Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Experimental Design
2.3. Lactate Dehydrogenase (LDH) Assay
2.4. Nitric Oxide (NO) Measurement
2.5. May-Grünwald–Giemsa (MGG) Staining
2.6. ELISA Assay
2.7. Labelling of Cytoskeletal F-Actin Filaments
2.8. Assessment of ROS Using 2′,7′-Dichlorodihydrofluorescein Diacetate (H2DCF/DA)
2.9. Cytometric Analysis of the Cell Cycle
2.10. Statistical Analysis
3. Results
3.1. LDH Release in Co-Cultures
3.2. Cell Cycle
3.3. IL-4, IL-10 and IL-13 Levels in Co-Cultures
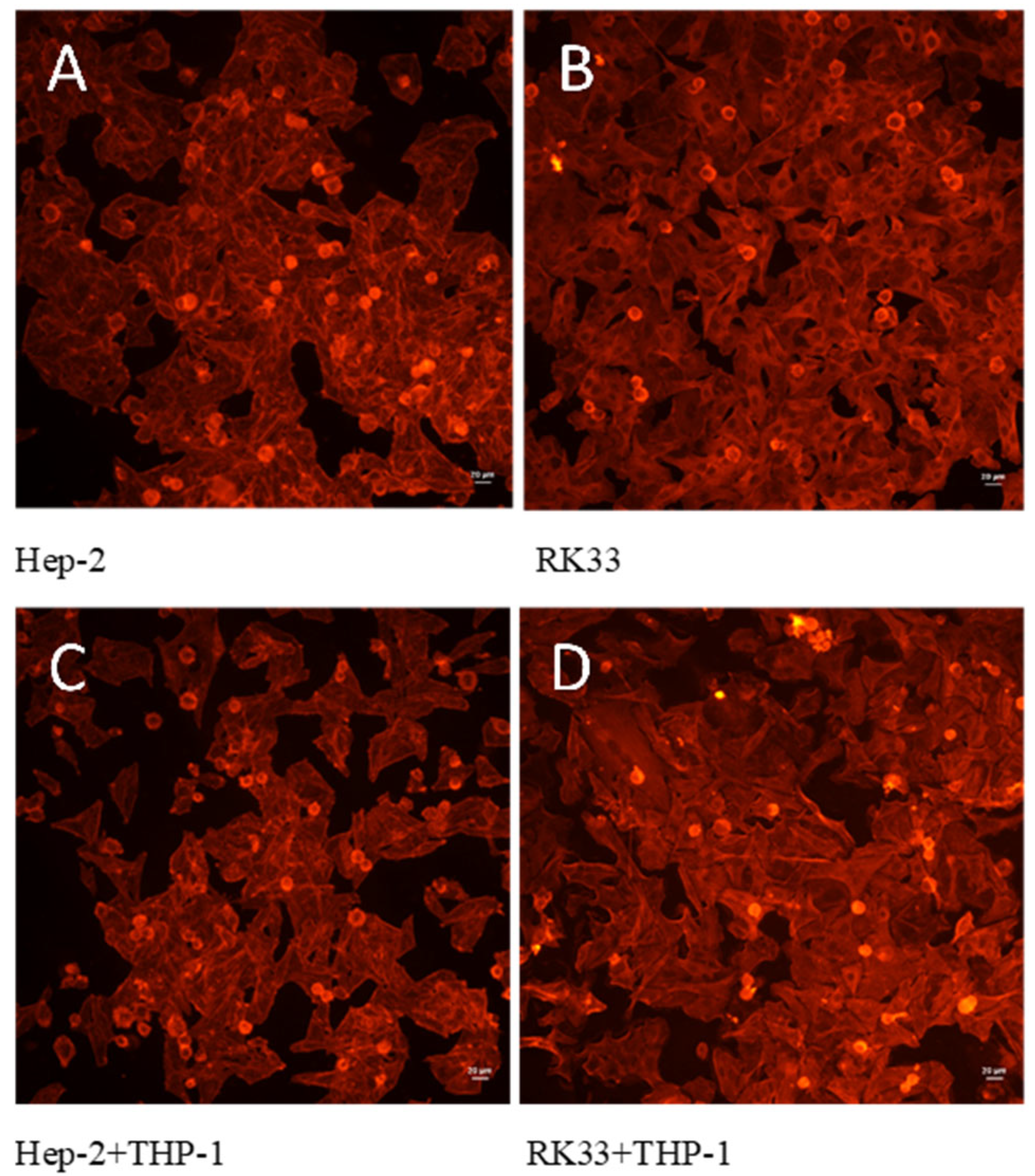
3.4. Lack of Morphological Changes in Tumor Cells in Tested Co-Cultures
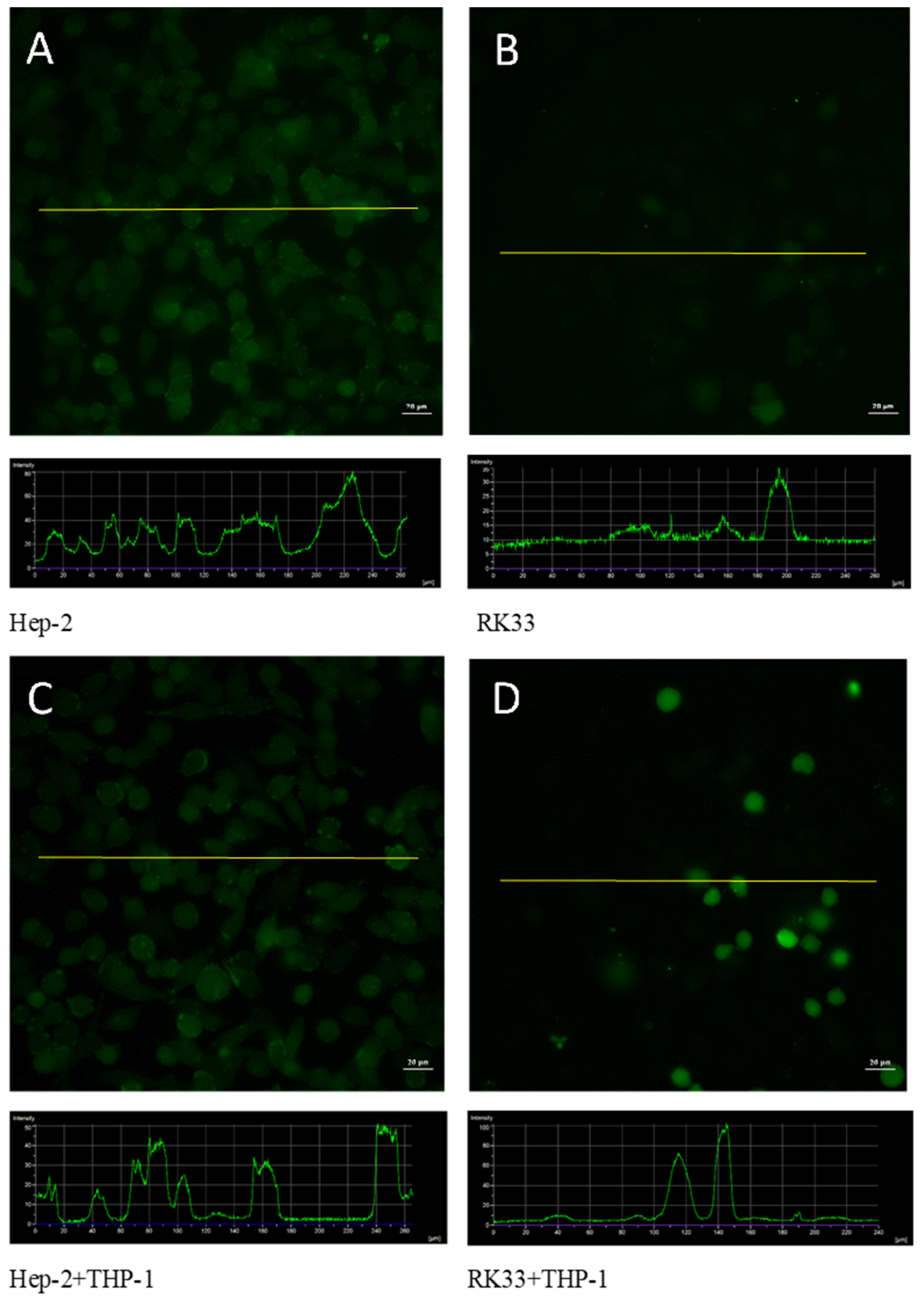
3.5. Fluorescent Analysis of ROS Formation in Tumor/Monocyte Co-Culture
3.6. Nitric Oxide Production Decreases in Co-Culture of Tumor Cells with Monocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Cheng, X.; Dai, K.; Bao, W.; Ding, R.; Wan, Y. Identification of immunocell infiltrates and effective diagnostic biomarkers in laryngeal carcinoma. Medicine 2023, 102, e32548. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zuo, N.; Pan, S.; Wang, Y.; Wang, Q.; Ma, J. The exosomal miR-1246 of laryngeal squamous cell carcinoma induces polarization of M2 type macrophages and promotes the invasiveness of laryngeal squamous cell carcinoma. J. Oncol. 2022, 2022, 4424221. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, H.; Zhang, T.; Ye, X.; Yang, H.; Shen, Y. Comprehensive analysis of macrophage-related multigene signature in the tumor microenvironment of head and neck squamous cancer. Aging 2021, 13, 5718–5747. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Li, X.-Y.; Tadashi, N.; Dong, P. Clinical significance of tumor-associated macrophage infiltration in supraglottic laryngeal carcinoma. Chin. J. Cancer 2011, 30, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lu, X.; Li, Z.; Sun, Y.; He, Z.; Li, X. Dihydroartemisinin prevents progression and metastasis of head and neck squamous cell carcinoma by inhibiting polarization of macrophages in tumor microenvironment. OncoTargets Ther. 2020, 13, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, T.; Liu, J.; Tai, J.; Wang, B.; Chen, Z.; Quan, Z. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp. Hematol. Oncol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Lin, H.; Li, M.; Lin, B. Tumor-associated macrophage polarization in the inflammatory tumor microenvironment. Front. Oncol. 