The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review
Abstract
1. Introduction
2. Method
2.1. Search Strategy
2.2. Study Selection, Study Quality, and Data Extraction
2.3. Inclusion Criteria
2.4. Exclusion Criteria
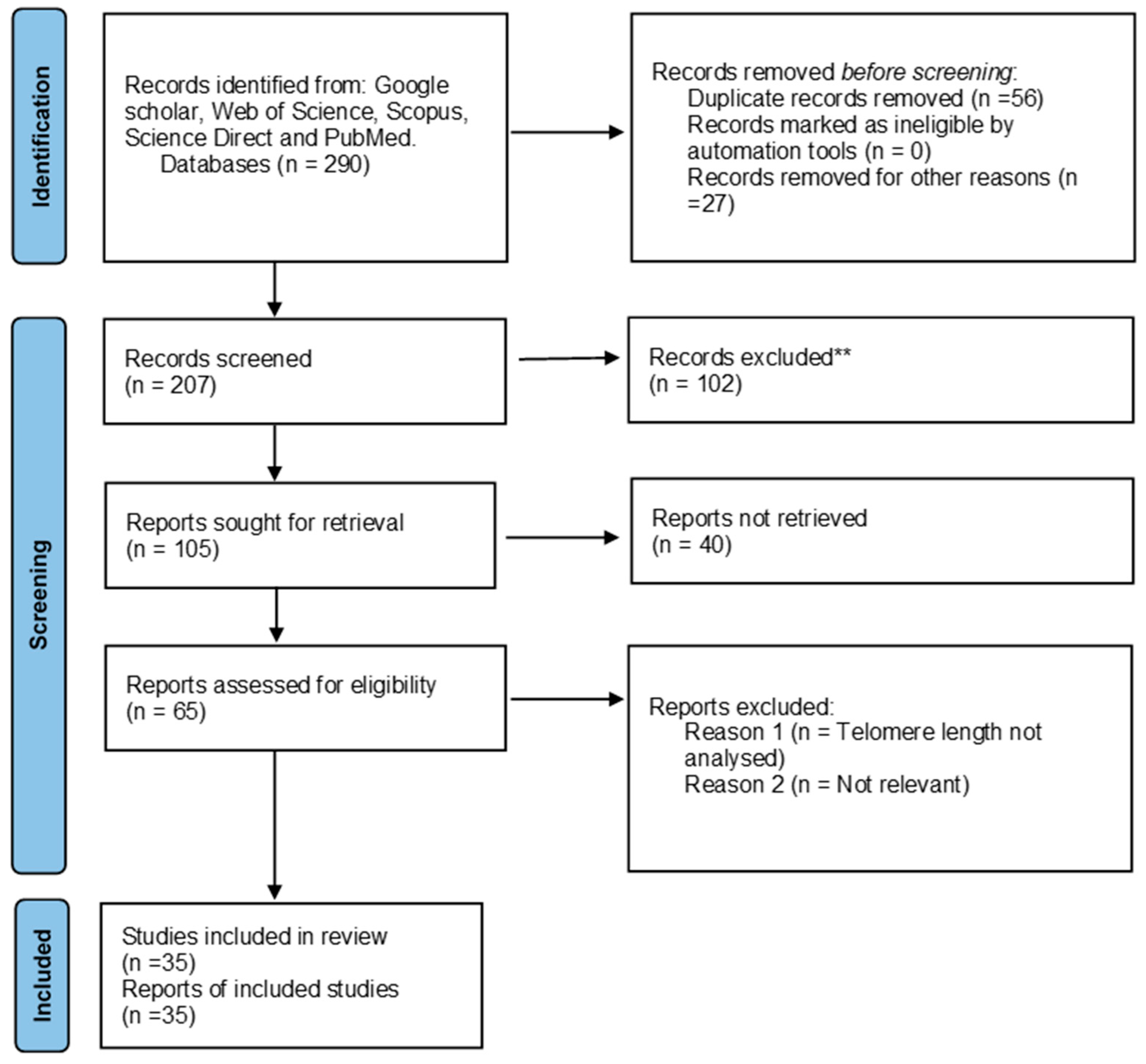
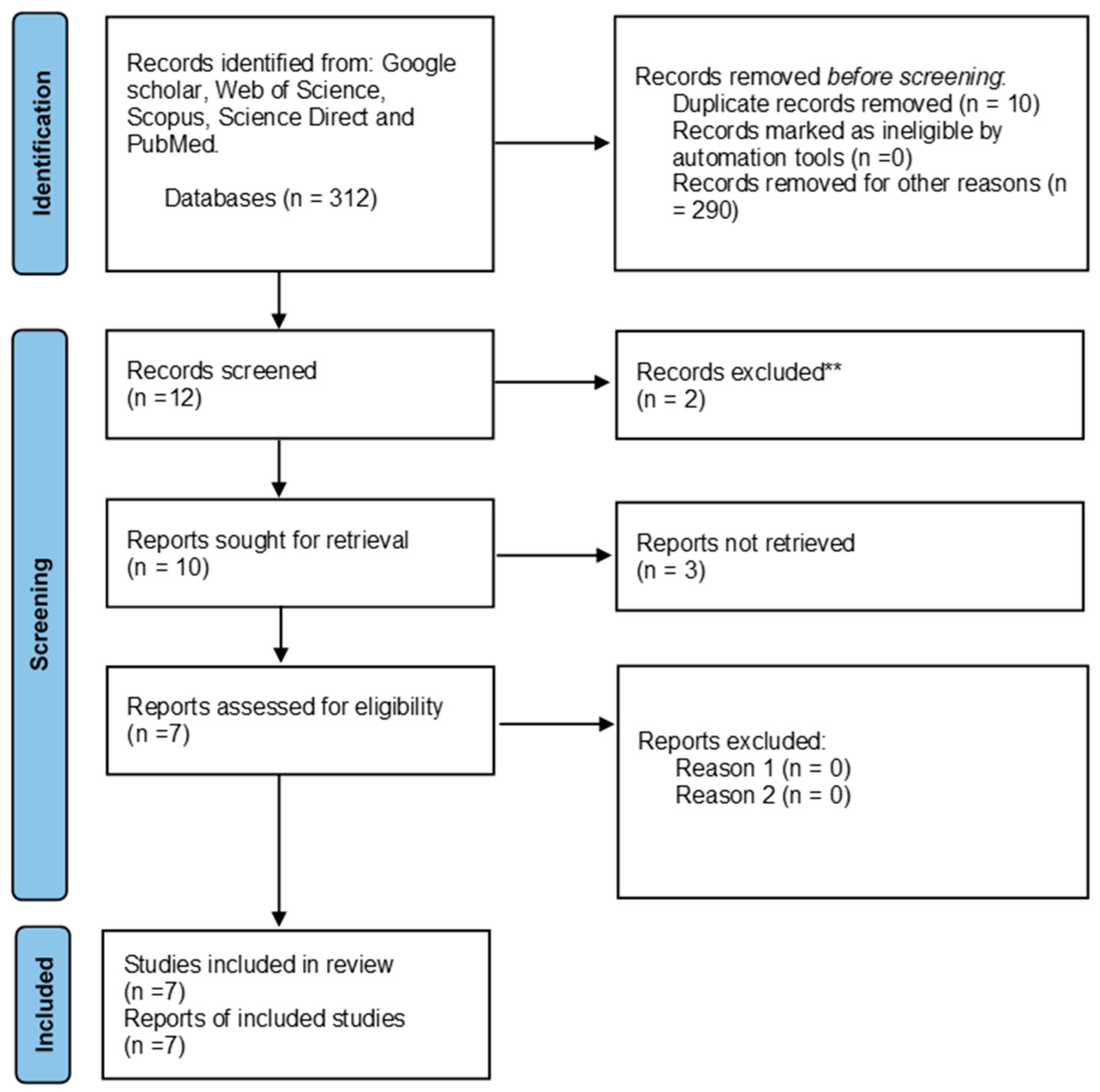
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, W.; Kowal, P.; Naidoo, N. Trends in Health and Well-Being of the Older Populations in SAGE Countries: 2014–2015. International Population Reports. 2018. Available online: https://www.researchgate.net/profile/Wan-He-5/publication/330205643_Trends_in_Health_and_Well-Being_of_the_Older_Populations_in_SAGE_Countries/links/5c33dcc792851c22a36382b2/Trends-in-Health-and-Well-Being-of-the-Older-Populations-in-SAGE-Countries.pdf (accessed on 9 March 2024).
- Gyasi, R.M.; Phillips, D.R.; Meeks, S. Aging and the Rising Burden of Noncommunicable Diseases in Sub-Saharan Africa and other Low-and Middle-Income Countries: A Call for Holistic Action. Gerontologist 2020, 60, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Annison, L.; Hackman, H.; Eshun, P.F.; Annison, S.; Forson, P.; Antwi-Baffour, S. Seroprevalence and effect of HBV and HCV co-infections on the immuno-virologic responses of adult HIV-infected persons on anti-retroviral therapy. PLoS ONE 2022, 17, e0278037. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013, 58, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Bettin, N.; Oss Pegorar, C.; Cusanelli, E. The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability. Cells 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.M.; Gussekloo, J.; Van Heemst, D.; Von Zglinicki, T.; Westendorp, R.G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell 2005, 4, 287–290. [Google Scholar] [CrossRef]
- Kong, C.M.; Lee, X.W.; Wang, X. Telomere shortening in human diseases. FEBS J. 2013, 280, 3180–3193. [Google Scholar] [CrossRef]
- Booth, J.S.; Toapanta, F.R. B and T cell immunity in tissues and across the ages. Vaccines 2021, 9, 24. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Ayavoo, T.; Murugesan, K.; Gnanasekaran, A. Roles and mechanisms of stem cell in wound healing. Stem Cell Investig. 2021, 8, 4. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, Z.; Liu, J.P. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef]
- Giraudeau, M.; Heidinger, B.; Bonneaud, C.; Sepp, T. Telomere shortening as a mechanism of long-term cost of infectious diseases in natural animal populations. Biol. Lett. 2019, 15, 20190190. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023, 8, 1–29. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.; Van Kessel, K.; Snippe, H. Immune Response in Human Pathology: Infections Caused by Bacteria, Viruses, Fungi, and Parasites. Nijkamp Parnham’s Princ. Immunopharmacol. 2019, 165–178. [Google Scholar] [CrossRef]
- Costa-Da-Silva, A.C.; Nascimento, D.D.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-De-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-De-Lima, C.G. Immune Responses in Leishmaniases: An Overview. Trop. Med. Infect. Dis. 2022, 7, 54. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and telomere length: A general overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. Int. J. Mol. Sci. 2019, 20, 4258. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Zhao, J.; Nguyen, L.N.T.; Dang, X.; Cao, D.; Khanal, S.; Schank, M.; Thakuri, B.K.C.; Ogbu, S.C.; Morrison, Z.D.; Wu, X.Y.; et al. ATM Deficiency Accelerates DNA Damage, Telomere Erosion, and Premature T Cell Aging in HIV-Infected Individuals on Antiretroviral Therapy. Front. Immunol. 2019, 10, 2531. [Google Scholar] [CrossRef] [PubMed]
