Muscarinic Receptors and Alzheimer’s Disease: New Perspectives and Mechanisms
Abstract
:1. Introduction
1.1. Alzheimer’s Disease Impact
1.2. Alzheimer’s Disease Pathophysiology
1.3. Early Hallmarks of AD
1.4. AD and Diabetes
1.5. Treating Alzheimer’s Disease: Managing Symptoms and Exploring New Targets
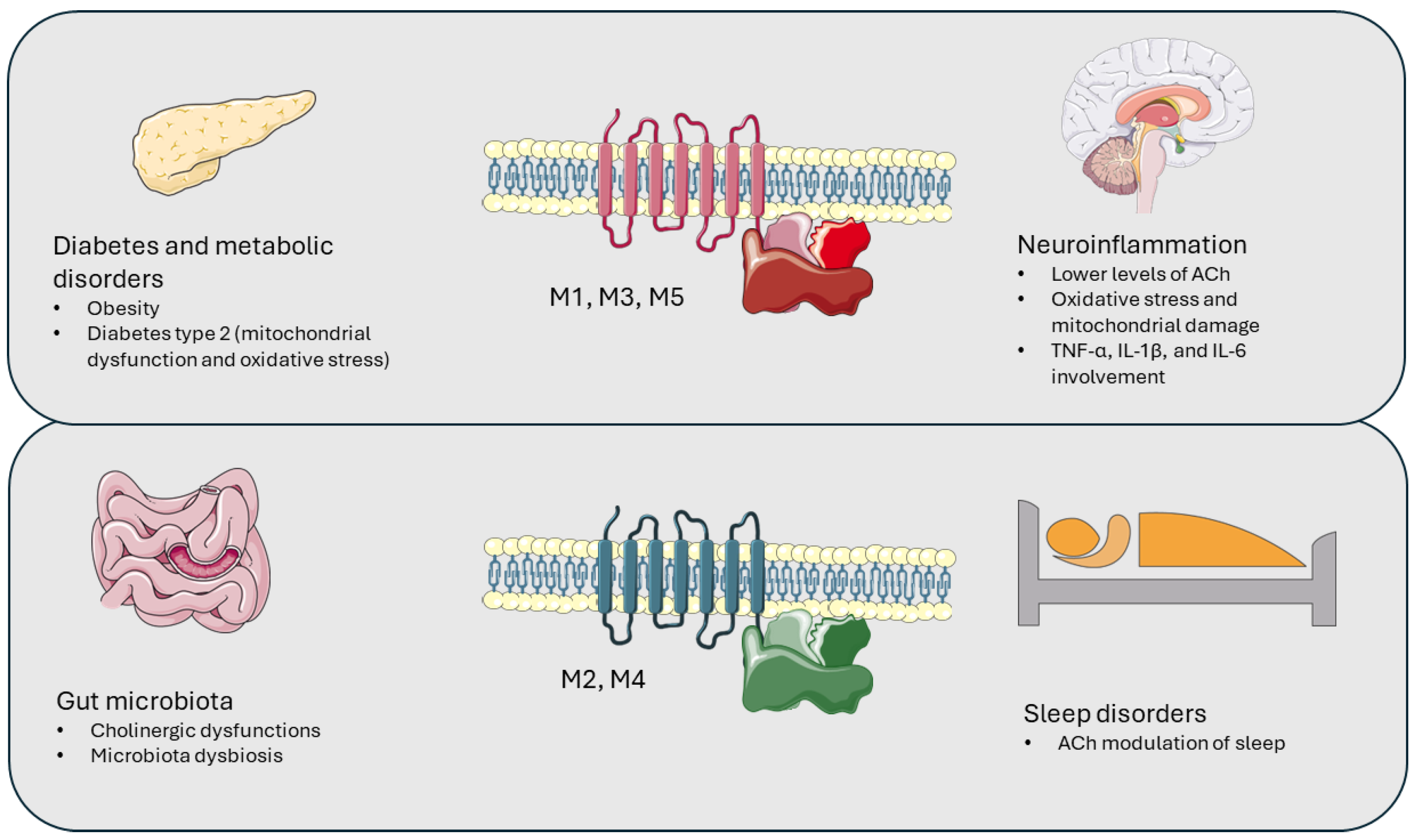
2. Muscarinic Receptors’ Involvement in Pathological Conditions
2.1. Muscarinic Receptors and Neuroinflammation
2.2. Muscarinic Receptors and Gut Microbiota
2.3. Muscarinic Receptors and Sleep Disorders
2.4. Muscarinic Receptors and Metabolic Disorders
3. Muscarinic Receptors as a Pharmacological Target
3.1. M1: Conceivable AD Target
3.2. M2: Complex Non-Selective Target
3.3. M3: Involvement in Mood Disorders and Sleep Functions
3.4. M4: Promising AD Target
3.5. M5: Relevant Role in Memory Impairment in AD
4. Supporting Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skaria, A.P. The Economic and Societal Burden of Alzheimer Disease: Managed Care Considerations. Am. J. Manag. Care 2022, 28, S188–S196. [Google Scholar] [CrossRef] [PubMed]
- Dwomoh, L.; Tejeda, G.S.; Tobin, A.B. Targeting the M1 Muscarinic Acetylcholine Receptor in Alzheimer’s Disease. Neuronal Signal. 2022, 6, 20210004. [Google Scholar] [CrossRef] [PubMed]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Theerasri, A.; Janpaijit, S.; Tencomnao, T.; Prasansuklab, A. Beyond the Classical Amyloid Hypothesis in Alzheimer’s Disease: Molecular Insights into Current Concepts of Pathogenesis, Therapeutic Targets, and Study Models. WIREs Mech. Dis. 2023, 15, e1591. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting Neuroinflammation in Alzheimer’s Disease: From Mechanisms to Clinical Applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation Context in Alzheimer’s Disease, a Relationship Intricate to Define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaiyrlykyzy, A.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Tsoy, A.; Askarova, S. The Expression Levels of Pro-inflammatory and Anti-inflammatory Cytokines and Chemokines in Blood Serum of Alzheimer’s Dementia Patients: Report from a Pilot Study in Kazakhstan. Alzheimer’s Dement. 2022, 18, 2–3. [Google Scholar] [CrossRef]
- Leonardo, S.; Fregni, F. Association of Inflammation and Cognition in the Elderly: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2023, 15, 1069439. [Google Scholar] [CrossRef]
- Anwar, M.M. Oxidative Stress-A Direct Bridge to Central Nervous System Homeostatic Dysfunction and Alzheimer’s Disease. Cell Biochem. Funct. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic Correlations between Alzheimer’s Disease and Gut Microbiome Genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef]
- Bostancıklıoğlu, M. Connectivity between Gut Microbiota and Terminal Awakenings in Alzheimer’s Disease. Curr. Alzheimer Res. 2023, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Zhang, H.; Zhao, W.; Ding, J.; Dai, R.; Xu, K.; He, C.; Liu, J.; Yang, L.; et al. Dynamic Distribution of Gut Microbiota during Alzheimer’s Disease Progression in a Mice Model. APMIS 2023, 131, 480–490. [Google Scholar] [CrossRef]
- Krishaa, L.; Ng, T.K.S.; Wee, H.N.; Ching, J. Gut-Brain Axis through the Lens of Gut Microbiota and Their Relationships with Alzheimer’s Disease Pathology: Review and Recommendations. Mech. Ageing Dev. 2023, 211, 111787. [Google Scholar] [CrossRef]
- Murray, E.R.; Kemp, M.; Nguyen, T.T. The Microbiota-Gut-Brain Axis in Alzheimer’s Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions. Arch. Clin. Neuropsychol. 2022, 37, 595–607. [Google Scholar] [CrossRef]
- Ameen, A.O.; Freude, K.; Aldana, B.I. Fats, Friends or Foes: Investigating the Role of Short- and Medium-Chain Fatty Acids in Alzheimer’s Disease. Biomedicines 2022, 10, 2778. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Zhang, C.; Zhao, Y.; Bu, G.; Xu, H.; Zhang, Y.W. M1 Muscarinic Acetylcholine Receptor in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 295–307. [Google Scholar] [CrossRef]
- Wysocka, A.; Palasz, E.; Steczkowska, M.; Niewiadomska, G. Dangerous Liaisons: Tau Interaction with Muscarinic Receptors. Curr. Alzheimer Res. 2020, 17, 224–237. [Google Scholar] [CrossRef]
