Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment
Abstract
1. Introduction
2. Pathogenesis
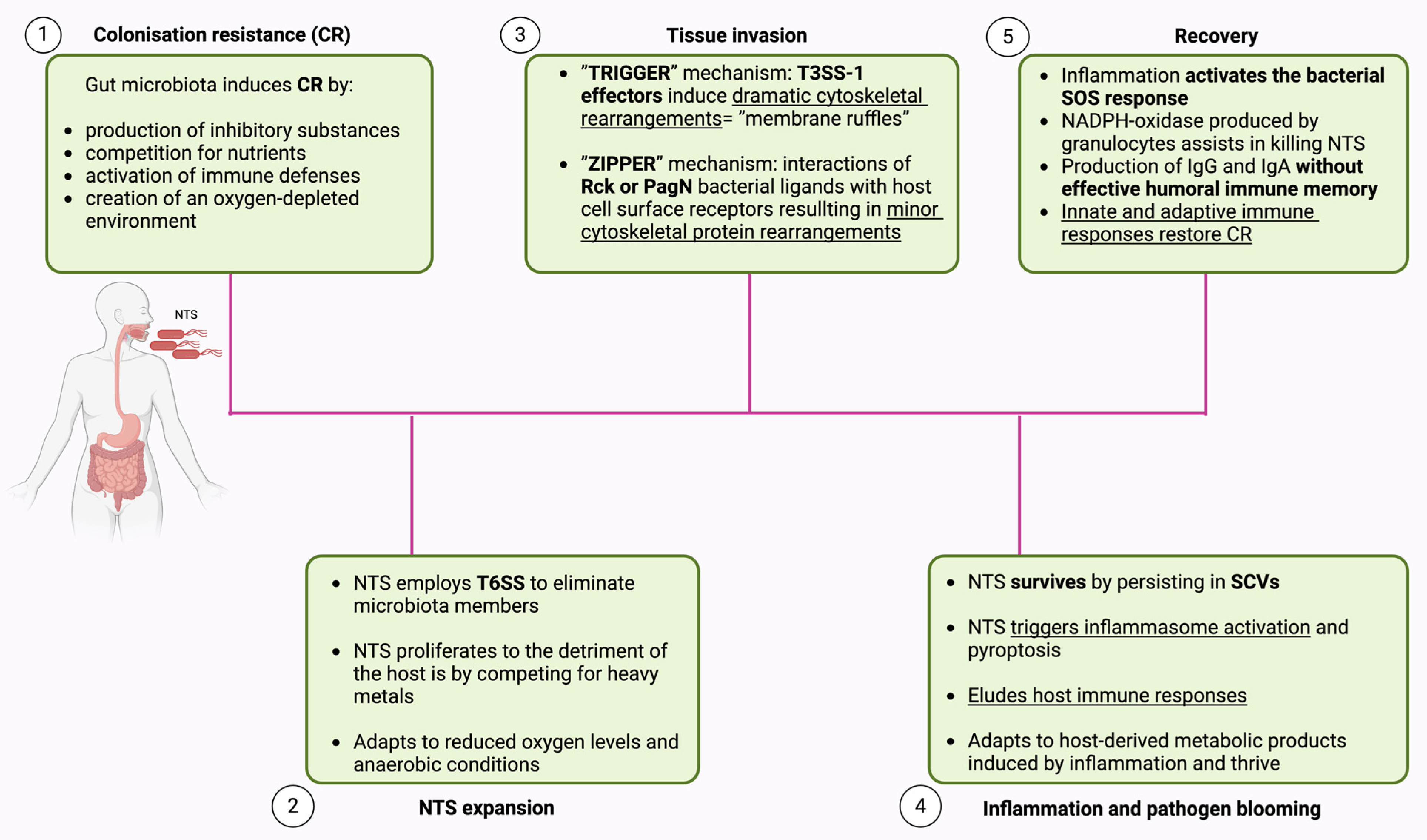
2.1. Colonization Resistance (CR)
2.2. NTS Expansion
2.3. Tissue Invasion
2.4. Inflammation and Pathogen Blooming
2.5. Recovery
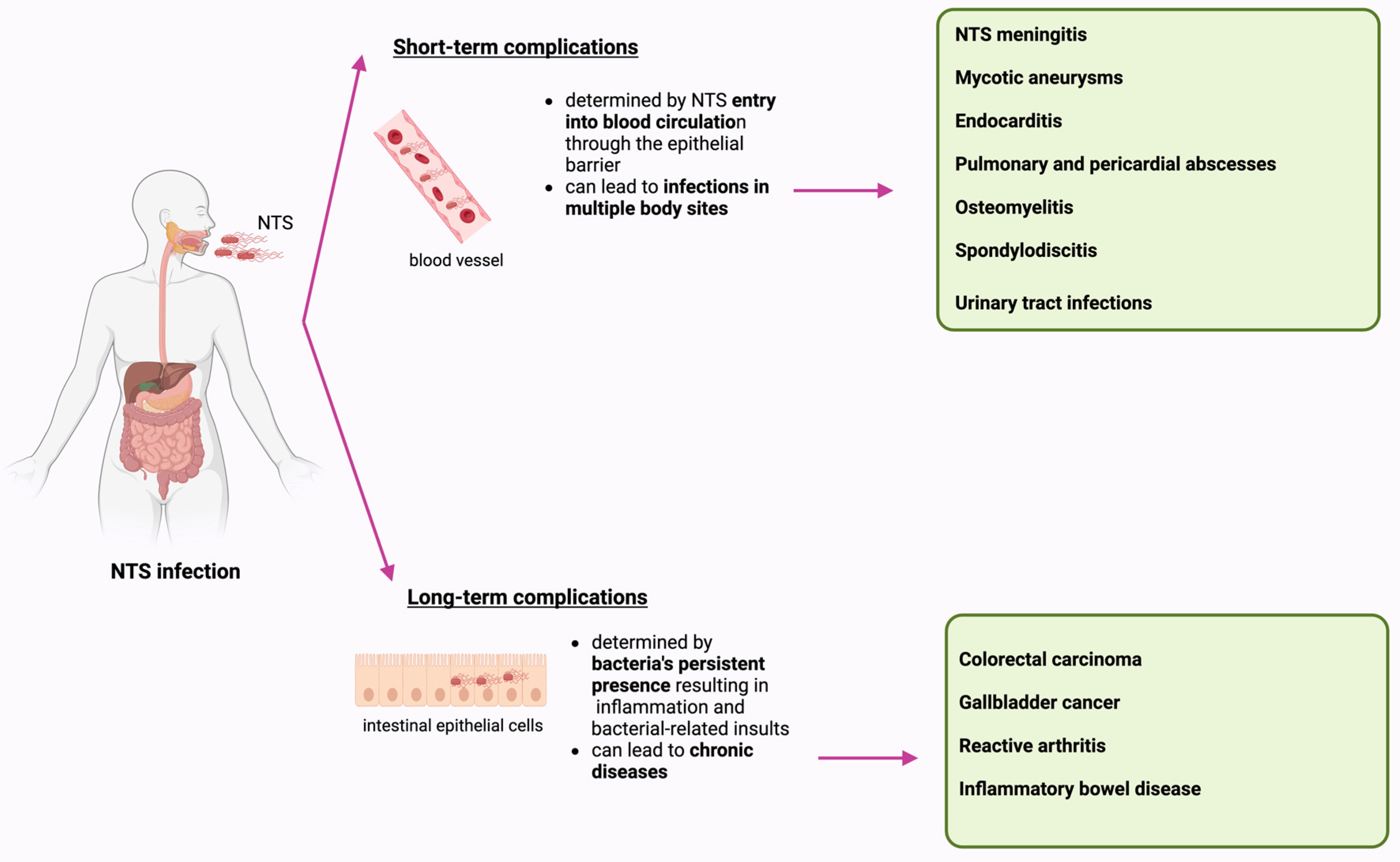
3. Complications Associated with NTS Infection
3.1. Short-Term Complications
3.2. Long-Term Complications
3.2.1. Colorectal Carcinoma
3.2.2. Gallbladder Cancer (GBC)
3.2.3. Reactive Arthritis (ReA)
3.2.4. Inflammatory Bowel Disease (IBD)