2023, 13, 1103149. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Takahashi, H.; Kaira, K.; Toyoda, M.; Murata, T.; Ohnishi, H.; Oyama, T.; Chikamatsu, K. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab. Investig. 2016, 96, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, N.; Zheng, Z.; Che, Y.; Suzuki, M.; Kano, S.; Lu, J.; Wang, P.; Sun, Y.; Homma, A. Exosomal lncRNA HOTAIR induce macrophages to M2 polarization via PI3K/ p-AKT /AKT pathway and promote EMT and metastasis in laryngeal squamous cell carcinoma. BMC Cancer 2022, 22, 1208. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Lin, C.-C.; Huang, Y.-W.; Chen, J.-H.; Tsou, Y.-A.; Chang, L.-C.; Fan, C.-C.; Lin, C.-Y.; Chang, W.-C. Macrophage secretory IL-1β promotes docetaxel resistance in head and neck squamous carcinoma via SOD2/CATICAM1 signaling. JCI Insight 2022, 7, e157285. [Google Scholar] [CrossRef]
- Maldonado, L.A.G.; Nascimento, C.R.; Fernandes, N.A.R.; Silva, A.L.P.; D’Silva, N.J.; Rossa, C., Jr. Influence of tumor cell-derived TGF-β on macrophage phenotype and macrophage-mediated tumor cell invasion. Int. J. Biochem. Cell Biol. 2022, 153, 106330. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Paduch, R.; Klatka, J.; Kandefer-Szerszeń, M.; Stepulak, A.; Pożarowski, P.; Zdzisińska, B. Establishment and preliminary characterization of two cell lines derived from larynx carcinoma. Folia Histochem. Cytobiol. 2002, 40, 195–196. [Google Scholar] [PubMed]
- Paduch, R.; Kandefer-Szerszeń, M.; Piersiak, T. The importance of release of proinflammatory cytokines, ROS, and NO in different stages of colon carcinoma growth and metastasis after treatment with cytotoxic drugs. Oncol. Res. 2010, 18, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cheng, J.; Xue, K.; Zhao, X.; Wen, L.; Xu, C. Expression of programmed death-ligand 1 in laryngeal carcinoma and its effects on immune cell subgroup infiltration. Pathol. Oncol. Res. 2019, 25, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Luthria, G.; Li, R.; Wang, S.; Prytyskach, M.; Kohler, R.H.; Lauffenburger, D.A.; Mitchison, T.J.; Weissleder, R.; Miller, M.A. In vivo microscopy reveals macrophage polarization locally promotes coherent microtubule dynamics in migrating cancer cells. Nat. Commun. 2020, 11, 3521. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, P.; Wei, S.; Wang, J.; Li, C. The role of macrophages-mediated communications among cell compositions of tumor microenvironment in cancer progression. Front. Immunol. 