- Lombardero, L.; Llorente-Ovejero, A.; Manuel, I.; Rodríguez-Puertas, R. Neurotransmitter Receptors in Alzheimer’s Disease: From Glutamatergic to Cholinergic Receptors. In Genetics, Neurology, Behavior, and Diet in Dementia: The Neuroscience of Dementia; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 441–456. [Google Scholar] [CrossRef]
- Ahmad, F.; Liu, P. Synaptosome as a Tool in Alzheimer’s Disease Research. Brain Res. 2020, 1746, 147009. [Google Scholar] [CrossRef]
- Takata, K.; Kimura, H.; Yanagisawa, D.; Harada, K.; Nishimura, K.; Kitamura, Y.; Shimohama, S.; Tooyama, I. Nicotinic Acetylcholine Receptors and Microglia as Therapeutic and Imaging Targets in Alzheimer’s Disease. Molecules 2022, 27, 2780. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Tripathi, T.; Kumar, A. Muscarinic and Nicotinic Acetylcholine Receptor Agonists: Current Scenario in Alzheimer’s Disease Therapy. J. Pharm. Pharmacol. 2018, 70, 985–993. [Google Scholar] [CrossRef]
- Sharma, M.; Pal, P.; Gupta, S.K. The Neurotransmitter Puzzle of Alzheimer’s: Dissecting Mechanisms and Exploring Therapeutic Horizons. Brain Res. 2024, 1829, 148797. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.F.; Cao, Y.; Im, W.; Nichols, R.A.; Lukas, R.J.; George, A.A. Neuroprotective Amyloid β N-Terminal Peptides Differentially Alter Human A7- and A7β2-Nicotinic Acetylcholine (NACh) Receptor Single-Channel Properties. Br. J. Pharmacol. 2024, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zappettini, S.; Grilli, M.; Olivero, G.; Mura, E.; Preda, S.; Govoni, S.; Salamone, A.; Marchi, M. Beta Amyloid Differently Modulate Nicotinic and Muscarinic Receptor Subtypes Which Stimulate in Vitro and in Vivo the Release of Glycine in the Rat Hippocampus. Front. Pharmacol. 2012, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Govoni, S.; Mura, E.; Preda, S.; Racchi, M.; Lanni, C.; Grilli, M.; Zappettini, S.; Salamone, A.; Olivero, G.; Pittaluga, A.; et al. Dangerous Liaisons between Beta-Amyloid and Cholinergic Neurotransmission. Curr. Pharm. Des. 2014, 20, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Mishra, W.; Kheradpezhouh, E.; Arabzadeh, E. Activation of M1 Cholinergic Receptors in Mouse Somatosensory Cortex Enhances Information Processing and Detection Behaviour. Commun. Biol. 2024, 7, 3. [Google Scholar] [CrossRef]
- Cantone, A.F.; Burgaletto, C.; Di Benedetto, G.; Pannaccione, A.; Secondo, A.; Bellanca, C.M.; Augello, E.; Munafò, A.; Tarro, P.; Bernardini, R.; et al. Taming Microglia in Alzheimer’s Disease: Exploring Potential Implications of Choline Alphoscerate via A7 NAChR Modulation. Cells 2024, 13, 309. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New Insights into Atypical Alzheimer’s Disease in the Era of Biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Schneider, J.A.; Jicha, G.A.; Duong, M.T.; Wolk, D.A. When Alzheimer’s Is LATE: Why Does It Matter? Ann. Neurol. 2023, 94, 211–222. [Google Scholar] [CrossRef]
- Farvadi, F.; Hashemi, F.; Amini, A.; Alsadat Vakilinezhad, M.; Raee, M.J. Early Diagnosis of Alzheimer’s Disease with Blood Test; Tempting but Challenging. Int. J. Mol. Cell Med. 2023, 12, 172–210. [Google Scholar] [CrossRef]
- Smirnov, D.S.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Simrén, J.; Lantero-Rodriguez, J.; Karikari, T.K.; Hiniker, A.; Rissman, R.A.; Salmon, D.P.; et al. Plasma Biomarkers for Alzheimer’s Disease in Relation to Neuropathology and Cognitive Change. Acta Neuropathol. 2022, 143, 487–503. [Google Scholar] [CrossRef]