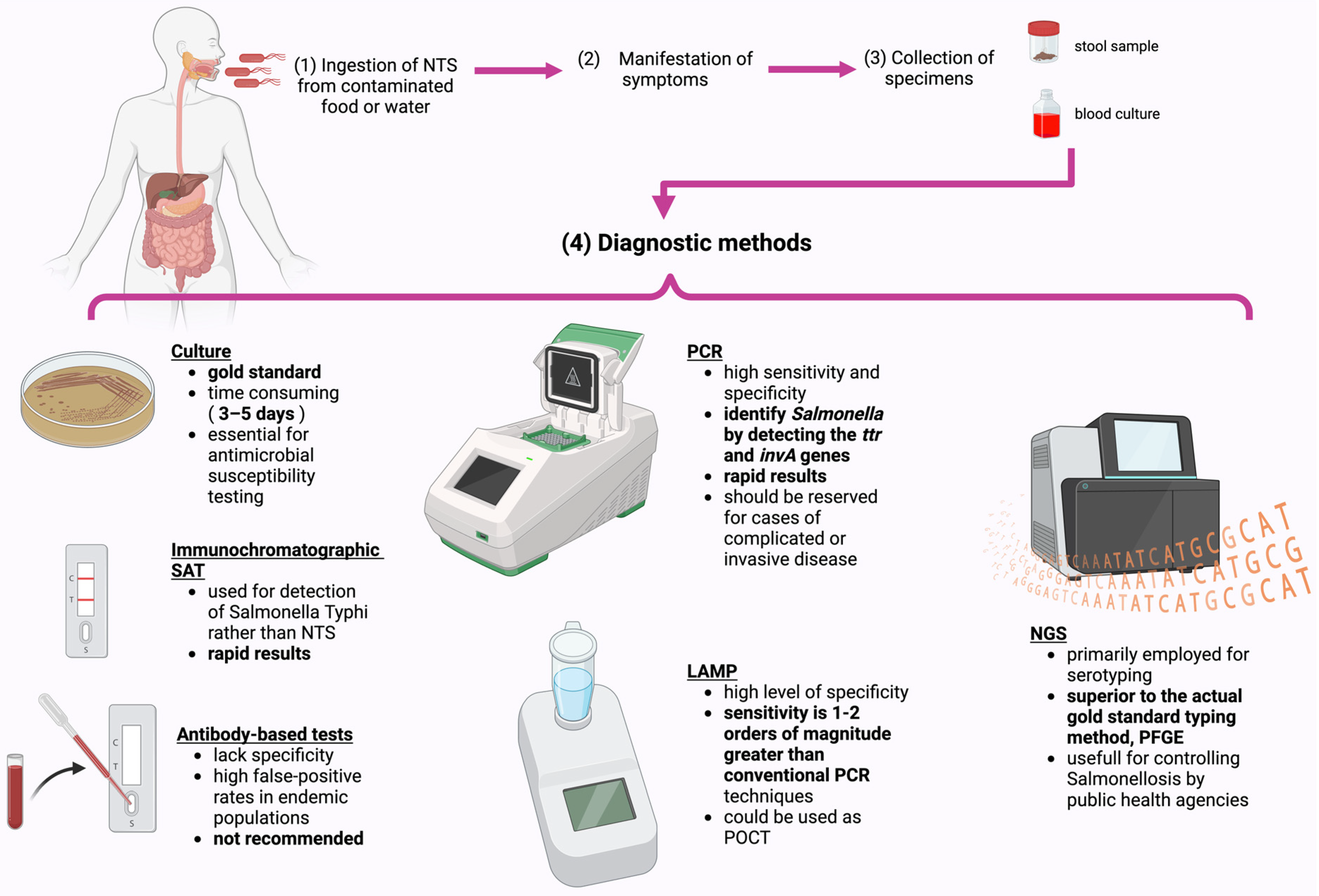
4. Diagnostic Methods
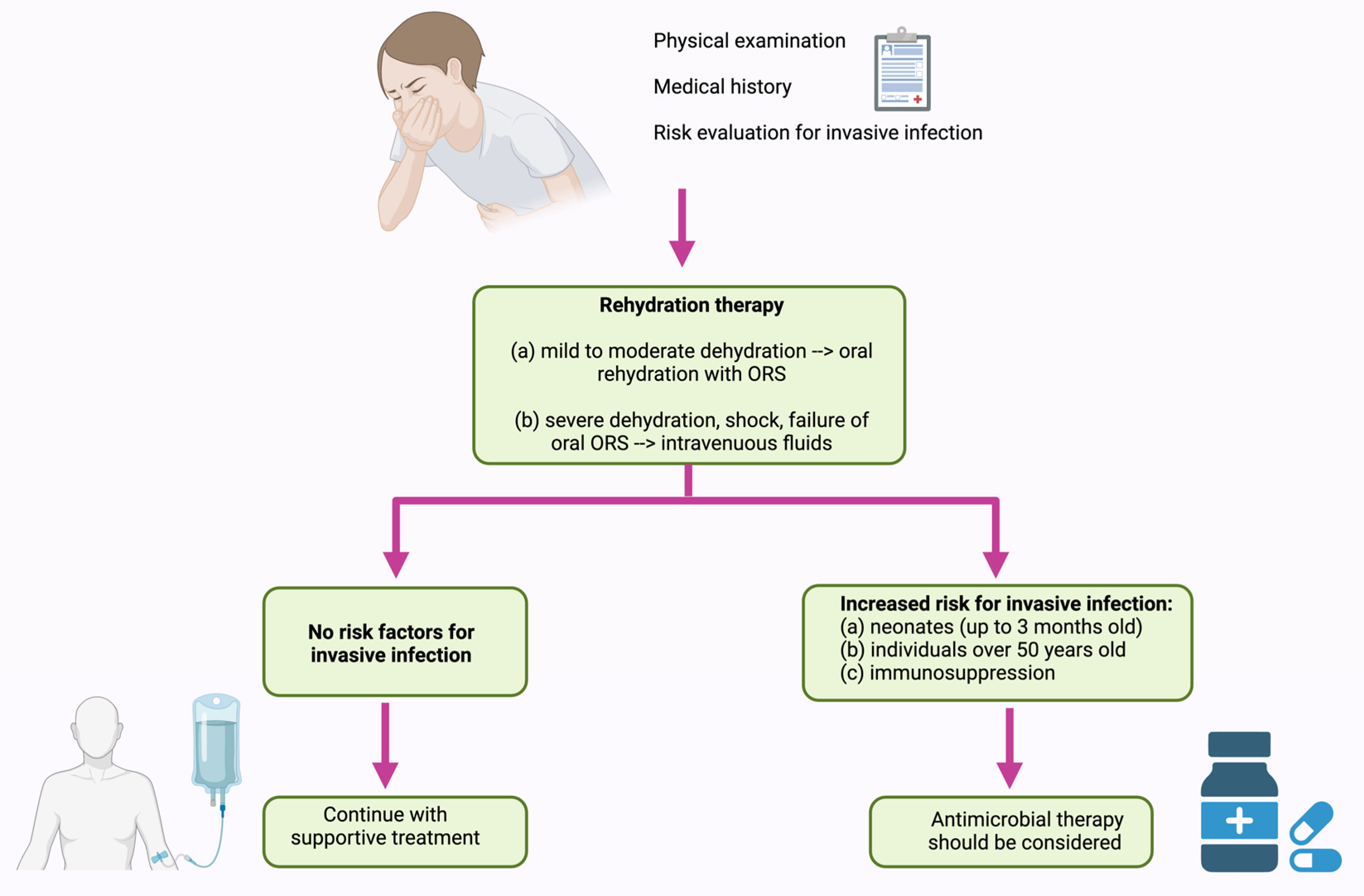
5. Treatment of NTS Infection
5.1. Current Disease Management
5.2. Antimicrobial Resistance (AMR)
5.3. Alternative Therapeutics
5.3.1. Probiotics
5.3.2. Vaccination
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ghazy, A.; Nyarku, R.; Faraj, R.; Bentum, K.; Woube, Y.; Williams, M.; Alocilja, E.; Abebe, W. Gold Nanoparticle-Based Plasmonic Detection of Escherichia Coli, Salmonella Enterica, Campylobacter Jejuni, and Listeria Monocytogenes from Bovine Fecal Samples. Microorganisms 2024, 12, 1069. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Appl. Environ. Microbiol. 2019, 85, E00591-19. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Nemhauser, J.B. (Ed.) CDC Yellow Book 2024: Health Information for International Travel; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Aung, K.T.; Khor, W.C.; Ong, K.H.; Tan, W.L.; Wong, Z.N.; Oh, J.Q.; Wong, W.K.; Tan, B.Z.Y.; Maiwald, M.; Tee, N.W.S.; et al. Characterisation of Salmonella Enteritidis ST11 and ST1925 Associated with Human Intestinal and Extra-Intestinal Infections in Singapore. Int. J. Environ. Res. Public. Health 2022, 19, 5671. [Google Scholar] [CrossRef] [PubMed]
- Uelze, L.; Bloch, A.; Borowiak, M.; Grobbel, M.; Deneke, C.; Fischer, M.; Malorny, B.; Pietsch, M.; Simon, S.; Szabó, I.; et al. What WGS Reveals about Salmonella Enterica Subsp. Enterica in Wildlife in Germany. Microorganisms 2021, 9, 1911. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- National Enteric Disease Surveillance: Salmonella Annual Report, 2016; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016.
