velichko, S.; Thai, P.; Hung, L.-Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-ΚB Mediates IL-1β- and IL-17A-Induced MUC5B Expression in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Hams, E.; Fallon, P.G. Innate Type 2 Cells and Asthma. Curr. Opin. Pharmacol. 2012, 12, 503–509. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Chen, A.C.H.; Radicioni, G.; Lourie, R.; Martin, M.; Broomfield, A.; Sheng, Y.H.; Hasnain, S.Z.; Radford-Smith, G.; Simms, L.A.; et al. Airway Mucus Hyperconcentration in Non–Cystic Fibrosis Bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 201, 661–670. [Google Scholar] [CrossRef]
- Evans, C.M.; Kim, K.; Tuvim, M.J.; Dickey, B.F. Mucus Hypersecretion in Asthma: Causes and Effects. Curr. Opin. Pulm. Med. 2009, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Sheehan, J.K. From Mucins to Mucus: Toward a More Coherent Understanding of This Essential Barrier. Proc. Am. Thorac. Soc. 2004, 1, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Rochman, Y.; Spolski, R.; Samsel, L.; Leonard, W.J. Thymic Stromal Lymphopoietin Is Produced by Dendritic Cells. J. Immunol. 2011, 187, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
| Genetic Variant | Comment |
|---|---|
| MBL deficiency | Mannose-binding lectin (MBL) is a key component of innate immunity involved in the clearance of bacteria and apoptotic cells. Genetic MBL deficiency is common in the general population and related to disease severity in bronchiectasis, including quality of life and frequency of exacerbations and admission to hospital. |
| Telomere | Telomere attrition is an established ageing biomarker. Lim et al. [4] reported that shortened telomere length was significantly relevant in sputum immune cells of bronchiectasis patients. |
| GBP5 | GBP5 upregulation, a positive regulator of the NLRP3 inflammasome, led to exaggerated immune response upon bacterial infections. |
| FUT2 | Secretion of a (1,2) fucosylated glycans elicits a dichotomous effect on host–microbe interactions, making the secretor genotype (FUT2) a risk factor underlying variation in infection type and disease severity in bronchiectasis. Homozygous secretors exhibit lower lung function, higher exacerbation rate, and more frequent P. aeruginosa-dominated infection |
| CFTR | Mutations associated with the following phenotypes:
|
| DAW1 | Motile ciliopathy laterality disorder. |
| DNAH5 | Mutations associated with the following phenotypes:
|
| PIK3CD | Immunodeficiency; autosomal dominant |
| SCNN1A | Mutations associated with the following phenotypes:
|
| SCNN1B | Mutations associated with the following phenotypes:
|
| SCNN1G | Mutations associated with the following phenotypes:
|
| DNAI1 | Mutations associated with the following phenotypes:
|
| DNAI2 | Mutations associated with the following phenotypes:
|
| DNAL1 | Mutations associated with the following phenotypes:
|
| RSPH4A | Mutations associated with the following phenotypes: Bronchiectasis with rhinorrhoea, rhinitis, nasal blockage, sinusitis, otitis media, hearing problems, deafness, low weight, and short stature. |
| PCDHGA7 and PCDHGB4 | Members of the protocadherin-γ gene cluster; decreased expression may be related to airway dilation in early bronchiectasis. |
| Proteasome 20S subunit-β (PSMB)five | The immunoproteasome has been shown to enhance the generation of major-histocompatibility-complex-I-associated peptide and facilitate the antiviral immune response in the lung. Furthermore, IFN-γ has previously been shown in mice to play an essential role in protective immunity against tuberculosis and mycobacterial infection, both of which are relevant to infection in bronchiectasis airways. |
| Immunoproteasome subunits PSMB8, PSMB9, PSMB10 | The immunoproteasome has been shown to enhance the generation of major-histocompatibility-complex-I-associated peptide and facilitate the antiviral immune response in the lung. Furthermore, IFN-γ has previously been shown in mice to play an essential role in protective immunity against tuberculosis and mycobacterial infection, both of which are relevant to infection in bronchiectasis airway. |
| LGR5 | Highly expressed in basal cells; may alter the structure of the airway or the ability to repair damaged airway epithelium. |
| Wnt signalling pathway | Wnt signalling pathway activity decreases with age and may explain age-associated severity in bronchiectasis. |
| SERPINA1 | Association between these genetic variants and α1-antitrypsin deficiency, which can lead to multiple pulmonary pathologies including chronic bronchitis and bronchiectasis. |
| DEUP1 | High expressions of DEUP1, FOXN4, and CDC20B, have been reported as precursors of multiciliated cells. A hypothesis is that the overproduction of certain ciliary proteins contributes to faulty cilia assembly, reduced clearing capacity of the lung, and bronchiectasis pathogenesis. |
| FOXN4 | High expressions of DEUP1, FOXN4, and CDC20B, have been reported as precursors of multiciliated cells. A hypothesis is that the overproduction of certain ciliary proteins contributes to faulty cilia assembly, reduced clearing capacity of the lung, and bronchiectasis pathogenesis. |
| CDC20B | High expressions of DEUP1, FOXN4, and CDC20B, have been reported as precursors of multiciliated cells. A hypothesis is that the overproduction of certain ciliary proteins contributes to faulty cilia assembly, reduced clearing capacity of the lung and bronchiectasis pathogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccio, F.R.; Baio, N.; Montini, S.; Ferroni, V.; Chino, V.; Pisanu, L.; Russo, M.; Giana, I.; Cascina, A.; Conio, V.; et al. Potential New Inflammatory Markers in Bronchiectasis: A Literature Review. Curr. Issues Mol. Biol. 2024, 46, 6675-6689. https://doi.org/10.3390/cimb46070398
Bertuccio FR, Baio N, Montini S, Ferroni V, Chino V, Pisanu L, Russo M, Giana I, Cascina A, Conio V, et al. Potential New Inflammatory Markers in Bronchiectasis: A Literature Review. Current Issues in Molecular Biology. 2024; 46(7):6675-6689. https://doi.org/10.3390/cimb46070398
Chicago/Turabian StyleBertuccio, Francesco Rocco, Nicola Baio, Simone Montini, Valentina Ferroni, Vittorio Chino, Lucrezia Pisanu, Marianna Russo, Ilaria Giana, Alessandro Cascina, Valentina Conio, and et al. 2024. "Potential New Inflammatory Markers in Bronchiectasis: A Literature Review" Current Issues in Molecular Biology 46, no. 7: 6675-6689. https://doi.org/10.3390/cimb46070398
APA StyleBertuccio, F. R., Baio, N., Montini, S., Ferroni, V., Chino, V., Pisanu, L., Russo, M., Giana, I., Cascina, A., Conio, V., Grosso, A., Gini, E., Albicini, F., Corsico, A. G., & Stella, G. M. (2024). Potential New Inflammatory Markers in Bronchiectasis: A Literature Review. Current Issues in Molecular Biology, 46(7), 6675-6689. https://doi.org/10.3390/cimb46070398