- Dogan, F.; Forsyth, N.R. Telomerase Regulation: A Role for Epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Şerifoğlu, N.; Lopes-Bastos, B.; Ferreira, M.G. Lack of telomerase reduces cancer incidence and increases lifespan of zebrafish tp53M214K mutants. Sci. Rep. 2024, 14, 5382. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef]
- Rajendran, P.; Alzahrani, A.M.; Hanieh, H.N.; Kumar, S.A.; Ben Ammar, R.; Rengarajan, T.; Alhoot, M.A. Autophagy and senescence: A new insight in selected human diseases. J. Cell. Physiol. 2019, 234, 21485–21492. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Faivre, B. Inflammation and oxidative stress in vertebrate host–parasite systems. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 71–83. [Google Scholar] [CrossRef]
- Buckley, S.; Byrnes, S.; Cochrane, C.; Roche, M.; Estes, J.D.; Selemidis, S.; Angelovich, T.A.; Churchill, M.J. The role of oxidative stress in HIV-associated neurocognitive disorders. Brain Behav. Immun.-Health 2021, 13, 100235. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Meyerson, M. Telomerase activation, cellular immortalization and cancer. Ann. Med. 2001, 33, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh Dhakad, M. Study of TH1/TH2 Cytokine Profiles in HIV/AIDS Patients in a Tertiary Care Hospital in India. J. Med. Microbiol. Diagn. 2016, 5, 2–7. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.C.; Sehl, M.E.; Shih, R.; Langfelder, P.; Wang, R.; Horvath, S.; Bream, J.H.; Duggal, P.; Martinson, J.; Wolinsky, S.M.; et al. Accelerated aging with HIV begins at the time of initial HIV infection. iScience 2022, 26, 107381. [Google Scholar] [CrossRef]
- Montano, M.; Oursler, K.K.; Xu, K.; Sun, Y.V.; Marconi, V.C. Biological ageing with HIV infection: Evaluating the geroscience hypothesis. Lancet Health Longev. 2022, 3, e194–e205. [Google Scholar] [CrossRef]
- Sehl, M.E.; Breen, E.C.; Shih, R.; Chen, L.; Wang, R.; Horvath, S.; Bream, J.H.; Duggal, P.; Martinson, J.; Wolinsky, S.M.; et al. Increased Rate of Epigenetic Aging in Men Living with HIV Prior to Treatment. Front. Genet. 2022, 12, 796547. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, I.C.; Thorball, C.W.; Ledergerber, B.; Kootstra, N.A.; Reiss, P.; Raffenberg, M.; Engel, T.; Braun, D.L.; Hasse, B.; Thurnheer, C.; et al. Telomere Length Declines in Persons with Human Immunodeficiency Virus Before Antiretroviral Therapy Start but Not After Viral Suppression: A Longitudinal Study Over >17 Years. J. Infect. Dis. 2022, 225, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Iudicello, J.E.; Lin, J.; Ellis, R.J.; Morgan, E.; Okwuegbuna, O.; Cookson, D.; Karris, M.; Saloner, R.; Heaton, R.; et al. Telomere length is associated with HIV infection, methamphetamine use, inflammation, and comorbid disease risk. Drug Alcohol Depend. 2021, 221, 108639. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Fishbane, N.; Jones, M.; Morin, A.; Xu, S.; Liu, J.C.; MacIsaac, J.; Milloy, M.-J.; Hayashi, K.; Montaner, J.; et al. Longitudinal study of surrogate aging measures during human immunodeficiency virus seroconversion. Aging 2017, 9, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Zanet, D.L.; Thorne, A.; Singer, J.; Maan, E.J.; Sattha, B.; Le Campion, A.; Soudeyns, H.; Pick, N.; Murray, M.; Money, D.M.; et al. Association between short leukocyte telomere length and hiv infection in a cohort study: No evidence of a relationship with antiretroviral therapy. Clin. Infect. Dis. 2014, 58, 1322–1332. [Google Scholar] [CrossRef]