2023, 14, 1113312. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, W.; Zhong, W.-Q.; Liu, Z.-J.; Li, H.-M.; Yu, Z.-L.; Zhao, Y.-F. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol. Rep. 2018, 40, 2558–2572. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Moriyama, M.; Haque, A.S.M.R.; Hattori, T.; Chinju, A.; Hu, C.; Kubota, K.; Miyahara, Y.; Kakizoe-Ishiguro, N.; Kawano, S.; et al. Oral squamous cell carcinoma contributes to differentiation of monocyte-derived tumor-associated macrophages via PAI-1 and IL-8 production. Int. J. Mol. Sci. 2021, 22, 9475. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Zhang, S.; Jing, Z.; Shang, L.; Jin, S.; Liu, F.; Shen, J.; Li, Y.; Hu, J.; Meng, Q.; et al. Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE2/β-catenin signalling pathway. Mol. Immunol. 2017, 90, 197–210. [Google Scholar] [CrossRef]
- Gunassekaran, G.R.; Vadevoo, S.M.P.; Baek, M.-C.; Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Mehta, H.; Lovell, A.; Papaioannou, E.; Fairbanks, L. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8+ T cells. Eur. J. Immunol. 2016, 46, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, M.; Shi, Q.; Fang, Y.; Fu, D.; Shen, Z.-x.; Yi, H.; Wang, L.; Zhao, W. Elevated serum IL-13 level is associated with increased Treg cells in tumor microenvironment and disease progression of diffuse large B-cell lymphoma. Hematol. Oncol. 2023, 41, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Park, J.M.; Berzofsky, J.A. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol. Immunother. 2004, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Zhang, M.; Li, Z. IL-13 and IL-13Ra1 are overexpressed in extranodal natural killer/T cell lymphoma and mediate tumor cell proliferation. Biochem. Biophys. Res. Commun. 2018, 503, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhu, Q.; Li, Z.; Peng, Y.; Yu, X.; Yuan, B.; Liu, Y.; Liu, Y.; Yin, L.; Peng, Y.; et al. The FOXM1–ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis. 2017, 8, e2659. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Shi, X.; Jiang, M.; Liu, H. Cross-talk between cancer stem cells and immune cells: Potential therapeutic targets in the tumor immune microenvironment. Mol. Cancer 2023, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, H.; Liang, R.; Yi, H.; Meng, X.; Chen, Z.; Pan, H.; Ma, Y.; Cai, L. ROS-inducing micelles sensitize tumor-associated macrophages to TLR3 stimulation for potent immunotherapy. Biomacromolecules 2018, 19, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Mu, W.; Barry, J.; Han, A.; Carpenter, R.L.; Jiang, B.