Conformational Alterations of the Cell Surface of Monomeric and Dimeric β2m-Free HLA-I (Proto-HLA) May Enable Novel Immune Functions in Health and Disease
Abstract
:1. Introduction
2. Human and Murine Cell Surfaces May also Express HLA-I HCs without B2m
3. Early Documentation of HLA-I B2m-Free HC Monomers on the Human Cell Surface
4. Does the Cell Surface of B2m-Free HLA HCs Represent a Separate Entity (as Proto-HLA) of the HLA Class?
- HLA molecules recognized by W6/32 as free α chains (even after boiling in SDS) are as follows: B7, B8, B13, B15, B18, B22, B35, B38, B39, B45, B50, B51, B55, B57, B60, B62, B63.
- HLA molecules recognized by W6/32 only as SDS-resistant noncovalent complexes with β2m (but not as free HCs) are as follows: B27, B*2701, B*2702, B*2705, B49.
5. Expression of Cell Surface B2m-Free HLA-B27 HCs in Transgenic Mice
- (1)
- A low-level expression of the HCs of HLA-B27 was detected on the cell surface of Con A-stimulated splenocytes.
- (2)
- The presence of HLA-B27 HCs on Con A-stimulated splenocytes was further confirmed by observing >50% of lysis of B27+ B2m−/− targets with anti-HLA-B27 CTLs in a 51Cr release assay.
6. Expression of Cell Surface B2m-Free HCs of Other Alleles of HLA-I in Humans
7. B2m-Free Monomeric HCs (Face-2) Expressed on Cancer Cell Surfaces
| Type of Cancer | HLA-E mAbs * | Citation |
|---|---|---|
| Melanoma | MEM-E/02 | Derré L et al. 2006 [56] |
| Lip squamosal cell carcinoma | MEM-E/02 | Goncalves et al. 2016 [57] |
| Laryngeal carcinoma | MEM-E/02 | Silva TG et al. 2011 [58] |
| Vulvar intraepithelial carcinoma | MEM-E/02 | van Esch EM et al. 2014 [59] |
| Penile cancer | MEM-E/02 | Djajadiningrat et al. 2015 [60] |
| Glioblastoma | MEM-E/02 | Mittelbronn M. et al. 2007 [61] |
| Glioblastoma | MEM-E/02 | Kren L et al. 2010 [62] |
| Glioblastoma | MEM-E/02 | Kren L et al. 2011 [63] |
| Oral osteosarcoma | MEM-E/02 | Costa Arantes et al. 2017 [64] |
| Intraoral mucoepidermoid carcinoma | MEM-E/02 | Mosconi C et al. 2017 [65] |
| Rectal cancer | MEM-E/02 | Reimers et al. 2014 [66] |
| Colorectal carcinoma | MEM-E/02 | Benevolo M et al. 2011 [67] |
| Colorectal carcinoma | MEM-E/02 | Zeestraten et al.2014 [68] |
| Colorectal carcinoma | MEM-E/02 | Guo et al. 2015 [69] |
| Colorectal carcinoma | MEM-E/02 | Huang et al. 2017 [70] |
| Gastric cancer | MEM-E/02 | Sasaki et al. 2014 [55] |
| Hepatocellular carcinoma | MEM-E/02 | Chen et al. 2011. [71] |
| Non-small-cell lung carcinoma | MEM-E/02 | Talebian-Yazdi et al. 2016 [72] |
| Breast cancer | MEM-E/02 | de Kruijf EM et al. 2010 [73] |
| Breast cancer | MEM-E/02 | da Silva et al. 2012. [74] |
| Ovarian cancer/cervical cancer | MEM-E/02 | Gooden M et al. 2011 [75] |
| Cervical cancer | MEM-E/02 | Gonçalves MA et al. 2008 [76] |
| Cervical cancer | MEM-E/02 | Spaans VM et al. 2012 [77] |
| Cervical squamous and adenocarcinoma | MEM-E/02 | Ferns et al. 2016 [78] |
| Serous ovarian adenocarcinoma | MEM-E/02 | Andersson et al. 2015 [79] |
| Serous ovarian Adenocarcinoma | MEM-E/02 | Zheng et al. 2015 [80] |
| Renal cell carcinoma | MEM-E/02 | Hanak L et al. 2009 [81] |
| Renal cell carcinoma | MEM-E/02 | Kren L et al., 2012 [82] |
| Thyroid cancer | MEM-E/02 | Zanetti et al. 2013 [83] |
| Hodgkin lymphoma | MEM-E/02 | Kren L et al., 2012 [84] |
| Melanoma, cervical cancer | 3D12 | Marín R et al. 2003 [85] |
| Glioblastoma stem cells | 3D12 | Wolpert et al. 2012 [86] |
| Glioblastoma | 3D12 | Wischhusen J et al. 2012 [87] |
| Neuroblastoma | 3D12 | Morandi et al. 2016 [88] |
| Hodgkin lymphoma | MEM-E/02 | Kren L et al., 2012 [84] |
| Chronic lymphocytic leukemia | 3D12 | McWilliams et al. 2016 [89] |
| Chronic lymphocytic leukemia | 3D12 | Wagner et al. 2017 [90] |
| Many cancers | 3D12 | Sensi M et al.2009 [91] |
8. Formation of Cell-Surface B2m-Free Face-3 (Homodimers) by Face-2 (HC Monomers) in Humans and Mice
9. B2m-Free Face-3 (Homodimers) and Face-4 (Heterodimers) on Exosomes and on the Cell Surface
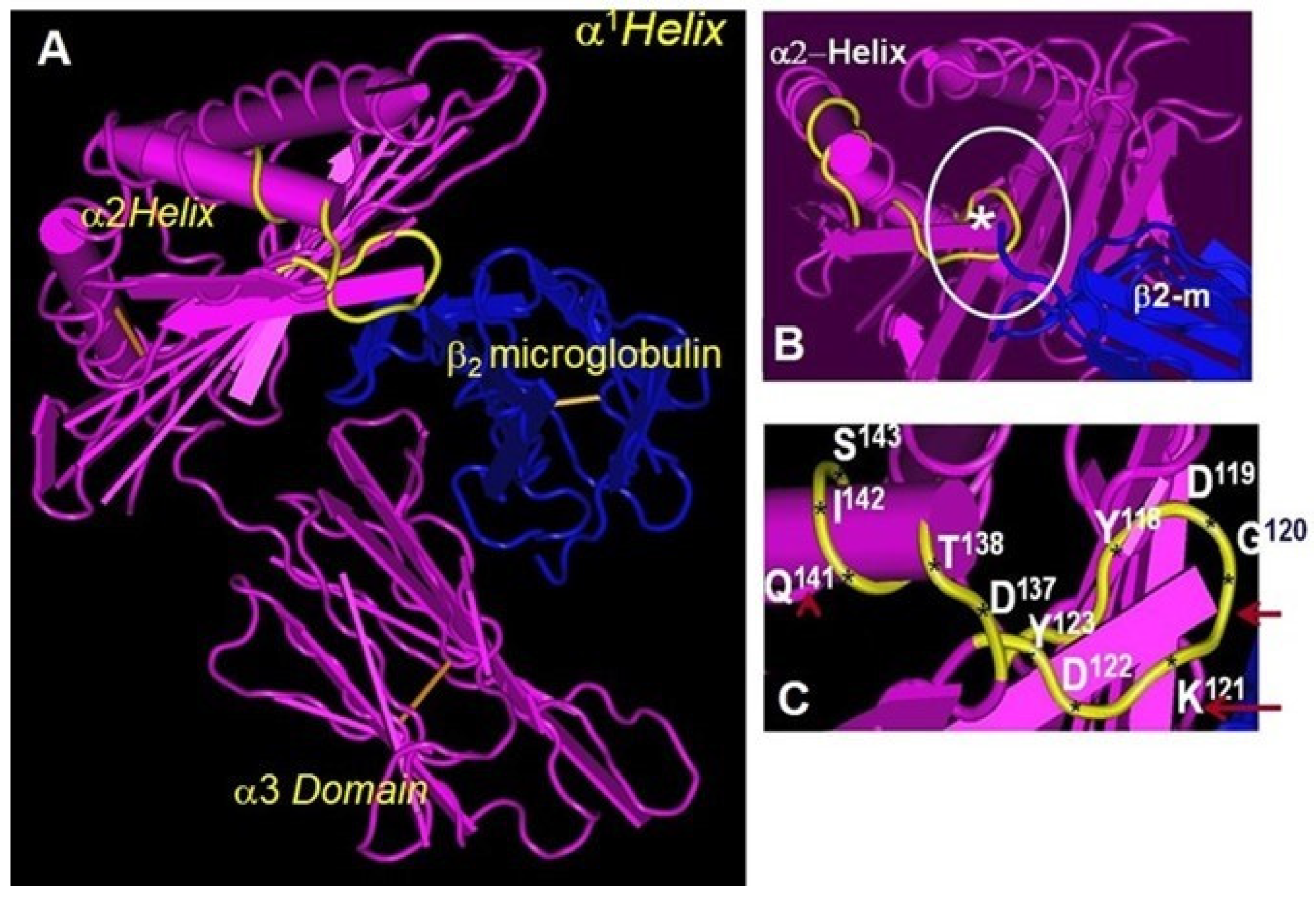
10. Folding and Conformational Orientation of HLA-I HCs with or without B2m
11. Do Conformational Alterations of the Cell Surface B2m-Free Human HLA HCs (Proto-HLA: Faces-2, -3, and -4) Enable Novel Immunomodulating Functions?