- Filardi, M.; Gnoni, V.; Tamburrino, L.; Nigro, S.; Urso, D.; Vilella, D.; Tafuri, B.; Giugno, A.; De Blasi, R.; Zoccolella, S.; et al. Sleep and Circadian Rhythm Disruptions in Behavioral Variant Frontotemporal Dementia. Alzheimer’s Dement. 2024, 20, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Joyce, K.K.; Harper, K.M.; Harp, S.J.; Cohen, T.J.; Martin, S.C.; Joyce, K.K.; Harper, K.M.; Harp, S.J.; Cohen, T.J.; et al. Evaluating Fatty Acid Amide Hydrolase as a Suitable Target for Sleep Promotion in a Transgenic TauP301S Mouse Model of Neurodegeneration. Pharmaceuticals 2024, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Cuello, A.C.; Hall, H.; Do Carmo, S. Experimental Pharmacology in Transgenic Rodent Models of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.X.; Baratono, S.; Drew, W.; Tetreault, A.M.; Fox, M.D.; Darby, R.R. Increased Cortical Thickness in Alzheimer’s Disease. Ann. Neurol. 2024, 95, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Voit-Bak, K.; Rosenthal, P.; Tselmin, S.; Julius, U.; Schatz, U.; Boehm, B.O.; Thuret, S.; Kempermann, G.; Reichmann, H.; et al. Extracorporeal Apheresis Therapy for Alzheimer Disease—Targeting Lipids, Stress, and Inflammation. Mol. Psychiatry 2019, 25, 275–282. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Liu, Y. Navigating the Metabolic Maze: Anomalies in Fatty Acid and Cholesterol Processes in Alzheimer’s Astrocytes. Alzheimer’s Res. Ther. 2024, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, K.; Czarnecka, K.; Szymański, P. A Review of the Mechanisms Underlying Selected Comorbidities in Alzheimer’s Disease. Pharmacol. Rep. 2021, 73, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Nday, C.M.; Eleftheriadou, D.; Jackson, G. Shared Pathological Pathways of Alzheimer’s Disease with Specific Comorbidities: Current Perspectives and Interventions. J. Neurochem. 2018, 144, 360–389. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahmood, Z.; Zahid, S. Linking Insulin with Alzheimer’s Disease: Emergence as Type III Diabetes. Neurol. Sci. 2015, 36, 1763–1769. [Google Scholar] [CrossRef]
- Schubert, M. The Correlation between Diabetes Mellitus and Neurodegenerative Diseases. Klin. Monatsblätter Augenheilkd. 2023, 240, 130–135. [Google Scholar] [CrossRef]
- Dubey, S.K.; Lakshmi, K.K.; Krishna, K.V.; Agrawal, M.; Singhvi, G.; Saha, R.N.; Saraf, S.; Saraf, S.; Shukla, R.; Alexander, A. Insulin Mediated Novel Therapies for the Treatment of Alzheimer’s Disease. Life Sci. 2020, 249, 117540. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Meng, X.; Yao, Y.; Xu, J. The Mechanism and Efficacy of GLP-1 Receptor Agonists in the Treatment of Alzheimer’s Disease. Front. Endocrinol. 2022, 13, 1033479. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, G.; Tehrani, S.S.; Fana, S.E.; Moradi-Sardareh, H.; Panahi, G.; Maniati, M.; Meshkani, R. Crosstalk between Alzheimer’s Disease and Diabetes: A Focus on Anti-Diabetic Drugs. Metab. Brain Dis. 2023, 38, 1769–1800. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining Cholinergic Therapy for Alzheimer’s Disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Gallrein, C.; Williams, A.B.; Meyer, D.H.; Messling, J.E.; Garcia, A.; Schumacher, B. Baz-2 Enhances Systemic Proteostasis in Vivo by Regulating Acetylcholine Metabolism. Cell Rep. 2023, 42, 113577. [Google Scholar] [CrossRef]
- Nguyen, H.T.M.; van der Westhuizen, E.T.; Langmead, C.J.; Tobin, A.B.; Sexton, P.M.; Christopoulos, A.; Valant, C. Opportunities and Challenges for the Development of M1 Muscarinic Receptor Positive Allosteric Modulators in the Treatment for Neurocognitive Deficits. Br. J. Pharmacol. 2022, 181, 2114–2142. [Google Scholar] [CrossRef]
- McDonald, A.J. Colocalization of M1 Muscarinic Cholinergic Receptors and NMDA Receptors in Dendrites and Spines of Pyramidal Neurons of the Mouse Basolateral Amygdala: An Ultrastructural Analysis. Neurosci. Lett. 2022, 779, 136624. [Google Scholar] [CrossRef]
- Scarpa, M.; Hesse, S.; Bradley, S.J. M1 Muscarinic Acetylcholine Receptors: A Therapeutic Strategy for Symptomatic and Disease-Modifying Effects in Alzheimer’s Disease? Adv. Pharmacol. 2020, 88, 277–310. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef]
- Abd-Elrahman, K.; Colson, T.-L.L.; Ferguson, S.S. A Positive Allosteric Modulator of M1 Acetylcholine Receptors Improves Cognitive Deficits in Male and Female APPswe/PSEN1ΔE9 Mice via Divergent Mechanisms. J. Pharmacol. Exp. Ther. 2023, 385, 479. [Google Scholar] [CrossRef]
- Rook, J.; Xiang, Z.; Dickerson, J.; Dogra, S.; Jensen, K.; Kim, I.; Ohler, M.; Weiss, K.; Conn, P.J. M1 Positive Allosteric Modulators Enhance Domains of Cognitive Function by Activation of SsT-Expressing Interneurons in the Prefrontal Cortex. J. Pharmacol. Exp. Ther. 2023, 385, 407. [Google Scholar] [CrossRef]