- Naveed, A.; Abdullah, S. Impact of parasitic infection on human gut ecology and immune regulations. Transl. Med. Commun. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Singh, R.; Bhengu, K.N.; Nembe-mafa, N.; Pillay, R.; Duma, Z.; Niehaus, A.J.; Mkhize-kwitshana, Z.L.; Africa, S.; et al. Immunological interaction during helminth and HIV co-infection: Integrative research needs for sub-Saharan Africa. S. Afr. J. Sci. 2023, 119, 16–19. [Google Scholar] [CrossRef]
- Koyasu, S.; Moro, K. Type 2 innate immune responses and the natural helper cell. Immunology 2011, 132, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, J.; Gantin, R.G.; Lechner, C.J.; Huang, X.; Agosssou, A.; Agbeko, Y.F.; Soboslay, P.T.; Köhler, C. Cellular cytokine and chemokine responses to parasite antigens and fungus and mite allergens in children co-infected with helminthes and protozoa parasites. J. Inflamm. 2015, 12, 5. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.-D. Immune responses against protozoan parasites: A focus on the emerging role of Nod-like receptors. Cell. Mol. Life Sci. 2016, 73, 3035–3051. [Google Scholar] [CrossRef]
- Stauffer, J.; Bruneaux, M.; Panda, B.; Visse, M.; Vasemägi, A.; Ilmonen, P. Telomere length and antioxidant defense associate with parasite-induced retarded growth in wild brown trout. Oecologia 2017, 185, 365–374. [Google Scholar] [CrossRef]
- Miglar, A.; Reuling, I.J.; Yap, X.Z.; Färnert, A.; Sauerwein, R.W.; Asghar, M. Biomarkers of cellular aging during a controlled human malaria infection. Sci. Rep. 2021, 11, 18733. [Google Scholar] [CrossRef] [PubMed]
- Sudyka, J.; Podmokła, E.; Drobniak, S.M.; Dubiec, A.; Arct, A.; Gustafsson, L.; Cichoń, M. Sex-specific effects of parasites on telomere dynamics in a short-lived passerine—The blue tit. Sci. Nat. 2019, 106, 1–8. [Google Scholar] [CrossRef]
- Asghar, M.; Palinauskas, V.; Zaghdoudi-Allan, N.; Valkiūnas, G.; Mukhin, A.; Platonova, E.; Färnert, A.; Bensch, S.; Hasselquist, D. Parallel telomere shortening in multiple body tissues owing to malaria infection. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161184. [Google Scholar] [CrossRef]
- Asghar, M.; Yman, V.; Homann, M.V.; Sondén, K.; Hammar, U.; Hasselquist, D.; Färnert, A. Cellular aging dynamics after acute malaria infection: A 12-month longitudinal study. Aging Cell 2018, 17, e12702. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Karell, P.; Bensch, S.; Ahola, K.; Asghar, M. Pale and dark morphs of tawny owls show different patterns of telomere dynamics in relation to disease status. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171127. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Bentwich, Z. HIV and helminth co-infection: Is deworming necessary? Parasite Immunol. 2006, 28, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Eggena, M.P.; Barugahare, B.; Okello, M.; Mutyala, S.; Jones, N.; Ma, Y.; Kityo, C.; Mugyenyi, P.; Cao, H. T cell activation in HIV-seropositive Ugandans: Differential associations with viral load, CD4+ T cell depletion, and coinfection. J. Infect Dis. 2005, 191, 694–701. [Google Scholar] [CrossRef][Green Version]
- Walson, J.L.; John-Stewart, G. Treatment of helminth co-infection in HIV-1 infected individuals in resource-limited settings. Cochrane Database Syst. Rev. 2008, CD006419. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.; Singh, R.; Mkhize-Kwitshana, Z.L. Anaemia and Nutritional Status during HIV and Helminth Coinfection among Adults in South Africa. Nutrients 2022, 14, 4970. [Google Scholar] [CrossRef]
- Brown, M.; Mawa, P.A.; Kaleebu, P.; Elliott, A.M. Helminths and HIV infection: Epidemiological observations on immunological hypotheses. Parasite Immunol. 2006, 28, 613–623. [Google Scholar] [CrossRef]