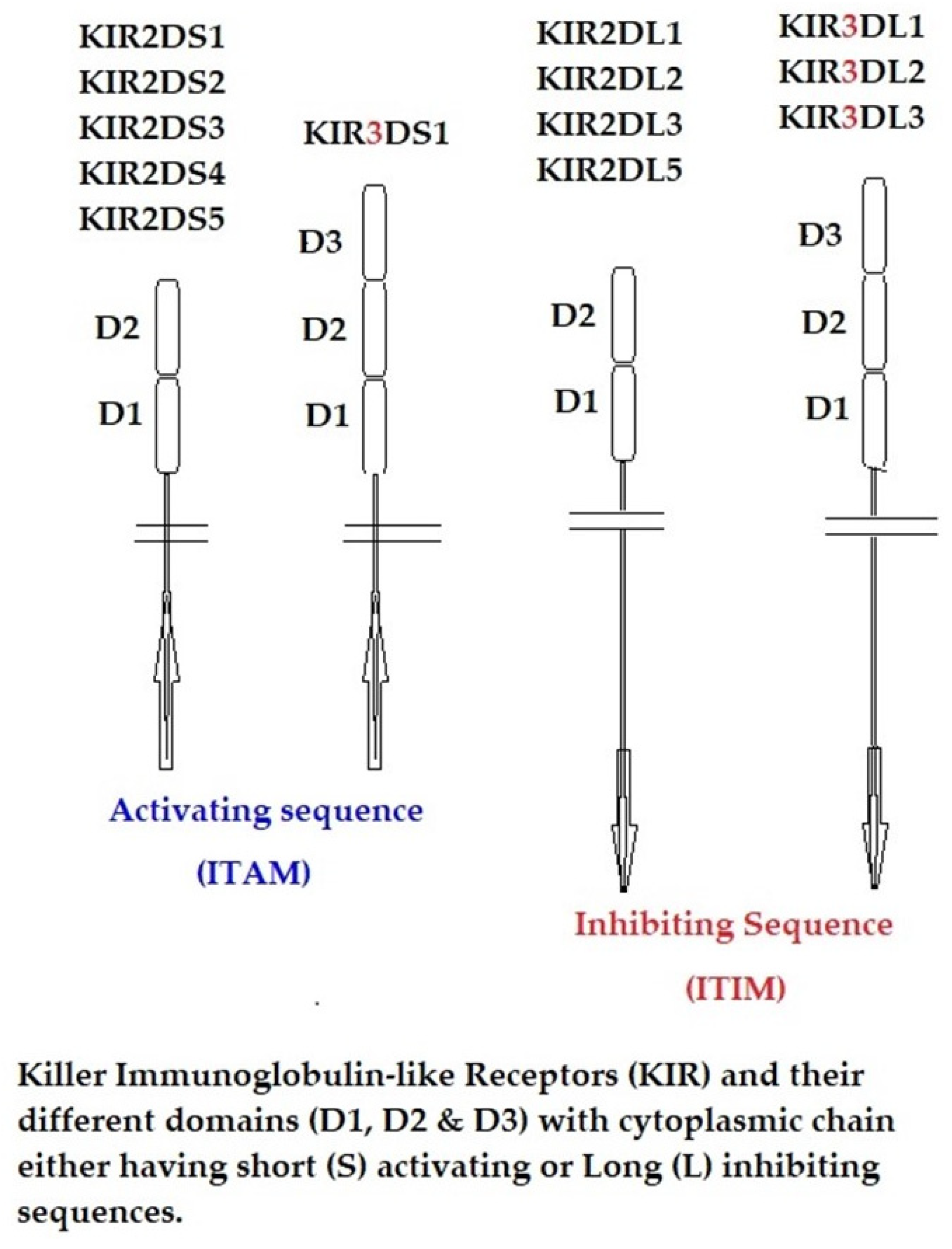
12. The α1 and α2 Domains of B2m-Free HCs as Ligands for KIR and LIR
- B2m-free B27 HCs (HC-B27) are first induced by bacterial infection.
- Subsequently, KIR3DL2 binding to HC-B27 affects the polarization of IL-17-producing immune cells by inhibiting the IL-2 and IFN-γ brakes on IL-17 production.
- Stronger binding of HC-B27 to KIR3DL2 and LILR compared with other B2m-associated HCs of HLA is capable of promoting the differentiation of pathogenic T and innate lymphocyte cell subsets, initially at mucosal sites.
- Subsequently, these cells migrate to affected joints, where they could amplify inflammation by activating fibroblast-like synoviocytes (FLSs) and antigen-presenting cells (APCs), releasing inflammatory mediators that damage joints.
- B27–KIR3DL2 interactions between FLSs and/or APCs and leukocytes promote the expansion and differentiation of these cells and recruit other immune cells to inflamed joints.
- Differentiated T and innate lymphocyte cells amplify immune responses by promoting antigen-presenting cell polarization [146].
- HC-B27-KIR3DL2 interactions could promote the differentiation of IL-17-producing immune cells by reducing T cell signaling strength and/or by inhibiting immune cell production of IFN-γ.
- HC-B27 could promote the differentiation of IL-17-producing immune cells by influencing immune synapse formation.
- HC-B27-LILRB2 or other LILR interactions could amplify immune responses by inhibiting the formation of regulatory T cells and tolerogenic dendritic cells.
- Both Th17 cells and NK cell subsets stimulate bone turnover by stimulating osteoclasts [147].