- Nguyen, H.T.M.; van der Westhuizen, E.T.; Khajehali, E.; Valant, C.; Christopoulos, A. Investigating Drivers for M1 Muscarinic Acetylcholine Receptor-Mediated Adverse Events by M1 Positive Allosteric Modulators. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Alzheimer’s Disease: From Immunotherapy to Immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef]
- Wagemann, O.; Liu, H.; Wang, G.; Shi, X.; Bittner, T.; Scelsi, M.A.; Farlow, M.R.; Clifford, D.B.; Supnet-Bell, C.; Santacruz, A.M.; et al. Downstream Biomarker Effects of Gantenerumab or Solanezumab in Dominantly Inherited Alzheimer Disease the DIAN-TU-001 Randomized Clinical Trial. JAMA Neurol. 2024, 81, 582–593. [Google Scholar] [CrossRef]
- Perneczky, R.; Dom, G.; Chan, A.; Falkai, P.; Bassetti, C. Anti-Amyloid Antibody Treatments for Alzheimer’s Disease. Eur. J. Neurol. 2024, 31, e16049. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Rosas, V.H.; Rendón-Ochoa, E.A.; Hernández-Flores, T.; Flores-León, M.; Arias, C.; Galarraga, E.; Bargas, J. Role of M4 -Receptor Cholinergic Signaling in Direct Pathway Striatal Projection Neurons during Dopamine Depletion. Synapse 2024, 78, e22287. [Google Scholar] [CrossRef]
- Brandl, K.; Laiken, N. Chapter 12—Acetylcholine and Muscarinic Receptors. In Primer on the Autonomic Nervous System, 4th ed.; Biaggioni, I., Browning, K., Fink, G., Jordan, J., Low, P.A., Paton, J.F.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 73–76. ISBN 978-0-323-85492-4. [Google Scholar]
- Benarroch, E.E. Glutamatergic Synaptic Plasticity and Dysfunction in Alzheimer Disease: Emerging Mechanisms. Neurology 2018, 91, 125–132. [Google Scholar] [CrossRef]
- Huff, A.E.; O’Neill, O.S.; Messer, W.S.; Winters, B.D. Muscarinic Receptor Activation Promotes Destabilization and Updating of Object Location Memories in Mice. Behav. Brain Res. 2024, 461, 114847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Ge, Y.H.; Li, J.B.; Xiong, C.H.; Law, P.Y.; Xu, J.R.; Qiu, Y.; Chen, H.Z. M1 Muscarinic Receptors Regulate the Phosphorylation of AMPA Receptor Subunit GluA1 via a Signaling Pathway Linking CAMP-PKA and PI3K-Akt. FASEB J. 2019, 33, 6622–6631. [Google Scholar] [CrossRef] [PubMed]
- Lahmy, V.; Meunier, J.; Malmström, S.; Naert, G.; Givalois, L.; Kim, S.H.; Villard, V.; Vamvakides, A.; Maurice, T. Blockade of Tau Hyperphosphorylation and Aβ1–42 Generation by the Aminotetrahydrofuran Derivative ANAVEX2-73, a Mixed Muscarinic and Σ1 Receptor Agonist, in a Nontransgenic Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2013, 38, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Sprouse, J.; Rebowe, N.; Hanania, T.; Klamer, D.; Missling, C.U. ANAVEX®2-73 (Blarcamesine), a Sigma-1 Receptor Agonist, Ameliorates Neurologic Impairments in a Mouse Model of Rett Syndrome. Pharmacol. Biochem. Behav. 2019, 187, 172796. [Google Scholar] [CrossRef] [PubMed]
- Sabbir, M.G.; Swanson, M.; Albensi, B.C. Loss of Cholinergic Receptor Muscarinic 1 Impairs Cortical Mitochondrial Structure and Function: Implications in Alzheimer’s Disease. Front. Cell Dev. Biol. 2023, 11, 1158604. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Iulita, M.F.; Gubert, P.; Flores Aguilar, L.; Ducatenzeiler, A.; Fisher, A.; Cuello, A.C. AF710B, an M1/Sigma-1 Receptor Agonist with Long-Lasting Disease-Modifying Properties in a Transgenic Rat Model of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Fadiran, E.O.; Hammond, E.; Tran, J.; Missling, C.U.; Ette, E. Population-Based Characterization of the Pharmacokinetics and Food Effect of ANAVEX3-71, a Novel Sigma-1 Receptor and Allosteric M1 Muscarinic Receptor Agonist in Development for Treatment of Frontotemporal Dementia, Schizophrenia, and Alzheimer Disease. Orig. Artic. Clin. Pharmacol. Drug Dev. 2023, 2024, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Fadiran, E.O.; Hammond, E.; Tran, J.; Xue, H.; Chen, J.; Kaufmann, W.E.; Missling, C.U.; Darpo, B. Concentration–QTc Relationship from a Single Ascending Dose Study of ANAVEX3-71, a Novel Sigma-1 Receptor and Allosteric M1 Muscarinic Receptor Agonist in Development for the Treatment of Frontotemporal Dementia, Schizophrenia, and Alzheimer’s Disease. Clin. Pharmacol. Drug Dev. 2023, 12, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.I. Muscarinic Acetylcholine Receptor Expression in Memory Circuits: Implications for Treatment of Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1996, 93, 13541–13546. [Google Scholar] [CrossRef]