-H.; Peiper, S.C.; Mahoney, M.G.; Aplin, A.E.; et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J. Exp. Clin. Cancer Res. 2022, 41, 41. [Google Scholar] [CrossRef] [PubMed]
- Fauskanger, M.; Haabeth, O.A.W.; Skjeldal, F.M.; Bogen, B.; Tveita, A.A. Tumor killing by CD4+ T cells is mediated via induction of inducible nitric oxide synthase-dependent macrophage cytotoxicity. Front. Immunol. 2018, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, D.; Markovic, M.; Bogdanovic, N.; Stojkovic, M.M.; Trajkovic, V. Necrotic tumor cells oppositely affect nitric oxide production in tumor cell lines and macrophages. Cell. Immunol. 2002, 215, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Rahat, M.A.; Hemmerlein, B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front. Physiol. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, D.; Luiz, J.P.M.; Hernandez, C.A.S.; Alves-Filho, J.C.; Hussell, T.; Townsend, P.A.; Moncada, S. Nitric oxide favours tumour-promoting inflammation through mitochondria-dependent and -independent actions on macrophages. Redox Biol. 2022, 54, 102350. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Schubert, K.; Pace, S.; Ziereisen, J.; Nikels, F.; Scherer, O.; Hüttel, S.; Zahler, S.; Vollmar, A.M.; Weinigel, C.; et al. Modulation of actin dynamics as potential macrophage subtype-targeting anti-tumour strategy. Sci. Rep. 2017, 7, 41434. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ahn, J.-H.; Lee, K.-T.; Jang, D.S.; Choi, J.-H. Deoxyschizandrin, isolated from Schisandra berries, induces cell cycle arrest in ovarian cancer cells and inhibits the protumoural activation of tumour-associated macrophages. Nutrients 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Rittá, M.; De Andrea, M.; Mondini, M.; Mazibrada, J.; Giordano, C.; Pecorari, G.; Garzaro, M.; Landolfo, V.; Schena, M.; Chiusa, L.; et al. Cell cycle and viral and immunologic profiles of head and neck squamous cell carcinoma as predictable variables of tumor progression. Head Neck 2009, 31, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Aljabery, F.; Shabo, I.; Saudi, A.; Holmbom, M.; Olson, H.; Jahnson, S. The emerging role of cell cycle protein p53 expression by tumor cells and M2-macrophage infiltration in urinary bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 148.e9–148.e16. [Google Scholar] [CrossRef] [PubMed]