- Nissapatorn, V.; Sawangjaroen, N. Parasitic infections in HIV infected individuals: Diagnostic & therapeutic challenges. Indian J. Med. Res. 2011, 134, 878–897. [Google Scholar] [PubMed]
- Schmid-Hempel, P. Immune defence, parasite evasion strategies and their relevance for “macroscopic phenomena” such as virulence. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Meuser, M.E.; Cunanan, C.J.; Cocklin, S. Structure, Function, and Interactions of the HIV-1 Capsid Protein. Life 2021, 11, 100. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Nano, M.; Montell, D.J. Apoptotic signaling: Beyond cell death. Semin. Cell Dev. Biol. 2024, 156, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.-Y.; Lee, C.-Y. Inhibiting viral replication and prolonging survival of hosts by attenuating stress responses to viral infection. J. Invertebr. Pathol. 2022, 190, 107753. [Google Scholar] [CrossRef]
- Martinez-Picado, J.; Deeks, S. Persistent HIV-1 replication during antiretroviral therapy. Curr. Opin. HIV AIDS 2016, 11, 417–423. [Google Scholar] [CrossRef]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J. Immunol. Res. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pommier, J.P.; Gauthier, L.; Livartowski, J.; Galanaud, P.; Boué, F.; Dulioust, A.; Marcé, D.; Ducray, C.; Sabatier, L.; Lebeau, J.; et al. Immunosenescence in HIV pathogenesis. Virology 1997, 231, 148–154. [Google Scholar] [CrossRef]
- Kurashova, N.A.; Vanyarkina, A.S.; Petrova, A.G.; Rychkova, L.V.; Kolesnikov, S.I.; Darenskaya, M.A.; Moskaleva, E.V.; Kolesnikova, L.I. Length of leukocyte telomeres in newborn from HIV-infected mothers. Bull. Exp. Biol. Med. 2023, 175, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen, C.E.; Hsieh, A.Y.Y.; Albert, A.Y.; Kimmel, E.R.; Cote, H.C.F.; Maan, E.J.; Prior, J.C.; Pick, N.; Murray, M.C.M. Lower anti-Müllerian hormone levels are associated with HIV in reproductive age women and shorter leukocyte telomere length among late reproductive age women. Aids 2023, 37, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Bukic, E.; Milasin, J.; Toljic, B.; Jadzic, J.; Jevtovic, D.; Obradovic, B.; Dragovic, G. Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus. Biology 2023, 12, 1210. [Google Scholar] [CrossRef]
- Lombardi, F.; Sanfilippo, A.; Fabbiani, M.; Borghetti, A.; Ciccullo, A.; Tamburrini, E.; Di Giambenedetto, S. Blood telomere length gain in people living with HIV switching to dolutegravir plus lamivudine versus continuing triple regimen: A longitudinal, prospective, matched, controlled study. J. Antimicrob. Chemother. 2023, 78, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Raffenberg, M.; Engel, T.; Schoepf, I.C.; Kootstra, N.A.; Reiss, P.; Braun, D.L.; Thorball, C.W.; Fellay, J.; Kouyos, R.D.; Ledergerber, B.; et al. Impact of Delaying Antiretroviral Treatment During Primary Human Immunodeficiency Virus Infection on Telomere Length. J. Infect. Dis. 2021, 224, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Monnin, A.; Vizeneux, A.; Nagot, N.; Eymard-Duvernay, S.; Meda, N.; Singata-Madliki, M.; Ndeezi, G.; Tumwine, J.K.; Kankasa, C.; Goga, A.; et al. Longitudinal Follow-Up of Blood Telomere Length in HIV-Exposed Uninfected Children Having Received One Year of Lopinavir/Ritonavir or Lamivudine as Prophylaxis. Children 2021, 8, 796. [Google Scholar] [CrossRef]
- Dalzini, A.; Ballin, G.; Dominguez-Rodriguez, S.; Rojo, P.; Petrara, M.R.; Foster, C.; Cotugno, N.; Ruggiero, A.; Nastouli, E.; Klein, N.; et al. Size of HIV-1 reservoir is associated with telomere shortening and immunosenescence in early-treated European children with perinatally acquired HIV-1. J. Int. AIDS Soc. 2021, 24, e25847. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.Y.; Kimmel, E.; Pick, N.; Sauvé, L.; Brophy, J.; Kakkar, F.; Bitnun, A.; Murray, M.C.; Côté, H.C. Inverse relationship between leukocyte telomere length attrition and blood mitochondrial DNA content loss over time. Aging 2020, 12, 15196–15221. [Google Scholar] [CrossRef] [PubMed]