- Messerer, R.; Dallanoce, C.; Matera, C.; Wehle, S.; Flammini, L.; Chirinda, B.; Bock, A.; Irmen, M.; Tränkle, C.; Barocelli, E.; et al. Novel Bipharmacophoric Inhibitors of the Cholinesterases with Affinity to the Muscarinic Receptors M1 and M2. Medchemcomm 2017, 8, 1346–1359. [Google Scholar] [CrossRef]
- Lai, M.K.P.; Lai, O.F.; Keene, J.; Esiri, M.M.; Francis, P.T.; Hope, T.; Chen, C.P.L.H. Psychosis of Alzheimer’s Disease Is Associated with Elevated Muscarinic M2 Binding in the Cortex. Neurology 2001, 57, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.D.; Lachowicz, J.E. Orally Active and Selective Benzylidene Ketal M2 Muscarinic Receptor Antagonists for the Treatment of Alzheimer’s Disease. Drug Dev. Res. 2002, 56, 310–320. [Google Scholar] [CrossRef]
- Gibbons, A.S.; Scarr, E.; McLean, C.; Sundram, S.; Dean, B. Decreased Muscarinic Receptor Binding in the Frontal Cortex of Bipolar Disorder and Major Depressive Disorder Subjects. J. Affect. Disord. 2009, 116, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Thal, L.J.; Forrest, M.; Loft, H.; Mengel, H. Lu 25-109, a Muscarinic Agonist, Fails to Improve Cognition in Alzheimer’s Disease. Lu25-109 Study Group. Neurology 2000, 54, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Arnt, J.; Didriksen, M.; Dragsted, N.; Moltzen Lenz, S.; Matz, J. In Vivo Muscarinic Cholinergic Mediated Effects of Lu 25-109, a M1 Agonist and M2/M3 Antagonist in Vitro. Psychopharmacology 1998, 137, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Giuliano, L.; Castrogiovanni, P.; Imbesi, R.; Ulivieri, M.; Fazio, F.; Blennow, K.; Zetterberg, H.; Di Rosa, M. Sex, Age, and Regional Differences in CHRM1 and CHRM3 Genes Expression Levels in the Human Brain Biopsies: Potential Targets for Alzheimer’s Disease-Related Sleep Disturbances. Curr. Neuropharmacol. 2022, 21, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Akaiwa, M.; Negoro, K.; Kawaminami, E.; Mihara, H.; Fuji, H.; Okimoto, R.; Ino, K.; Ishizu, K.; Takahashi, T. Design, Synthesis, and Structure–Activity Relationships Study of N-Pyrimidyl/Pyridyl-2-Thiazolamine Analogues as Novel Positive Allosteric Modulators of M3 Muscarinic Acetylcholine Receptor. Chem. Pharm. Bull. 2021, 69, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wess, J.; Alzheimer, C. Long-Term-But Not Short-Term-Plasticity at the Mossy Fiber-CA3 Pyramidal Cell Synapse in Hippocampus Is Altered in M1/M3 Muscarinic Acetylcholine Receptor Double Knockout Mice. Cells 2023, 12, 1890. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.; Fedorova, I.; Zheng, F.; Miyakawa, T.; Koustova, E.; Gomeza, J.; Basile, A.S.; Alzheimer, C.; Wess, J. M2 Muscarinic Acetylcholine Receptor Knock-out Mice Show Deficits in Behavioral Flexibility, Working Memory, and Hippocampal Plasticity. J. Neurosci. 2004, 24, 10117–10127. [Google Scholar] [CrossRef]
- Erskine, D.; Taylor, J.-P.; Bakker, G.; Brown, A.J.H.; Tasker, T.; Nathan, P.J. Cholinergic Muscarinic M1 and M4 Receptors as Therapeutic Targets for Cognitive, Behavioural, and Psychological Symptoms in Psychiatric and Neurological Disorders. Drug Discov. Today 2019, 24, 2307–2314. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Chen, Z.; Wu, L.; Wang, T.; Cao, D.; Wang, H.; Liu, S.; Xu, Y.; Li, F.; et al. The Unconventional Activation of the Muscarinic Acetylcholine Receptor M4R by Diverse Ligands. Nat. Commun. 2022, 13, 2855. [Google Scholar] [CrossRef] [PubMed]