| Hep-2 Mono-Culture (% of Cell Death) | RK33 Mono-Culture (% of Cell Death) | THP-1 Mono-Culture (% of Cell Death) | Cell Culture and Type of Interactions | % Change in Co-Cultures Compared to a Cancer Cell Mono-Cultures |
|---|---|---|---|---|
| 5.7% ± 1.6% | ---------- | 9.5% ± 1.4% | Hep-2 + THP-1 (direct) | 6.2% ± 3.7% ↓ |
| ---------- | 7.9% ± 0.8% | 9.5% ± 1.4% | RK33 + THP-1 (direct) | 5.9% ± 3.2% ↑ |
| 5.7% ± 1.6% | ---------- | 9.5% ± 1.4% | Hep-2 + THP-1 (indirect) | 0.8% ± 3.0% ↓ |
| ---------- | 7.9% ± 0.8% | 9.5% ± 1.4% | RK33 + THP-1 (indirect) | 12.2% * ± 1.4% ↑ |
| Cell Cycle | Cell Culture | ||||||
|---|---|---|---|---|---|---|---|
| Hep-2 | RK33 | THP-1 | Hep-2 +THP-1 | RK33 +THP-1 | Hep-2 +THP-1 (Insert) | RK33 +THP-1 (Insert) | |
| Sub-G1 | 5.99 ± 0.34 | 7.75 ± 0.71 | 11.57 ± 0.43 | 2.59 ± 0.15 | 6.00 ± 0.59 | 4.26 ± 0.26 | 4.15 ± 0.36 |
| G1 | 67.76 ± 0.54 | 60.02 ± 0.20 | 51.29 ± 0.49 | 68.50 ± 1.01 | 61.38 ± 1.09 | 68.88 ± 0.51 | 62.17 ± 1.29 |
| S | 8.40 ± 0.60 | 18.67 ± 0.24 | 18.61 ± 0.25 | 12.95 ± 0.62 | 20.83 ± 0.93 | 9.44 ± 0.29 | 19.67 ± 0.58 |
| G2 | 18.02 ± 0.39 | 13.94 ± 0.79 | 18.88 ± 0.35 | 16.23 ± 1.12 | 12.06 ± 0.70 | 17.50 ± 0.54 | 14.17 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduch, R.; Klatka, M.; Pieniądz, P.; Wertel, I.; Pawłowska, A.; Klatka, J. Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro. Curr. Issues Mol. Biol. 2024, 46, 6836-6852. https://doi.org/10.3390/cimb46070408
Paduch R, Klatka M, Pieniądz P, Wertel I, Pawłowska A, Klatka J. Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro. Current Issues in Molecular Biology. 2024; 46(7):6836-6852. https://doi.org/10.3390/cimb46070408
Chicago/Turabian StylePaduch, Roman, Maria Klatka, Paulina Pieniądz, Iwona Wertel, Anna Pawłowska, and Janusz Klatka. 2024. "Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro" Current Issues in Molecular Biology 46, no. 7: 6836-6852. https://doi.org/10.3390/cimb46070408
APA StylePaduch, R., Klatka, M., Pieniądz, P., Wertel, I., Pawłowska, A., & Klatka, J. (2024). Reciprocal Interactions of Human Monocytes and Cancer Cells in Co-Cultures In Vitro. Current Issues in Molecular Biology, 46(7), 6836-6852. https://doi.org/10.3390/cimb46070408