13. Conclusions: Evolutionary Implications of B2m-Free HCs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cresswell, P.; Springer, T.; Strominger, J.L.; Turner, M.J.; Grey, H.M.; Kubo, R.T. Immunological identity of the small subunit of HL-A antigens and beta2-microglobulin and its turnover on the cell membrane. Proc. Natl. Acad. Sci. USA 1974, 71, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A.; Strominger, J.L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc. Natl. Acad. Sci. USA 1976, 73, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.; Alpert, B.N.; Orr, H.T.; Strominger, J.L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J. Biol. Chem. 1977, 252, 7555–7567. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Ravindranath, N.M.; Selvan, S.R.; Hilali, F.E.; Amato-Menker, C.J.; Filippone, E.J. Cell Surface B2m-Free Human Leukocyte Antigen (HLA) Monomers and Dimers: Are They Neo-HLA Class and Proto-HLA? Biomolecules 2023, 13, 1178. [Google Scholar] [CrossRef]
- Goodfellow, P.N.; Jones, E.A.; van Heyningen, V.; Solomon, E.; Bobrow, M.; Miggiano, V.; Bodmer, W.F. The B2-microglobulin gene is on chromosome 15 and not in the HLA region. Nature 1975, 254, 267–269. [Google Scholar] [CrossRef]
- Arce-Gomez, B.; Jones, E.A.; Barnstable, C.J.; Solomon, E.; Bodmer, W.F. The genetic control of HLA-A and B antigens in somatic cell hybrids: Requirement for beta2 microglobulin. Tissue Antigens 1978, 11, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.; Fraser, J.; Flyer, D.; Calvin, S.; Flavell, R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc. Natl. Acad. Sci. USA 1986, 83, 7447–7451. [Google Scholar] [CrossRef] [PubMed]
- Parnes, J.R.; Seidman, J.G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell 1982, 29, 661–669. [Google Scholar] [CrossRef]
- Ploegh, H.L.; Cannon, L.E.; Strominger, J.L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc. Natl. Acad. Sci. USA 1979, 76, 2273–2277. [Google Scholar] [CrossRef]
- Potter, T.A.; Boyer, C.; Verhulst, A.M.; Golstein, P.; Rajan, T.V. Expression of H-2Db on the cell surface in the absence of detectable beta 2 microglobulin. J. Exp. Med. 1984, 160, 317–322. [Google Scholar] [CrossRef]
- Potter, T.A.; Zeff, R.A.; Schmitt-Verhulst, A.M.; Rajan, T.V. Molecular analysis of an EL4 cell line that expresses H-2Db but not H-2Kb or beta 2-microglobulin. Proc. Natl. Acad. Sci. USA 1985, 82, 2950–2954. [Google Scholar] [CrossRef]
- Höglund, P.; Glas, R.; Menard, C.; Kase, A.; Johansson, M.H.; Franksson, L.; Lemmonier, F.; Kärre, K. Beta2-microglobulin-deficient NK cells show increased sensitivity to MHC class I-mediated inhibition, but self tolerance does not depend upon target cell expression of H-2Kb and Db heavy chains. Eur. J. Immunol. 1998, 28, 370–378. [Google Scholar] [CrossRef]
- Hansen, T.H.; Myers, N.B.; Lee, D.R. Studies of two antigenic forms of Ld with disparate beta 2-microglobulin (beta 2m) associations suggest that beta 2m facilitate the folding of the alpha 1 and alpha 2 domains during de novo synthesis. J. Immunol. 1988, 140, 3522–3527. [Google Scholar] [CrossRef]
- Rock, K.L.; Gamble, S.; Rothstein, L.; Gramm, C.; Benacerraf, B. Dissociation of beta 2-microglobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell 1991, 65, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Myer, N.B.; Lie, W.R.; Nett, M.; Rubocki, R.J.; Hansen, T.H. The conformation of Ld induced by beta 2-microglobulin is fixed during de novo synthesis and irreversible by exchange or dissociation. J. Immunol. 1989, 142, 2751–2758. [Google Scholar] [CrossRef]
- Williams, D.B.; Barber, B.H.; Flavell, R.A.; Allen, H. Role of beta 2-microglobulin in the intracellular transport and surface expression of murine class I histocompatibility molecules. J. Immunol. 1989, 142, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Krangel, M.S.; Orr, H.T.; Strominger, J.L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell 1979, 18, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Barnstable, C.J.; Bodmer, W.F.; Brown, G.; Galfre, G.; Milstein, C.; Williams, A.F.; Ziegler, A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 1978, 14, 9–20. [Google Scholar] [CrossRef]
- Parham, P.; Barnstable, C.J.; Bodmer, W.F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 1979, 123, 342–349. [Google Scholar] [CrossRef]
- Bushkin, Y.; Tung, J.S.; Pinter, A.; Michaelson, J.; Boyse, E.A. Unusual association of beta 2-microglobulin with certain class I heavy chains of the murine major histocompatibility complex. Proc. Natl. Acad. Sci. USA 1986, 83, 432–436. [Google Scholar] [CrossRef]
- Bushkin, Y.; Posnett, D.N.; Pernis, B.; Wang, C.Y. A new HLA-linked T cell membrane molecule, related to the beta chain of the clonotypic receptor, is associated with T3. J. Exp. Med. 1986, 164, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Bushkin, Y.; Demaria, S.; Le, J.M.; Schwab, R. Physical association between the CD8 and HLA class I molecules on the surface of activated human T lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 3985–3989. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, E.; Stockinger, H.; Majdic, O.; Gaugitsch, H.; Lindley, I.J.; Maurer, D.; Hajek-Rosenmayr, A.; Knapp, W. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J. Exp. Med. 1990, 171, 1431–1442. [Google Scholar] [CrossRef]
- Demaria, S.; Schwab, R.; Bushkin, Y. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell Immunol. 1992, 142, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Bushkin, Y. CD8 and beta 2-microglobulin-free MHC class I molecules in T cell immunoregulation. Int. J. Clin. Lab. Res. 1993, 23, 61–69. [Google Scholar] [CrossRef]
- Tran, T.M.; Ivanyi, P.; Hilgert, I.; Brdicka, T.; Pla, M.; Breur, B.; Flieger, M.; Ivasková, E.; Horejsí, V. The epitope recognized by pan-HLA class I-reactive monoclonal antibody W6/32 and its relationship to unusual stability of the HLA-B27/beta2-microglobulin complex. Immunogenetics 2001, 53, 440–446. [Google Scholar] [CrossRef]
- Bluestone, R. Seronegative spondylarthropathies. Hosp. Pract. 1979, 14, 87–97. [Google Scholar] [CrossRef]
- Bluestone, R. Diagnosis of rheumatic disease. 2. Laboratory tests. Postgrad. Med. 1979, 65, 78–81, 84–87. [Google Scholar] [CrossRef]
- Khare, S.D.; Luthra, H.S.; David, C.S. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: A model of human spondyloarthropathies. J. Exp. Med. 1995, 182, 1153–1158. [Google Scholar] [CrossRef]
- Khare, S.D.; Hansen, J.; Luthra, H.S.; David, C.S. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human beta2-microglobulin (beta2m) double transgenic mice with disrupted mouse beta2m. J. Clin. Investig. 1996, 98, 2746–2755. [Google Scholar] [CrossRef]