- Minami, R.; Takahama, S.; Yamamoto, M. Correlates of telomere length shortening in peripheral leukocytes of HIV-infected individuals and association with leukoaraiosis. PLoS ONE 2019, 14, e0218996. [Google Scholar] [CrossRef] [PubMed]
- Auld, E.; Lin, J.; Chang, E.; Byanyima, P.; Ayakaka, I.; Musisi, E.; Worodria, W.; Davis, J.L.; Segal, M.; Blackburn, E.; et al. HIV infection is associated with shortened telomere length in ugandans with suspected tuberculosis. PLoS ONE 2016, 11, e0163153. [Google Scholar] [CrossRef] [PubMed]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Akusjärvi, S.S.; Palaniappan, A.N.; Sundaraj, V.; Mupanni, N.R.; Sperk, M.; Cheedarla, N.; Sridhar, R.; et al. Systemic Inflammation and the Increased Risk of Inflamm-Aging and Age-Associated Diseases in People Living with HIV on Long Term Suppressive Antiretroviral Therapy. Front. Immunol. 2019, 10, 1965. [Google Scholar] [CrossRef] [PubMed]
- Alejos, B.; Stella-Ascariz, N.; Montejano, R.; Rodriguez-Centeno, J.; Schwimmer, C.; Bernardino, J.I.; Rodes, B.; Esser, S.; Goujard, C.; Sarmento-Castro, R.; et al. Determinants of blood telomere length in antiretroviral treatment-naïve HIV-positive participants enrolled in the NEAT 001/ANRS 143 clinical trial. HIV Med. 2019, 20, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Stella-Ascariz, N.; Montejano, R.; Rodriguez-Centeno, J.; Alejos, B.; Schwimmer, C.; Bernardino, J.I.; Rodes, B.; Allavena, C.; Hoffmann, C.; Gisslén, M.; et al. Blood Telomere Length Changes After Ritonavir-Boosted Darunavir Combined with Raltegravir or Tenofovir-Emtricitabine in Antiretroviral-Naive Adults Infected with HIV-1. J. Infect. Dis. 2018, 218, 1523–1530. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-Garcia, J.; et al. Impact of Nucleos(t)ide Reverse Transcriptase Inhibitors on Blood Telomere Length Changes in a Prospective Cohort of Aviremic HIV-Infected Adults. J. Infect. Dis. 2018, 218, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, S.; Pick, N.; Mai, A.; Murray, M.C.M.; Kidson, K.; Chu, J.; Albert, A.Y.K.; Côté, H.C.F.; Maan, E.J.; Goshtasebi, A.; et al. Premature Spinal Bone Loss in Women Living with HIV is Associated with Shorter Leukocyte Telomere Length. Int. J. Environ. Res. Public Health 2018, 15, 1018. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.; Strehlau, R.; Shen, J.; Violari, A.; Patel, F.; Liberty, A.; Foca, M.; Wang, S.; Terry, M.B.; Yin, M.T.; et al. Biomarkers of Aging in HIV-Infected Children on Suppressive Antiretroviral Therapy. Am. J. Ther. 2018, 78, 549–556. [Google Scholar] [CrossRef]
- Gianesin, K.; Noguera-Julian, A.; Zanchetta, M.; Del Bianco, P.; Petrara, M.R.; Freguja, R.; Rampon, O.; Fortuny, C.; Camós, M.; Mozzo, E.; et al. Premature aging and immune senescence in HIV-infected children. AIDS 2016, 30, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Jimnez, V.C.; Wit, F.W.N.M.; Joerink, M.; Maurer, I.; Harskamp, A.M.; Schouten, J.; Prins, M.; Van Leeuwen, E.M.M.; Booiman, T.; Deeks, S.G.; et al. T-Cell Activation Independently Associates with Immune Senescence in HIV-Infected Recipients of Long-term Antiretroviral Treatment. J. Infect. Dis. 2016, 214, 216–225. [Google Scholar]