- Chambers, N.E.; Millett, M.; Moehle, M.S. The Muscarinic M4 Acetylcholine Receptor Exacerbates Symptoms of Movement Disorders. Biochem. Soc. Trans. 2023, 51, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.K.; Ingram, S.M.; Teal, L.B.; Lindsley, C.W.; Jones, C.K. M1/M4-Preferring Muscarinic Cholinergic Receptor Agonist Xanomeline Reverses Wake and Arousal Deficits in Nonpathologically Aged Mice. ACS Chem. Neurosci. 2023, 14, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Popiolek, M.; Mandelblat-Cerf, Y.; Young, D.; Garst-Orozco, J.; Lotarski, S.M.; Stark, E.; Kramer, M.; Butler, C.R.; Kozak, R. In Vivo Modulation of Hippocampal Excitability by M4 Muscarinic Acetylcholine Receptor Activator: Implications for Treatment of Alzheimer’s Disease and Schizophrenic Patients. ACS Chem. Neurosci. 2019, 10, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Araya, R.; Noguchi, T.; Yuhki, M.; Kitamura, N.; Higuchi, M.; Saido, T.C.; Seki, K.; Itohara, S.; Kawano, M.; Tanemura, K.; et al. Loss of M5 Muscarinic Acetylcholine Receptors Leads to Cerebrovascular and Neuronal Abnormalities and Cognitive Deficits in Mice. Neurobiol. Dis. 2006, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Wierońska, J.M.; Cieślik, P.; Burnat, G.; Kalinowski, L. Activation of Metabotropic Glutamate Receptor (MGlu2) and Muscarinic Receptors (M1, M4, and M5), Alone or in Combination, and Its Impact on the Acquisition and Retention of Learning in the Morris Water Maze, NMDA Expression and CGMP Synthesis. Biomolecules 2023, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- Dwomoh, L.; Rossi, M.; Scarpa, M.; Khajehali, E.; Molloy, C.; Herzyk, P.; Mistry, S.N.; Bottrill, A.R.; Sexton, P.M.; Christopoulos, A.; et al. M1 Muscarinic Receptor Activation Reduces the Molecular Pathology and Slows the Progression of Prion-Mediated Neurodegenerative Disease. Sci. Signal. 2022, 15, eabm3720. [Google Scholar] [CrossRef]
- Gatta, V.; Mengod, G.; Reale, M.; Tata, A.M. Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis. Biomedicines 2020, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, M.; Di Pinto, G.; Reale, M.; Mengod, G.; Tata, A.M. Cholinergic System and Neuroinflammation: Implication in Multiple Sclerosis. Cent. Nerv. Syst. Agents Med. Chem. 2016, 17, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Frinchi, M.; Giardina, C.; Scordino, M.; Zuccarini, M.; De Simone, C.; Di Carlo, M.; Belluardo, N.; Mudò, G.; Di Liberto, V. Neuroprotective and Antioxidant Role of Oxotremorine-M, a Non-Selective Muscarinic Acetylcholine Receptors Agonist, in a Cellular Model of Alzheimer Disease. Cell Mol. Neurobiol. 2023, 43, 1941–1956. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic System during the Progression of Alzheimer’s Disease: Therapeutic Implications. Expert. Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- Binning, W.; Hogan-Cann, A.E.; Yae Sakae, D.; Maksoud, M.; Ostapchenko, V.; Al-Onaizi, M.; Matovic, S.; Lu, W.-Y.; Prado, M.A.M.; Inoue, W.; et al. Chronic HM3Dq Signaling in Microglia Ameliorates Neuroinflammation in Male Mice. Brain Behav. Immun. 2020, 88, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The Liver–Brain–Gut Neural Arc Maintains the Treg Cell Niche in the Gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, M.; Li, D.; He, X.; Zang, W. Cholinergic Drugs Reduce Metabolic Inflammation and Diabetic Myocardial Injury by Regulating the Gut Bacterial Component Lipopolysaccharide-Induced ERK/Egr-1 Pathway. FASEB J. 2023, 37, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bruno, J.P. Cortical Cholinergic Inputs Mediating Arousal, Attentional Processing and Dreaming: Differential Afferent Regulation of the Basal Forebrain by Telencephalic and Brainstem Afferents. Neuroscience 1999, 95, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Hughes, L.F.; Toth, L.A. Sleep, Activity, Temperature and Arousal Responses of Mice Deficient for Muscarinic Receptor M2 or M4. Life Sci. 2010, 86, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Salín-Pascual, R.J.; Díaz-Muñoz, M.; Rivera-Valerdi, L.; Ortiz-López, L.; Blanco-Centurión, C. Decrease in Muscarinic M2 Receptors from Synaptosomes in the Pons and Hippocampus after REM Sleep Deprivation in Rats. Sleep. Res. Online 1998, 1, 19–23. [Google Scholar]
- Pooladgar, P.; Sakhabakhsh, M.; Taghva, A.; Soleiman-Meigooni, S. Donepezil Beyond Alzheimer’s Disease? A Narrative Review of Therapeutic Potentials of Donepezil in Different Diseases. Iran. J. Pharm. Res. 2022, 21, e128408. [Google Scholar] [CrossRef]