- Stam, N.J.; Spits, H.; Ploegh, H.L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 1986, 137, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Perosa, F.; Luccarelli, G.; Prete, M.; Favoino, E.; Ferrone, S.; Dammacco, F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J. Immunol. 2003, 171, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Payeli, S.K.; Kollnberger, S.; Belaunzaran, O.M.; Thiel, M.; McHugh, K.; Giles, J.; Shaw, J.; Kleber, S.; Ridley, A.; Wong-Baeza, I.; et al. Inhibiting HLA-B27 homodimer-driven immune cell inflammation in spondylarthritis. Arthritis Rheum. 2012, 64, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; Rysnik, O.; Kollnberger, S.; Shaw, J.; Utriainen, L.; Al-Mossawi, M.H.; Payeli, S.; Belaunzaran, O.M.; Milling, S.; Renner, C.; et al. Expression of aberrant HLA-B27 molecules is dependent on B27 dosage and peptide supply. Ann Rheum Dis. 2014, 73, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Chen, C.J.; Yen, J.H.; Ou, T.T.; Tsai, J.J.; Liu, C.S.; Liu, H.W. Free HLA class I heavy chain-carrying monocytes—A potential role in the pathogenesis of spondyloarthropathies. J. Rheumatol. 2002, 29, 966–972. [Google Scholar] [PubMed]
- Lan, C.C.; Tsai, W.C.; Wu, C.S.; Yu, C.L.; Yu, H.S. Psoriatic patients with arthropathy show significant expression of free HLA class I heavy chains on circulating monocytes: A potential role in the pathogenesis of psoriatic arthropathy. Br. J. Dermatol. 2004, 151, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Brown, D.; Bowness, P.; Hill Gaston, J.S.; Moffett, A.; Trowsdale, J.; Allen, R.L. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology 2006, 45, 1338–1344. [Google Scholar] [CrossRef]
- Santos, S.G.; Lynch, S.; Campbell, E.C.; Antoniou, A.N.; Powis, S.J. Induction of HLA-B27 heavy chain homodimer formation after activation in dendritic cells. Arthritis Res. Ther. 2008, 10, R100. [Google Scholar] [CrossRef]
- Boyson, J.E.; Erskine, R.; Whitman, M.C.; Chiu, M.; Lau, J.M.; Koopman, L.A.; Valter, M.M.; Angelisova, P.; Horejsi, V.; Strominger, J.L. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc. Natl. Acad. Sci. USA 2002, 99, 16180–16185. [Google Scholar] [CrossRef]
- Seitz, C.; Uchanska-Ziegler, B.; Zank, A.; Ziegler, A. The monoclonal antibody HCA2 recognises a broadly shared epitope on selected classical as well as several non-classical HLA class I molecules. Mol. Immunol. 1998, 35, 819–827. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Kaneku, H.; El-Awar, N.; Morales-Buenrostro, L.E.; Terasaki, P.I. Antibodies to HLA-E in non-alloimmunized Males: Pattern of HLA-Ia-reactivity of anti–HLA-E–positive sera. J. Immunol. 2010, 185, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F complex without peptide binds to MHC class I protein in the open conformer form. J. Immunol. 2010, 184, 6199–6208. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, C.; Löchte, S.; Hein, Z.; Kopicki, J.D.; Harders, A.R.; Linden, N.; Karner, A.; Preiner, J.; Weghuber, J.; Garcia-Alai, M.; et al. Dissociation of β2m from MHC class I triggers formation of noncovalent transient heavy chain dimers. J. Cell Sci. 2022, 135, jcs259489. [Google Scholar] [CrossRef]
- Giacomini, P.; Aguzzi, A.; Tecce, R.; Fisher, P.B.; Ferrone, S. A third polypeptide associated with heavy and light chain subunits of class I HLA antigens in immune interferon-treated human melanoma cells. Eur. J. Immunol. 1985, 15, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, P.; Beretta, A.; Nicotra, M.R.; Ciccarelli, G.; Martayan, A.; Cerboni, C.; Lopalco, L.; Bini, D.; Delfino, L.; Ferrara, G.B.; et al. HLA-C heavy chains free of beta2-microglobulin: Distribution in normal tissues and neoplastic lesions of non-lymphoid origin and interferon-y responsiveness. Tissue Antigens 1997, 50, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Marozzi, A.; Meneveri, R.; Bunone, G.; De Santis, C.; Lopalco, L.; Beretta, A.; Agresti, A.; Siccardi, A.G.; Della Valle, G.; Ginelli, E. Expression of beta 2m-free HLA class I heavy chains in neuroblastoma cell lines. Scand. J. Immunol. 1993, 37, 661–667. [Google Scholar] [CrossRef]
- Martayan, A.; Fiscella, M.; Setini, A.; Ciccarelli, G.; Gambari, R.; Feriotto, G.; Beretta, A.; Siccardi, A.G.; Appella, E.; Giacomini, P. Conformation and surface expression of free HLA-CW1 heavy chains in the absence of beta 2-microglobulin. Hum. Immunol. 1997, 53, 23–33. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Taniguchi, M.; Chen, C.W.; Ozawa, M.; Kaneku, H.; El-Awar, N.; Cai, J.; Terasaki, P.I. HLA-E monoclonal antibodies recognize shared peptide sequences on classical HLA class Ia: Relevance to human natural HLA antibodies. Mol. Immunol. 2010, 47, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Pham, T.; El-Awar, N.; Kaneku, H.; Terasaki, P.I. Anti-HLAE mAb 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: Web-tools validate the immunogenic epitopes of HLA-E recognized by the antibodies. Mol. Immunol. 2011, 48, 423–430. [Google Scholar] [CrossRef]
- Menier, C.; Saez, B.; Horejsi, V.; Martinozzi, S.; Krawice-Radanne, I.; Bruel, S.; Le Danff, C.; Reboul, M.; Hilgert, I.; Rabreau, M.; et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: New tools to analyze the expression of nonclassical HLA class I molecules. Hum. Immunol. 2003, 64, 315–326. [Google Scholar] [CrossRef]
- Lee, N.; Goodlett, D.R.; Ishitani, A.; Marquardt, H.; Geraghty, D.E. HLA-Esurface expression depends on binding of TAP-dependent peptides derived from certain HLAClass I signal sequences. J. Immunol. 1998, 160, 4951–4960. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Filippone, E.J.; Mahowald, G.; Callender, C.; Babu, A.; Saidman, S.; Ferrone, S. Significance of the intraindividual variability of HLA IgG antibodies in renal disease patients observed with different beadsets monitored with two different secondary antibodies on a Luminex platform. Immunol. Res. 2018, 66, 584–604. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Ravindranath, N.M.; Amato-Menker, C.J. Luminex Multiplex Bead Assay Monitoring HLA IgG Antibodies in Sensitized Pre- and Post-transplant Patients: Clonality of the Detection Antibody Impacts Specificity and Sensitivity. Appl. Sci. 2021, 11, 6430. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Hopfield, J.; Ferrone, S. HLA-E restricted monoclonal antibodies: Therapeutic potential as a double-edged sword against tumor progression. Internal Med. Rev. 2017, 3, 1–49. [Google Scholar]
- Sasaki, T.; Ravindranath, M.H.; Terasaki, P.I.; Freitas, M.C.; Kawakita, S.; Jucaud, V. Gastric cancer progression may involve a shift in HLA-E profile from an intact heterodimer to β2-microglobulin-free monomer. Int. J. Cancer 2014, 134, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Derré, L.; Corvaisier, M.; Charreau, B.; Moreau, A.; Godefroy, E.; Moreau-Aubry, A.; Jotereau, F.; Gervois, N. Expression and release of HLA-E by melanoma cells and melanocytes: Potential impact on the response of cytotoxic effector cells. J. Immunol. 2006, 177, 3100–3107. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; Oliveira, J.P.; Oliveira, C.F.; Silva, T.A.; Mendonça, E.F.; Wastowski, I.J.; Batista, A.C. Relevance of HLA-G, HLA-E and IL-10 expression in lip carcinogenesis. Hum. Immunol. 2016, 77, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.G.; Crispim, J.C.; Miranda, F.A.; Hassumi, M.K.; de Mello, J.M.; Simões, R.T.; Souto, F.; Soares, E.G.; Donadi, E.A.; Soares, C.P. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol. Histopathol. 2011, 26, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- van Esch, E.M.; Tummers, B.; Baartmans, V.; Osse, E.M.; Ter Haar, N.; Trietsch, M.D.; Hellebrekers, B.W.; Holleboom, C.A.; Nagel, H.T.; Tan, L.T.; et al. Alterations in classical and nonclassical HLA expression in recurrent and progressive HPV-induced usual vulvar intraepithelial neoplasia and implications for immunotherapy. Int. J. Cancer. 2014, 135, 830–842. [Google Scholar] [CrossRef]