- Blanco, J.R.; Jarrin, I.; Martinez, A.; Siles, E.; Larrayoz, I.M.; Canuelo, A.; Gutierrez, F.; Gonzalez-Garcia, J.; Vidal, F.; Moreno, S. Shorter telomere length predicts poorer immunological recovery in virologically suppressed hiv-1-infected patients treated with combined antiretroviral therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 68, 21–29. [Google Scholar] [CrossRef]
- Srinivasa, S.; Fitch, K.V.M.; Petrow, E.B.; Burdo, T.H.; Williams, K.C.; Lo, J.; Côté, H.C.F.; Grinspoon, S.K. Soluble CD163 Is Associated with Shortened Telomere Length in HIV-Infected Patients. Am. J. Ther. 2014, 67, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Malan-Müller, S.; Hemmings, S.M.J.; Spies, G.; Kidd, M.; Fennema-Notestine, C.; Seedat, S. Correction: Shorter Telomere Length—A Potential Susceptibility Factor for HIV-Associated Neurocognitive Impairments in South African Woman. PLoS ONE 2013, 8, e58351. [Google Scholar] [CrossRef] [PubMed]
- Leeansyah, E.; Cameron, P.U.; Solomon, A.; Tennakoon, S.; Velayudham, P.; Gouillou, M.; Spelman, T.; Hearps, A.; Fairley, C.; Smit, D.V.; et al. Inhibition of Telomerase Activity by Human Immunodeficiency Virus (HIV) Nucleos(t)ide Reverse Transcriptase Inhibitors: A Potential Factor Contributing to HIV-Associated Accelerated Aging. J. Infect. Dis. 2013, 207, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Pathai, S.; Lawn, S.D.; Gilbert, C.E.; McGuinness, D.; McGlynn, L.; Weiss, H.A.; Port, J.; Christ, T.; Barclay, K.; Wood, R.; et al. Accelerated biological ageing in HIV-infected individuals in South Africa: A case-control study. Aids 2013, 27, 2375–2384. [Google Scholar] [CrossRef]
- Côté, H.C.F.; Soudeyns, H.; Thorne, A.; Alimenti, A.; Lamarre, V.; Maan, E.J.; Sattha, B.; Singer, J.; Lapointe, N.; Money, D.M.; et al. Leukocyte Telomere Length in HIV-Infected and HIV-Exposed Uninfected Children: Shorter Telomeres with Uncontrolled HIV Viremia. PLoS ONE 2012, 7, e39266. [Google Scholar] [CrossRef] [PubMed]
- Bestilny, L.J.; Gill, M.J.; Mody, C.H.; Riabowol, K.T. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS 2000, 14, 771–780. [Google Scholar] [CrossRef]
- Wolthers, K.C.; Noest, A.J.; Otto, S.A.; Miedema, F.; De Boer, R.J. Normal Telomere Lengths in Naive and Memory CD4+ T Cells in HIV Type 1 Infection: A Mathematical Interpretation. AIDS Res. Hum. Retroviruses 1999, 15, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Landay, A.L.; Lederman, M.M.; Connick, E.; Spritzler, J.; Kuritzkes, D.R.; Kessler, H.; Levine, B.L.; Louis, D.C.S.; June, C.H. Increases in T Cell Telomere Length in HIV Infection after Antiretroviral Combination Therapy for HIV-1 Infection Implicate Distinct Population Dynamics in CD4+ and CD8+ T Cells. Clin. Immunol. 1999, 92, 14–24. [Google Scholar] [CrossRef][Green Version]
- Palmer, L.D.; Weng, N.P.; Levine, B.L.; June, C.H.; Lane, H.C.; Hodes, R.J. Telomere length, telomerase activity, and replicative potential in HIV infection: Analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 1997, 185, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Centeno, J.; Esteban-Cantos, A.; Montejano, R.; Stella-Ascariz, N.; De Miguel, R.; Mena-Garay, B.; Saiz-Medrano, G.; Alejos, B.; Jiménez-González, M.; Bernardino, J.I.; et al. Effects of tenofovir on telomeres, telomerase and T cell maturational subset distribution in long-term aviraemic HIV-infected adults. J. Antimicrob. Chemother. 2022, 77, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, R.W.; Roestenberg, M.; Moorthy, V.S. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat. Rev. Immunol. 2011, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- CLSI H20-A2; Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Methods; Approved Standard-Second Edition. Clinical And Laboratory Standards Institute: Wayne, PA, USA, 2007.