- Yamada, R.G.; Ueda, H.R. Molecular Mechanisms of REM Sleep. Front. Neurosci. 2020, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Lund, C.; Clemmensen, C. Muscarinic Receptors in Energy Homeostasis: Physiology and Pharmacology. Basic. Clin. Pharmacol. Toxicol. 2020, 126, 66–76. [Google Scholar] [CrossRef]
- Han, M.; Lian, J.; Su, Y.; Deng, C. Cevimeline Co-Treatment Attenuates Olanzapine-Induced Metabolic Disorders via Modulating Hepatic M3 Muscarinic Receptor: AMPKα Signalling Pathway in Female Rats. J. Psychopharmacol. 2022, 36, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Whitcomb, D.J.; Park, S.J.; Martinez-Perez, C.; Barbati, S.A.; Mitchell, S.J.; Cho, K. M1 Muscarinic Acetylcholine Receptor Dysfunction in Moderate Alzheimer’s Disease Pathology. Brain Commun. 2020, 2, fcaa058. [Google Scholar] [CrossRef]
- Basurto, E.; González-Flores, O.; Hoffman, K. Chronic MK-801 Administration Provokes Persistent Deficits in Social Memory in the Prairie Vole (Microtus Ochrogaster): A Potential Animal Model for Social Deficits of Schizophrenia. Behav. Brain Res. 2024, 465, 114948. [Google Scholar] [CrossRef]
- McDonald, A.J. Functional Neuroanatomy of Basal Forebrain Projections to the Basolateral Amygdala: Transmitters, Receptors, and Neuronal Subpopulations. J. Neurosci. Res. 2024, 102, 25318. [Google Scholar] [CrossRef]
- Zuchner, T.; Schliebs, R.; Perez-Polo, J.R. Down-Regulation of Muscarinic Acetylcholine Receptor M2 Adversely Affects the Expression of Alzheimer’s Disease-Relevant Genes and Proteins. J. Neurochem. 2005, 95, 20–32. [Google Scholar] [CrossRef]
- Wang, L.; Qu, F.; Yu, X.; Yang, S.; Zhao, B.; Chen, Y.; Li, P.; Zhang, Z.; Zhang, J.; Han, X.; et al. Cortical Lipid Metabolic Pathway Alteration of Early Alzheimer’s Disease and Candidate Drugs Screen. Eur. J. Med. Res. 2024, 29, 199. [Google Scholar] [CrossRef]
- Jha, D.; Blennow, K.; Zetterberg, H.; Savas, J.N.; Hanrieder, J. Spatial Neurolipidomics-MALDI Mass Spectrometry Imaging of Lipids in Brain Pathologies. J. Mass. Spectrom. 2024, 59, e5008. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodriguez, M.; Perez, S.E.; Martinez-Gardeazabal, J.; Manuel, I.; Malek-Ahmadi, M.; Rodriguez-Puertas, R.; Mufson, E.J. Frontal Cortex Lipid Alterations During the Onset of Alzheimer’s Disease. J. Alzheimers Dis. 2024, 98, 1515–1532. [Google Scholar] [CrossRef]
- Rampa, L.; Santangelo, R.; Gaspardone, C.; Cerutti, A.; Magnani, G.; Piscazzi, F.; Sgherzi, G.; Fiore, G.; Filippi, M.; Agosta, F.; et al. Potential Cardiologic Protective Effects of Acetylcholinesterase Inhibitors in Patients With Mild to Moderate Dementia. Am. J. Cardiol. 2023, 200, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Guerin, A.A.; Walker, L.C.; Lawrence, A.J. Clinical Effectiveness of Muscarinic Receptor-Targeted Interventions in Neuropsychiatric Disorders: A Systematic Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 36, ISBN 0123456789. [Google Scholar]
- Levey, A.I.; Edmunds, S.M.; Heilman, C.J.; Desmond, T.J.; Frey, K.A. Localization of Muscarinic M3 Receptor Protein and M3 Receptor Binding in Rat Brain. Neuroscience 1994, 63, 207–221. [Google Scholar] [CrossRef]
- Scarr, E. Muscarinic Receptors in Psychiatric Disorders—Can We Mimic “Health”? Neurosignals 2009, 17, 298–310. [Google Scholar] [CrossRef]
- Gomeza, J.; Zhang, L.U.; Kostenis, E.; Felder, C.; Bymaster, F.; Brodkin, J.; Shannon, H.; Xia, B.; Deng, C.-X.; Wess, J. Enhancement of D1 Dopamine Receptor-Mediated Locomotor Stimulation in M 4 Muscarinic Acetylcholine Receptor Knockout Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 10483–10488. [Google Scholar] [CrossRef]
- Myslivecek, J. Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum. Biomedicines 2021, 9, 25. [Google Scholar] [CrossRef]
- Jeon, J.; Dencker, D.; Wörtwein, G.; Woldbye, D.P.D.; Cui, Y.; Davis, A.A.; Levey, A.I.; Schütz, G.; Sager, T.N.; Mørk, A.; et al. A Subpopulation of Neuronal M4 Muscarinic Acetylcholine Receptors Plays a Critical Role in Modulating Dopamine-Dependent Behaviors. J. Neurosci. 2010, 30, 2396–2405. [Google Scholar] [CrossRef]