- Djajadiningrat, R.S.; Horenblas, S.; Heideman, D.A.; Sanders, J.; de Jong, J.; Jordanova, E.S. Classic and nonclassic HLA class I expression in penile cancer and relation to HPV status and clinical outcome. J. Urol. 2015, 193, 1245–1251. [Google Scholar] [CrossRef]
- Mittelbronn, M.; Simon, P.; Löffler, C.; Capper, D.; Bunz, B.; Harter, P.; Schlaszus, H.; Schleich, A.; Tabatabai, G.; Goeppert, B.; et al. Elevated HLA-Elevels in human glioblastomas but not in grade I to III astrocytomas correlate with infiltrating CD8+ cells. J. Neuroimmunol. 2007, 189, 50–58. [Google Scholar] [CrossRef]
- Kren, L.; Muckova, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Smrcka, M.; Slaby, O.; Lakomy, R.; et al. Production of immune-modulatory nonclassical molecules HLA-G and HLA-E by tumor infiltrating ameboid microglia/macrophages in glioblastomas: A role in innate immunity? J. Neuroimmunol. 2010, 220, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kren, L.; Slaby, O.; Muckova, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Lakomy, R.; Vanhara, P.; et al. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: An unexpected prognostic significance? Neuropathology 2011, 31, 129–134. [Google Scholar] [CrossRef]
- Costa Arantes, D.A.; Gonçalves, A.; Jham, B.C.; Duarte, E.C.B.; de Paula, É.C.; de Paula, H.M.; Mendonça, E.F.; Batista, A.C. Evaluation of HLA-G, HLA-E, and PD-L1 proteins in oral osteosarcomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, e188–e196. [Google Scholar] [CrossRef]
- Mosconi, C.; Arantes, D.A.C.; Gonçalves, A.S.; Alencar, R.C.G.; Oliveira, J.C.; Silva, T.A.; Mendonça, E.F.; Batista, A.C. Immunohistochemical investigations on the expression of programmed cell death ligand 1, human leukocyte antigens G and E, and granzyme B in intraoral mucoepidermoid carcinoma. Arch. Oral. Biol. 2017, 83, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.S.; Engels, C.C.; Putter, H.; Morreau, H.; Liefers, G.J.; van de Velde, C.J.; Kuppen, P.J. Prognostic value of HLA class I, HLA-E, HLA-G and Tregs in rectal cancer: A retrospective cohort study. BMC Cancer 2014, 14, 486. [Google Scholar] [CrossRef]
- Benevolo, M.; Mottolese, M.; Tremante, E.; Rollo, F.; Diodoro, M.G.; Ercolani, C.; Sperduti, I.; Lo Monaco, E.; Cosimelli, M.; Giacomini, P. High expression of HLA-E in colorectal carcinoma is associated with a favorable prognosis. J. Transl. Med. 2011, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Zeestraten, E.C.; Reimers, M.S.; Saadatmand, S.; Goossens-Beumer, I.J.; Dekker, J.W.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.; Kuppen, P.J. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br. J. Cancer. 2014, 110, 459–468. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Lv, Y.G.; Wang, L.; Shi, S.J.; Yang, F.; Zheng, G.X.; Wen, W.H.; Yang, A.G. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell. Immunol. 2015, 293, 10–16. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, D.; Li, F.; Xiao, Z.; Wu, M.; Shi, D.; Xiang, P.; Bao, Z. Loss of Fas expression and high expression of HLA-E promoting the immune escape of early colorectal cancer cells. Oncol. Lett. 2017, 13, 3379–3386. [Google Scholar] [CrossRef]
- Chen, A.; Shen, Y.; Xia, M.; Xu, L.; Pan, N.; Yin, Y.; Miao, F.; Shen, C.; Xie, W.; Zhang, J. Expression of the nonclassical HLA class I and MICA/B molecules in human hepatocellular carcinoma. Neoplasma 2011, 58, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Talebian-Yazdi, M.; van Riet, S.; van Schadewijk, A.; Fiocco, M.; van Hall, T.; Taube, C.; Hiemstra, P.S.; van der Burg, S.H. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget 2016, 7, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- de Kruijf, E.M.; Sajet, A.; van Nes, J.G.; Natanov, R.; Putter, H.; Smit, V.T.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.; Kuppen, P.J. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J. Immunol. 2010, 185, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.B.; Silva, T.G.; Duarte, R.A.; Neto, N.L.; Carrara, H.H.; Donadi, E.A.; Gonçalves, M.A.; Soares, E.G.; Soares, C.P. Expression of the Classical and Nonclassical HLA Molecules in Breast Cancer. Int. J. Breast Cancer 2013, 2013, 250435. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.A.; Le Discorde, M.; Simões, R.T.; Rabreau, M.; Soares, E.G.; Donadi, E.A.; Carosella, E.D. Classical and non-classical HLA molecules and p16 (INK4a) expression in precursors lesions and invasive cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Spaans, V.M.; Peters, A.A.; Fleuren, G.J.; Jordanova, E.S. HLA-E expression in cervical adenocarcinomas: Association with improved long-term survival. J. Transl. Med. 2012, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Ferns, D.M.; Heeren, A.M.; Samuels, S.; Bleeker, M.C.G.; de Gruijl, T.D.; Kenter, G.G.; Jordanova, E.S. Classical and nonclassical HLA class I aberrations in primary cervical squamous- and adenocarcinomas and paired lymph node metastases. J. Immunother. Cancer 2016, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Poschke, I.; Villabona, L.; Carlson, J.W.; Lundqvist, A.; Kiessling, R.; Seliger, B.; Masucci, G.V. Non-classical HLA-class I expression in serous ovarian carcinoma: Correlation with the HLA-genotype, tumor infiltrating immune cells and prognosis. Oncoimmunology 2015, 5, e1052213. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, R.; Xie, S.; Wen, X.; Wang, H.; Gao, X.; Guo, L. Human leukocyte antigen-E alleles and expression in patients with serous ovarian cancer. Cancer Sci. 2015, 106, 522–548. [Google Scholar] [CrossRef]
- Hanak, L.; Slaby, O.; Lauerova, L.; Kren, L.; Nenutil, R.; Michalek, J. Expression pattern of HLA class I antigens in renal cell carcinoma and primary cell line cultures: Methodological implications for immunotherapy. Med. Sci. Monit. 2009, 15, CR638–CR643. [Google Scholar]