- Paiardini, M.; Müller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef]
- Hukezalie, K.R.; Thumati, N.R.; Côté, H.C.F.; Wong, J.M.Y. In Vitro and Ex Vivo Inhibition of Human Telomerase by Anti-HIV Nucleoside Reverse Transcriptase Inhibitors (NRTIs) but Not by Non-NRTIs. PLoS ONE 2012, 7, e47505. [Google Scholar] [CrossRef]
| Aims of the Study | Methodology | Overall Findings | Reference |
|---|---|---|---|
| To examine the relative length of telomeric duplicates in infants with unrecognized perinatal HIV infection. |
|
| [72] |
| To investigate the relationship between leukocyte telomere length (LTL) and anti-Müllerian hormone (AMH) in order to better describe the relative contribution of clinical and sociodemographic variables to ovarian aging in women with HIV (WWH). |
|
| [73] |
| To evaluate the influence of specific HIV-related variables on patients’ relative telomere length (RTL) and to compare patients’ RTL within and between different cART classes. |
|
| [74] |
| To examine the impact on blood telomere length (BTL) after a year of transitioning from maintaining a standard triple therapy (TT) with an anchor medication and a two-NRTI backbone to a dual therapy (DT) with dolutegravir + lamivudine. |
|
| [75] |
| To determine if immunological reconstitution interference or telomerase inhibition is the cause of the adverse effects of tenofovir on telomere length (TL). |
|
| [100] |
| To investigate the pace of change in epigenetic age in PLWH after HIV infection but before startingActive Antiretroviral Therapy (HAART). |
|
| [41] |
| To determine the influence of clinical and HIV-related factors, such as specific ARVs, on longitudinal TL dynamics. |
|
| [42] |
| To study the effect of HIV and methamphetamine (METH) on leukocyte telomere lengths (LTLs) and the relationships between LTL and other aging biomarkers. |
|
| [43] |
| To assess any independent relationships between the initiation of ART and TL in individuals in the Zurich Primary HIV Infection Study (ZPHI) who have documented primary HIV infection (PHI), and to calculate the effect of starting ART early in comparison to other variables that are known to be associated with TL, like age. |
|
| [76] |
| To assess the acute and long-term effects on telomere shortening of two ARV prophylaxes: lopinavir/ritonavir (LPV/r) and lamivudine (3TC). |
|
| [77] |
| To research how the biological and immunological aging profile in PHIV is related to the HIV-1 reservoir. |
|
| [78] |
| To investigate the association between leukocyte telomere length (LTL) and whole-blood mitochondrial DNA (WB mtDNA) content in a group of HIV-positive and HIV-negative girls and women, both cross-sectionally and longitudinally. |
|
| [79] |
| To assess biological and environmental determinants of telomere length and assess peripheral leukocytes in HIV+ and healthy people. |
|
| [80] |
| To examine the factors linked to the baseline blood telomere length of participants enrolled in this randomized, open-label trial that compared ritonavir-boosted darunavir (DRV/r) plus raltegravir (RAL) with DRV/r plus tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in adults HIV-positive patients naïve to antiretroviral therapy (ART). |
|
| [83] |
| To assess the degree of systemic inflammation and comprehend the risk of age-associated illnesses in PLHIV on long-term suppressive ART by using a wide range of biomarkers for inflammation and immunological activation. |
|
| [82] |
| To assess blood TL changes in an adult ART-naïve population in a sub-study of the NEAT001/ANRS 143 clinical trial comparing ritonavir-boosted darunavir with raltegravir or tenofovir disoproxil fumarate/emtricitabine. |
|
| [84] |
| To examine how abacavir and tenofovir disoproxil fumarate (TDF) affected the length of blood telomeres in persons with HIV infection who were undergoing sustained virological suppression. |
|
| [85] |
| To investigate novel biological variables associated with poor bone mineral density (BMD) in women living with HIV (WLWH). |
|
| [86] |
| To evaluate two aging biomarkers, DNA methylation (DNAm) age and telomere length, in a cohort of children with HIV infection who received early treatment, and to compare these aging biomarkers to those of children who were HIV-exposed uninfected (HEU) and HIV-unexposed uninfected (HUU). |
|
| [87] |
| To determine the possible onset of age acceleration in HIV and outline the major biological pathways that may be disrupted during the HIV seroconversion phase. |
|
| [44] |
| To examine whether shortened telomere length was associated with HIV infection, tuberculosis diagnosis, and 2-month death in a single cohort of HIV-infected and HIV-uninfected adults hospitalized with pneumonia. |
|
| [81] |
| To investigate biological aging in connection to immunological activation and senescence markers in a perinatally HIV-infected kid cohort. |