- Jakubík, J.; Tuček, S.; El-Fakahany, E.E. Role of Receptor Protein and Membrane Lipids in Xanomeline Wash-Resistant Binding to Muscarinic M1 Receptors. J. Pharmacol. Exp. Ther. 2004, 308, 105–110. [Google Scholar] [CrossRef]
- Li, W.; Basile, A.S.; Tong, L.; Van Laere, K.; Masdeu, J.C.; Hostetler, E.D.; Marshall, F.; Uslaner, J.M. The Novel M4 PAM PET Tracer [11C] MK-6884: A Novel Biomarker for Measuring Target Engagement of Muscarinic M4 Positive Allosteric Modulators (PAMs) as Well as Cholinergic Tone in Patients with Alzheimer’s Disease. Alzheimer’s Dement. 2022, 18, e067249. [Google Scholar] [CrossRef]
- Lange, H.S.; Vardigan, J.D.; Cannon, C.E.; Puri, V.; Henze, D.A.; Uslaner, J.M. Effects of a Novel M4 Muscarinic Positive Allosteric Modulator on Behavior and Cognitive Deficits Relevant to Alzheimer’s Disease and Schizophrenia in Rhesus Monkey. Neuropharmacology 2021, 197, 108754. [Google Scholar] [CrossRef]
- d’Orchymont, F.; Narvaez, A.; Raymond, R.; Sachdev, P.; Charil, A.; Krause, S.; Vasdev, N. In Vitro Evaluation of PET Radiotracers for Imaging Synaptic Density, the Acetylcholine Transporter, AMPA-Tarp-Γ8 and Muscarinic M4 Receptors in Alzheimer’s Disease. Am. J. Nucl. Med. Mol. Imaging 2024, 14, 1. [Google Scholar] [CrossRef]
- Bender, A.M.; Garrison, A.T.; Lindsley, C.W. The Muscarinic Acetylcholine Receptor M5: Therapeutic Implications and Allosteric Modulation. ACS Chem. Neurosci. 2019, 10, 1025–1034. [Google Scholar] [CrossRef]
- Zuccolo, E.; Laforenza, U.; Negri, S.; Botta, L.; Berra-Romani, R.; Faris, P.; Scarpellino, G.; Forcaia, G.; Pellavio, G.; Sancini, G.; et al. Muscarinic M5 Receptors Trigger Acetylcholine-Induced Ca2+ Signals and Nitric Oxide Release in Human Brain Microvascular Endothelial Cells. J. Cell Physiol. 2019, 234, 4540–4562. [Google Scholar] [CrossRef]
| Subtype | Negative Effects | Reference | Positive Effects | Reference |
|---|---|---|---|---|
| M1 | Cognitive dysfunction | [62] | Improvement in object location in memory tasks | [63] |
| mAChRs dysregulations | [64] | σ1 chaperone collaboration, Ca2+ homeostasis (ANAVEX2-73) | [35,65,66] | |
| Mitochondrial function alterations | [67] | Modulation of APP metabolism (ANAVEX3-71) | [68,69,70] | |
| M2 | Interfere with APP processing | [71] | Bipharmacophoric inhibition of cholinesterase (10-C10) | [72] |
| Hallucination and behavioral symptoms | [73] | Enhancement of cognition by facilitating ACh release (SCH577790) | [74] | |
| M3 | Mood disorders | [75] | Cognitive and behavioral improvement (LU 25-109) | [76,77] |
| Sleep dysfunctions | [78] | PAM activity (N-pyrimidyl/pyridyl-2-thiazolamine) | [79] | |
| Reduced excitatory synaptic drive onto CA3 pyramidal neurons | [80,81] | |||
| M4 | Locomotor alterations | [82] | Cognitive and behavioral modulation (compound-110) | [82,83] |
| Treatment of movement disorders | [84] | |||
| mAChR agonist (Xanomeline) | [85] | |||
| Reduction in glutamate release from CA1 and CA3 pyramidal neurons (PT-3763) | [86] | |||
| M5 | Reduced blood flow in the hippocampus results in neuronal atrophy and memory impairments | [87] | M5 activation (VU0357017, VU0152100, VU0238429) | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, M.; Trebesova, H.; Grilli, M. Muscarinic Receptors and Alzheimer’s Disease: New Perspectives and Mechanisms. Curr. Issues Mol. Biol. 2024, 46, 6820-6835. https://doi.org/10.3390/cimb46070407
Monaco M, Trebesova H, Grilli M. Muscarinic Receptors and Alzheimer’s Disease: New Perspectives and Mechanisms. Current Issues in Molecular Biology. 2024; 46(7):6820-6835. https://doi.org/10.3390/cimb46070407
Chicago/Turabian StyleMonaco, Martina, Hanna Trebesova, and Massimo Grilli. 2024. "Muscarinic Receptors and Alzheimer’s Disease: New Perspectives and Mechanisms" Current Issues in Molecular Biology 46, no. 7: 6820-6835. https://doi.org/10.3390/cimb46070407
APA StyleMonaco, M., Trebesova, H., & Grilli, M. (2024). Muscarinic Receptors and Alzheimer’s Disease: New Perspectives and Mechanisms. Current Issues in Molecular Biology, 46(7), 6820-6835. https://doi.org/10.3390/cimb46070407