- Kren, L.; Valkovsky, I.; Dolezel, J.; Capak, I.; Pacik, D.; Poprach, A.; Lakomy, R.; Redova, M.; Fabian, P.; Krenova, Z.; et al. HLA-G and HLA-E specific mRNAs connote opposite prognostic significance in renal cell carcinoma. Diagn. Pathol. 2012, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, B.R.; Carvalho-Galano, D.F.; Feitosa, N.L.; Hassumi-Fukasawa, M.K.; Miranda-Camargo, F.A.; Maciel, L.M.; Ribeiro-Silva, A.; Soares, E.G. Differential expression of immunemodulatory molecule HLA-E in nonneoplastic and neoplastic lesions of the thyroid. Int. J. Immunopathol. Pharmacol. 2013, 26, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kren, L.; Fabian, P.; Slaby, O.; Janikova, A.; Soucek, O.; Sterba, J.; Krenova, Z.; Michalek, J.; Kral, Z. Multifunctional immune-modulatory protein HLA-E identified in classical Hodgkin lymphoma: Possible implications. Pathol. Res. Pract. 2012, 208, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Ruiz-Cabello, F.; Pedrinaci, S.; Méndez, R.; Jiménez, P.; Geraghty, D.E.; Garrido, F. Analysis of HLA-E expression in human tumors. Immunogenetics 2003, 54, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, F.; Roth, P.; Lamszus, K.; Tabatabai, G.; Weller, M.; Eisele, G. HLA-E contributes to an immune inhibitory phenotype of glioblastoma stem-like cells. J. Neuroimmunol. 2012, 250, 27–34. [Google Scholar] [CrossRef]
- Wischhusen, J.; Friese, M.A.; Mittelbronn, M.; Meyermann, R.; Weller, M. HLA-E protects glioma cells from NKG2D mediated immune responses in vitro: Implications for immune escape in vivo. J. Neuropathol. Exp. Neurol. 2005, 64, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Pozzi, S.; Carlini, B.; Amoroso, L.; Pistoia, V.; Corrias, M.V. Soluble HLA-G and HLA-E Levels in Bone Marrow Plasma Samples Are Related to Disease Stage in Neuroblastoma Patients. J. Immunol. Res. 2016, 2016, 7465741. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, E.M.; Mele, J.M.; Cheney, C.; Timmerman, E.A.; Fiazuddin, F.; Strattan, E.J.; Mo, X.; Byrd, J.C.; Muthusamy, N.; Awan, F.T. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology 2016, 5, e1226720. [Google Scholar] [CrossRef]
- Wagner, B.; da Silva Nardi, F.; Schramm, S.; Kraemer, T.; Celik, A.A.; Dürig, J.; Horn, P.A.; Dührsen, U.; Nückel, H.; Rebmann, V. HLA-E allelic genotype correlates with HLA-E plasma levels and predicts early progression in chronic lymphocytic leukemia. Cancer 2017, 123, 814–823. [Google Scholar] [CrossRef]
- Sensi, M.; Pietra, G.; Molla, A.; Nicolini, G.; Vegetti, C.; Bersani, I.; Millo, E.; Weiss, E.; Moretta, L.; Mingari, M.C.; et al. Peptides with dual binding specificity for HLA-A2 and HLA-E are encoded by alternatively spliced isoforms of the antioxidant enzyme peroxiredoxin 5. Int. Immunol. 2009, 21, 257–268. [Google Scholar] [CrossRef]
- Capps, G.G.; Robinson, B.E.; Lewis, K.D.; Zuniga, M.C. In vivo dimeric association of class I MHC heavy chains. Possible relationship to class I MHC heavy chain-beta 2-microglobulin dissociation. J. Immunol. 1993, 151, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Capps, G.G.; Zúñiga, M.C. The cytoplasmic domain of the H-2Ld class I major histocompatibility complex molecule is differentially accessible to immunological and biochemical probes during transport to the cell surface. J. Biol. Chem. 1993, 268, 21263–21270. [Google Scholar] [CrossRef] [PubMed]
- Lhotakova, K.; Grzelak, A.; Polakova, I.; Vackova, J.; Smahel, M. Establishment and characterization of a mouse tumor cell line with irreversible downregulation of MHC class I molecules. Oncol. Rep. 2019, 42, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Matko, J.; Rahman, N.A.; Barisas, B.G.; Edidin, M. Self-association of class I major histocompatibility complex molecules in liposome and cell surface membranes. Biochemistry 1992, 31, 7182–7189. [Google Scholar] [CrossRef] [PubMed]
- Matko, J.; Bushkin, Y.; Wei, T.; Edidin, M. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J. Immunol. 1994, 152, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M.; Achilles, S.; Zeff, R.; Wei, T. Probing the stability of class I major histocompatibility complex (MHC) molecules on the surface of human cells. Immunogenetics 1997, 46, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.L.; Bowness, P.; McMichael, A. The role of HLA-B27 in spondyloarthritis. Immunogenetics 1999, 50, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Gonen-Gross, T.; Achdout, H.; Gazit, R.; Hanna, J.; Mizrahi, S.; Markel, G.; Golcheman-Wohl, D.; Yagel, S.; Horejsí, V.; Levy, O.; et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J. Immunol. 2003, 171, 1343–1351. [Google Scholar] [CrossRef]
- Gonen-Gross, T.; Achdout, H.; Arnon, T.I.; Gazit, R.; Stern, N.; Horejsí, V.; Goldman-Wohl, D.; Yagel, S.; Mandelboim, O. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J. Immunol. 2005, 175, 4866–4874. [Google Scholar] [CrossRef]
- Dmjanovich, S.; Vereb, G.; Schaper, A.; Jenei, A.; Matkó, J.; Starink, P.; Fox, G.Q.; Arndt-Jovin, D.J.; Jovin, T.M. Structural hierarchy in the clustering of HLA class I molecules in the plasma membrane of human lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 1995, 92, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Bodnár, A.; Bacsó, Z.; Jenei, A.; Jovin, T.M.; Edidin, M.; Damjanovich, S.; Matkó, J. Class I HLA oligomerization at the surface of B cells is controlled by exogenous beta(2)-microglobulin: Implications in activation of cytotoxic T lymphocytes. Int. Immunol. 2003, 15, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.R.; Barthen, C.; Williamson, D.J.; Davies, D.M. HLA-B and HLA-C differ in their nanoscale organization at cell surfaces. Front. Immunol. 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Mear, J.P.; Schreiber, K.L.; Munz, C.; Zhu, X.; Stevanovi, S.; Rammensee, H.G.; Rowland-Hones, S.L.; Colbert, R.A. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 1999, 163, 6665–6670. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.A.; Peh, C.A.; Kollnberger, S.; Elliott, T.; McMichael, A.J.; Bowness, P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur. J. Immunol. 2003, 33, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Satumtira, N.; Dorris, M.L.; May, E.; Wang, A.; Furuta, E.; Taurog, J.D. HLA-B27in transgenic rats forms disulfide-linked heavy chain oligomers and multimers that bind to the chaperone BiP. J. Immunol. 2004, 172, 5110–5119. [Google Scholar] [CrossRef]
- van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Sadoul Exosomes are released by cultured cortical neurons. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Tenza, D.; Mecheri, S.; Peronet, R.; Bonnerot, C.; Desaymard, C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell 1997, 8, 2631–2645. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Wang, G.-J.; Liu, Y.; Qin, A.; Shah, S.V.; Deng, Z.-B.; Xiang, X.; Cheng, Z.; Liu, C.; Wang, J.; Zhang, L.; et al. Thymus exosomes-like particles induce regulatory T cells. J. Immunol. 2008, 181, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, J.; Chaput, N.; Zitvogel, L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit. Rev. Immunol. 2005, 25, 215–223. [Google Scholar] [CrossRef]
- Kim, S.H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Zitvogel Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Mignot, G.; Roux, S.; Thery, C.; Ségura, E.; Zitvogel, L. Prospects for exosomes in immunotherapy of cancer. J. Cell Mol. Med. 2006, 10, 376–388. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef]