|
| [88] |
| To investigate if T-cell activation in HIV-positive patients undergoing long-term antiretroviral treatment is associated with immunological senescence. |
|
| [89] |
| To investigate the possible association between TL and immunological response 48 weeks after cART initiation and the function of OS and nitrosative stress in HIV-1 immunorecovery following cART. |
|
| [90] |
| To assess the correlation between TL and immunological activation markers in a group of males who were either HIV-positive or not. |
|
| [91] |
| To investigate LTL in the population infected with HIV and HCV and factors associated with a shorter LTL. |
|
| [45] |
| To examine whether HIV infection and chronic stress associated with childhood trauma affect the length of the telomere and whether leukocyte TL (LTL) in particular is a risk factor for NCI. |
|
| [92] |
| To investigate the effects of the more widely used nucleoside reverse transcriptase inhibitors (NRTIs) on TL and telomerase activity in vitro in activated PBMCs and ex vivo in PBMCs from persons using NRTIs who were HIV-infected and HIV-uninfected. |
|
| [93] |
| To examine whether there is evidence that HIV-infected people have advanced biological aging relative to HIV-seronegative people by comparing the length of the telomere and CDKN2A expression. |
|
| [94] |
| To examine the potential effects of nucleoside reverse transcriptase inhibitor (NRTI) exposure during pregnancy or youth on leukocyte telomere length (LTL), a measure of cellular aging. |
|
| [95] |
| To examine telomere length in blood cell populations as an indicator of replicative history in a large number of HIV patients. |
|
| [96] |
| To examine if the telomere length data may still permit high turnover rates in CD4+ T cells, which would deplete renewal and ultimately result in the depletion of CD4+ T cells. |
|
| [97] |
| To investigate TRF variations as a measure of T-cell replicative history and relate these to long-term variations in lymphocyte subpopulations after the start of prospective antiretroviral therapy. |
|
| [98] |
| To measure the length of the telomeres in peripheral blood mononuclear cells (PBMCs) from HIV-positive individuals in order to assess the effect of HIV infection on PBMC mitotic division and determine whether cellular senescence may be involved in immunological suppression. |
|
| [71] |
| To investigate whether HIV infection causes telomere length variations among T-cell subpopulations as a reflection of these cells’ history of replication and whether the remaining potential for T-cell replication is changed in infected individuals. |
|
| [99] |
| Aims of the Study | Methodology | Overall Findings | Reference |
|---|---|---|---|
| * To analyze the relationship between telomere length, cellular senescence, telomerase expression and aging-related activities during a single malaria infection. |
|
| [52] |
| To investigate the association between blood parasite infection (from the genera Plasmodium and Haemoproteus) and telomere length (TL) in a natural population of the blue tit (Cyanistes caeruleus). |
|
| [53] |
| * To investigate the impact of a single acute Plasmodium falciparum malaria infection on the cellular aging dynamics of tourists. |
|
| [55] |
| To examine the possible functions of TL and antioxidant (AO) defense in T. bryosalmonae-infected fish, as well as their correlations with parasite burden and disease severity. |
|
| [51] |
| To investigate the effect of blood parasitic hemorrhage on telomere dynamics in tawny owls. |
|
| [57] |
| To determine whether malaria infection causes parallel telomere shortening in blood and tissue samples from various avian organs. |
|
| [54] |
| To determine the potential long-term effects of avian malaria in birds. |
|
| [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macamo, E.D.; Mkhize-Kwitshana, Z.L.; Mthombeni, J.; Naidoo, P. The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review. Curr. Issues Mol. Biol. 2024, 46, 7258-7290. https://doi.org/10.3390/cimb46070431
Macamo ED, Mkhize-Kwitshana ZL, Mthombeni J, Naidoo P. The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review. Current Issues in Molecular Biology. 2024; 46(7):7258-7290. https://doi.org/10.3390/cimb46070431
Chicago/Turabian StyleMacamo, Engelinah D., Zilungile L. Mkhize-Kwitshana, Julian Mthombeni, and Pragalathan Naidoo. 2024. "The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review" Current Issues in Molecular Biology 46, no. 7: 7258-7290. https://doi.org/10.3390/cimb46070431
APA StyleMacamo, E. D., Mkhize-Kwitshana, Z. L., Mthombeni, J., & Naidoo, P. (2024). The Impact of HIV and Parasite Single Infection and Coinfection on Telomere Length: A Systematic Review. Current Issues in Molecular Biology, 46(7), 7258-7290. https://doi.org/10.3390/cimb46070431