- Lynch, S.; Santos, S.G.; Campbell, E.C.; Nimmo, A.M.S.; Botting, C.; Prescott, A.; Antoniou, A.N.; Powis, S.J. Novel MHC class I structures on exosomes. J. Immunol. 2009, 183, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Makhadiyeva, D.; Lam, L.; Moatari, M.; Vallance, J.; Zheng, Y.; Campbell, E.C.; Powis, S.J. MHC class I dimer formation by alteration of the cellular redox environment and induction of apoptosis. Immunology 2012, 135, 133–139. [Google Scholar] [CrossRef]
- Morales, P.J.; Pace, J.L.; Platt, J.S.; Langat, D.K.; Hunt, J.S. Synthesis of beta(2)-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology 2007, 122, 179–188. [Google Scholar] [CrossRef]
- Schreiber, A.B.; Schlessinger, J.; Edidin, M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J. Cell Biol. 1984, 98, 725–731. [Google Scholar] [CrossRef]
- Kittur, D.; Shimizu, Y.; DeMars, R.; Edidin, M. Insulin binding to human B lymphoblasts is a function of HLA haplotype. Proc. Natl. Acad. Sci. USA 1987, 84, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, T.S.; Chakrabarti, A.; Edidin, M. Interaction of class I human leukocyte antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol. Biol. Cell 1997, 8, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H.J.; van der Poel, J.J.; Castelli-Visser, R.; Pool, J.; Renbiao, C.; Keyu, X.; van Rood, J.J. Rood Interaction between des-Tyr1-gamma-endorphin and HLA class I molecules: Serological detection of an HLA-A2 subtype. Immunogenetics 1985, 22, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Navarrete, V.; Hämmerling, G.J. Surface appearance and instability of empty H-2 class I molecules under physiological conditions. Proc. Natl. Acad. Sci. USA 1991, 88, 3594–3597. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Hansen, T.H. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur. J. Immunol. 1994, 24, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Activating and inhibitory NK cell receptors. Adv. Exp. Med. Biol. 1998, 452, 13–18. [Google Scholar] [CrossRef]
- Lanier, L.L. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell 1998, 92, 705–707. [Google Scholar] [CrossRef]
- Debska-Zielkowska, J.; Moszkowska, G.; Zielinski, M.; Zielinska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefanska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Snyder, M.R.; Nakajima, T.; Leibson, P.J.; Weyand, C.M.; Goronzy, J.J. Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J. Immunol. 2004, 173, 3725–3731. [Google Scholar] [CrossRef]
- Snyder, M.R.; Weyand, C.M.; Goronzy, J.J. The double life of NK receptors: Stimulation or co-stimulation? Trends Immunol. 2004, 25, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. Specificity and function of immunoglobulin superfamily NK cell inhibitory and stimulatory receptors. Immunol. Rev. 1997, 155, 127–133. [Google Scholar] [CrossRef]
- Colonna, M. Immunoglobulin superfamily inhibitory receptors: From natural killer cells to antigen-presenting cells. Res. Immunol. 1997, 148, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Wagtmann, N.; Biassoni, R.; Cantoni, C.; Verdiani, S.; Malnati, M.S.; Vitale, M.; Bottino, C.; Moretta, L.; Moretta, A.; Long, E.O. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity 1995, 2, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, F.; Borrego, F.; Brooks, A.G.; Parker, K.C.; Coligan, J.E. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc. Natl. Acad. Sci. USA 1997, 94, 6313–6318. [Google Scholar] [CrossRef]
- Boyington, J.C.; Brooks, A.G.; Sun, P.D. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol. Rev. 2001, 181, 66–78. [Google Scholar] [CrossRef]
- Kollnberger, S.; Bird, L.; Sun, M.Y.; Retiere, C.; Braud, V.M.; McMichael, A.; Bowness, P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002, 46, 2972–2982. [Google Scholar] [CrossRef] [PubMed]
- Kollnberger, S.; Chan, A.; Sun, M.Y.; Chen, L.Y.; Wright, C.; di Gleria, K.; McMichael, A.; Bowness, P. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers, is independent of the sequence of bound peptide. Eur. J. Immunol. 2007, 37, 1313–1322. [Google Scholar] [CrossRef]
- Shaw, J.; Kollnberger, S. New perspectives on the ligands and function of the killer cell immunoglobulin-like receptor KIR3DL2 in health and disease. Front. Immunol. 2012, 3, 339. [Google Scholar] [CrossRef]
- Kollnberger, S.; Bird, L.A.; Roddis, M.; Hacquard-Bouder, C.; Kubagawa, H.; Bodmer, H.C.; Breban, M.; McMichael, A.J.; Bowness, P. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J. Immunol. 2004, 173, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Kollnberger, S.; Bowness, P. The role of B27 heavy chain dimer immune receptor interactions in spondyloarthritis. Adv. Exp. Med. Biol. 2009, 649, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.; Shaw, J.; Piper, C.; Wong-Baeza, I.; McHugh, K.; Ridley, A.; Li, D.; Lenart, I.; Antoniou, A.N.; DiGleria, K.; et al. HLA-B27 homodimers and free H chains are stronger ligands for leukocyte Ig-like receptor B2 than classical HLA class I. J. Immunol. 2012, 188, 6184–6193. [Google Scholar] [CrossRef] [PubMed]
- Wong-Baeza, I.; Ridley, A.; Shaw, J.; Hatano, H.; Rysnik, O.; McHugh, K.; Piper, C.; Brackenbridge, S.; Fernandes, R.; Chan, A.; et al. KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J. Immunol. 2013, 190, 3216–3224. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Hatano, H.; Kollnberger, S. The biochemistry and immunology of non-canonical forms of HLA-B27. Mol. Immunol. 2014, 57, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.N.; Wong, M.T.; Zhang, A.L.; Winer, D.; Suhoski, M.M.; Tolentino, L.L.; Gaitan, J.; Davidson, M.G.; Kung, T.H.; Galel, D.M.; et al. T(H)1, T(H)2, and T(H)17 cells instruct monocytes to differentiate into specialized dendritic cell subsets. Blood 2011, 118, 3311–3320. [Google Scholar] [CrossRef]
- Söderström, K.; Stein, E.; Colmenero, P.; Purath, U.; Müller-Ladner, U.; de Matos, C.T.; Tarner, I.H.; Robinson, W.H.; Engleman, E.G. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc. Natl. Acad. Sci. USA 2010, 107, 13028–13033. [Google Scholar] [CrossRef]
- Brown, D.; Trowsdale, J.; Allen, R. The LILR family: Modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 2004, 64, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Umikawa, M.; Cui, C.; Li, J.; Chen, X.; Zhang, C.; Huynh, H.; Kang, X.; Silvany, R.; Wan, X.; et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 2012, 485, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Hsu, M.L.; Fanger, N.; Kubin, M.; Cosman, D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 1997, 159, 5192–5196. [Google Scholar] [CrossRef]
- Tedla, N.; Lee, C.; Borges, L.; Geczy, C.L.; Arm, J.P. Differential expression of leukocyte immunoglobulin-like receptors on cord-blood-derived human mast cell progenitors and mature mast cells. J. Leukoc. Biol. 2008, 83, 334–343. [Google Scholar] [CrossRef]
- Jones, D.C.; Kosmoliaptsis, V.; Apps, R.; Lapaque, N.; Smith, I.; Kono, A.; Chang, C.; Boyle, L.H.; Taylor, C.J.; Trowsdale, J.; et al. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J. Immunol. 2011, 186, 2990–2997. [Google Scholar] [CrossRef]
- Zhang, Z.; Hatano, H.; Shaw, J.; Nordkamp, O.M.; Jiang, G.; Li, D.; Kollnberger, S. The Leukocyte Immunoglobulin-Like Receptor Family Member LILRB5 Binds to HLA-Class I Heavy Chains. PLoS ONE 2015, 10, e0129063. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Wei, X.; Lu, S.; Li, S.; Hashimoto, K.; Dijkstra, J.M.; Xia, C.J. The Structure of a Peptide-Loaded Shark MHC Class I Molecule Reveals Features of the Binding between β2-Microglobulin and H Chain Conserved in Evolution. J. Immunol. 2021, 207, 308–321. [Google Scholar] [CrossRef]
- Zijlstra, M.; Bix, M.; Simister, N.E.; Loring, J.A.; Raulet, D.H.; Jaenisch, R. Beta 2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature 1990, 344, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Bix, M.; Raulet, D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J. Exp. Med. 1992, 176, 829–834. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Grimholt, U. Major Histocompatibility Complex (MHC) Fragment Numbers Alone—In Atlantic Cod and in General—Do Not Represent Functional Variability. F1000Res 2018, 7, 963. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.M.; Grimholt, U.; Leong, J.; Koop, B.F.; Hashimoto, K. Comprehensive Analysis of MHC Class II Genes in Teleost Fish Genomes Reveals Dispensability of the Peptide-Loading DM System in a Large Part of Vertebrates. BMC Evol. Biol. 2013, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Grimholt, U.; Tsukamoto, K.; Azuma, T.; Leong, J.; Koop, B.F.; Dijkstra, J.M. A Comprehensive Analysis of Teleost MHC Class I Sequences. BMC Evol. Biol. 2015, 15, 32. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Yamaguchi, T.; Grimholt, U. Conservation of Sequence Motifs Suggests That the Nonclassical MHC Class I Lineages CD1/PROCR and UT Were Established Before the Emergence of Tetrapod Species. Immunogenetics 2018, 70, 459–476. [Google Scholar] [CrossRef]
- Dijkstra, J.M.; Yamaguchi, T. Ancient Features of the MHC Class II Presentation Pathway, and a Model for the Possible Origin of MHC Molecules. Immunogenetics 2019, 71, 233–249. [Google Scholar] [CrossRef]
- Kaufman, J.F.; Auffray, C.; Korman, A.J.; Shackelford, D.A.; Strominger, J. The Class II Molecules of the Human and Murine Major Histocompatibility Complex. Cell 1984, 36, 1–13. [Google Scholar] [CrossRef]
- Kaufman, J. Vertebrates and the Evolution of the Major Histocompatibility Complex (MHC) Class I and Class II Molecules. Verh. Dtsch. Zool. Ges. 1988, 81, 131–144. [Google Scholar]
- Kaufman, J.; Andersen, R.; Avila, D.; Engberg, J.; Lambris, J.; Salomonsen, J.; Welinder, K.; Skjødt, K. Different Features of the MHC Class I Heterodimer Have Evolved at Different -Rates. Chicken B-F and Beta 2-Microglobulin Sequences Reveal Invariant Surface Residues. J. Immunol. 1992, 148, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nakanishi, T.; Kurosawa, Y. Identification of a shark sequence resembling the major histocompatibility complex class I alpha 3 domain. Proc. Natl. Acad. Sci. USA 1992, 89, 2209–2212. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H. HLA Class Ia and Ib Polyreactive Anti-HLA-E IgG2a Monoclonal Antibodies (TFL-006 and TFL-007) Suppress Anti-HLA IgG Production by CD19+ B Cells and Proliferation of CD4+ T Cells While Upregulating Tregs. J. Immunol. Res. 2017, 2017, 3475926. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Ravindranath, N.M.; El Hilali, F.; Selvan, S.R.; Filippone, E.J. Ramifications of the HLA-I Allelic Reactivity of Anti-HLA-E*01:01 and Anti-HLA-E*01:03 Heavy Chain Monoclonal Antibodies in Comparison with Anti-HLA-I IgG Reactivityin Non-Alloimmunized Males, Melanoma-Vaccine Recipients, and End-Stage Renal Disease Patients. Antibodies 2022, 11, 18. [Google Scholar]
| HLA-Ia alleles | mAb MEM-E/02 | mAb 3D12 | HLA-Ia alleles | mAb MEM-E/02 | mAb 3D12 | HLA-Ia alleles | mAb MEM-E/02 | mAb 3D12 |
|---|---|---|---|---|---|---|---|---|
| A*01:01 | B*07:02 | 1910 | B*52:01 | 928 | 1416 | |||
| A*03:01 | 789 | B*08:01 | 1062 | B*53:01 | 2754 | |||
| A*11:01 | 2940 | B*13:01 | 5700 | 1820 | B*54:01 | 1910 | ||
| A*11:02 | 559 | B*13:02 | 1326 | 1171 | B*55:01 | 1287 | ||
| A*23:01 | 4096 | B*14:01 | 3135 | B*56:01 | 5352 | |||
| A*24:02 | 2505 | B*14:02 | 942 | B*57:01 | 3626 | 588 | ||
| A*24:03 | 629 | B*15:01 | 832 | B*57:03 | 2586 | 1143 | ||
| A*25:01 | 1593 | B*15:02 | 3250 | B*58:01 | 1636 | 823 | ||
| A*29:02 | 526 | B*15:03 | 4731 | B*59:01 | 2803 | |||
| A*30:01 | B*15:10 | 768 | B*67:01 | 704 | 1856 | |||
| A*30:02 | 603 | B*15:12 | 1903 | B*73:01 | 5560 | 659 | ||
| A*32:01 | 3037 | B*15:13 | 3400 | 591 | B*78:01 | 4273 | ||
| A*33:01 | 1604 | B*15:16 | B*81:01 | 1097 | 579 | |||
| A*33:03 | 991 | B*18:01 | 4392 | B*82:01 | 5295 | |||
| A*34:01 | 1219 | B*27:05 | 942 | |||||
| A*36:01 | 571 | B*27:08 | 1175 | 3264 | ||||
| A*66:01 | 664 | B*35:01 | 8716 | 566 | ||||
| A*68:01 | 917 | B*37:01 | 3444 | |||||
| A*69:01 | 3125 | B*38:01 | 968 | 1672 | ||||
| C*01:02 | 2567 | 966 | B*39:01 | 3010 | ||||
| C*02:02 | 1713 | 720 | B*40:01 | 3478 | 800 | |||
| C*03:02 | 2358 | B*40:02 | 2442 | 712 | ||||
| C*0303 | 2585 | 571 | B*40:06 | 9898 | 3216 | |||
| C*03:04 | 1765 | B*41:01 | 4987 | |||||
| C*04:03 | 9263 | 3796 | B*42:01 | |||||
| C*05:01 | 3076 | 931 | B*44:02 | 2621 | ||||
| C*06:02 | 6680 | B*44:03 | 2654 | 1321 | ||||
| C*07:02 | 2481 | 1640 | B*45:01 | 3134 | 604 | |||
| C*08:01 | 1692 | 592 | B*46:01 | 3042 | ||||
| C*12:03 | 1889 | 1020 | B*47:01 | 777 | ||||
| C*14:02 | 2688 | B*48:01 | 3577 | |||||
| C*15:02 | 1128 | B*49:01 | 1588 | |||||
| C*16:01 | 1869 | 530 | B*50:01 | 769 | ||||
| C*17:01 | 7779 | 5554 | B*51:01 | 2485 | 841 | |||
| C*18:02 | 4096 | 1095 | B*51:02 | 2303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindranath, M.H.; Ravindranath, N.M.; Amato-Menker, C.J.; Hilali, F.E.; Filippone, E.J. Conformational Alterations of the Cell Surface of Monomeric and Dimeric β2m-Free HLA-I (Proto-HLA) May Enable Novel Immune Functions in Health and Disease. Curr. Issues Mol. Biol. 2024, 46, 6961-6985. https://doi.org/10.3390/cimb46070416
Ravindranath MH, Ravindranath NM, Amato-Menker CJ, Hilali FE, Filippone EJ. Conformational Alterations of the Cell Surface of Monomeric and Dimeric β2m-Free HLA-I (Proto-HLA) May Enable Novel Immune Functions in Health and Disease. Current Issues in Molecular Biology. 2024; 46(7):6961-6985. https://doi.org/10.3390/cimb46070416
Chicago/Turabian StyleRavindranath, Mepur H., Narendranath M. Ravindranath, Carly J. Amato-Menker, Fatiha El Hilali, and Edward J. Filippone. 2024. "Conformational Alterations of the Cell Surface of Monomeric and Dimeric β2m-Free HLA-I (Proto-HLA) May Enable Novel Immune Functions in Health and Disease" Current Issues in Molecular Biology 46, no. 7: 6961-6985. https://doi.org/10.3390/cimb46070416
APA StyleRavindranath, M. H., Ravindranath, N. M., Amato-Menker, C. J., Hilali, F. E., & Filippone, E. J. (2024). Conformational Alterations of the Cell Surface of Monomeric and Dimeric β2m-Free HLA-I (Proto-HLA) May Enable Novel Immune Functions in Health and Disease. Current Issues in Molecular Biology, 46(7), 6961-6985. https://doi.org/10.3390/cimb46070416