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S. Salmonella in the Pork Production Chain and Its Impact on Human Health in the European Union. Epidemiol. Infect. 2017, 145, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Lynne, A.M.; Nayak, R. Salmonella Challenges: Prevalence in Swine and Poultry and Potential Pathogenicity of Such Isolates 1,2. J. Anim. Sci. 2008, 86 (Suppl. 14), E149–E162. [Google Scholar] [CrossRef]
- Makendi, C.; Page, A.J.; Wren, B.W.; Le Thi Phuong, T.; Clare, S.; Hale, C.; Goulding, D.; Klemm, E.J.; Pickard, D.; Okoro, C.; et al. A Phylogenetic and Phenotypic Analysis of Salmonella Enterica Serovar Weltevreden, an Emerging Agent of Diarrheal Disease in Tropical Regions. PLoS Negl. Trop. Dis. 2016, 10, e0004446. [Google Scholar] [CrossRef]
- Uzairue, L.I.; Shittu, O.B.; Ojo, O.E.; Obuotor, T.M.; Olanipekun, G.; Ajose, T.; Arogbonlo, R.; Medugu, N.; Ebruke, B.; Obaro, S.K. Antimicrobial Resistance and Virulence Genes of Invasive Salmonella Enterica from Children with Bacteremia in North-Central Nigeria. SAGE Open Med. 2023, 11, 205031212311753. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Daigle, F. Special Issue “Salmonella: Pathogenesis and Host Restriction”. Microorganisms 2021, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Chen, Y.-C.; Chen, N.-W.; Hsu, Y.-J.; Chu, H.-H.; Chen, C.-L.; Chiu, C.-H. Changing Antimicrobial Resistance and Epidemiology of Non-Typhoidal Salmonella Infection in Taiwanese Children. Front. Microbiol. 2021, 12, 648008. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating What They Are and What They Are Not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Marchello, C.S.; Birkhold, M.; Crump, J.A.; Martin, L.B.; Ansah, M.O.; Breghi, G.; Canals, R.; Fiorino, F.; Gordon, M.A.; Kim, J.-H.; et al. Complications and Mortality of Non-Typhoidal Salmonella Invasive Disease: A Global Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2022, 22, 692–705. [Google Scholar] [CrossRef]
- Medalla, F.; Gu, W.; Friedman, C.R.; Judd, M.; Folster, J.; Griffin, P.M.; Hoekstra, R.M. Increased Incidence of Antimicrobial-Resistant Nontyphoidal Salmonella Infections, United States, 2004–2016. Emerg. Infect. Dis. 2021, 27, 1662–1672. [Google Scholar] [CrossRef]
- Cohen, D.; Muhsen, K. Vaccines for Enteric Diseases. Hum. Vaccines Immunother. 2019, 15, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, P.; Ng, V.; Murray, R.; Nesbitt, A. Forecasting the Incidence of Salmonellosis in Seniors in Canada: A Trend Analysis and the Potential Impact of the Demographic Shift. PLoS ONE 2018, 13, e0208124. [Google Scholar] [CrossRef] [PubMed]
- Pulford, C.V.; Perez-Sepulveda, B.M.; Canals, R.; Bevington, J.A.; Bengtsson, R.J.; Wenner, N.; Rodwell, E.V.; Kumwenda, B.; Zhu, X.; Bennett, R.J.; et al. Stepwise Evolution of Salmonella Typhimurium ST313 Causing Bloodstream Infection in Africa. Nat. Microbiol. 2020, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive Non-Typhoidal Salmonella Disease: An Emerging and Neglected Tropical Disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Marchello, C.S.; Fiorino, F.; Pettini, E.; Crump, J.A.; Martin, L.B.; Breghi, G.; Canals, R.; Gordon, M.A.; Hanumunthadu, B.; Jacobs, J.; et al. Incidence of Non-Typhoidal Salmonella Invasive Disease: A Systematic Review and Meta-Analysis. J. Infect. 2021, 83, 523–532. [Google Scholar] [CrossRef]
- Stecher, B. Establishing Causality in Salmonella-Microbiota-Host Interaction: The Use of Gnotobiotic Mouse Models and Synthetic Microbial Communities. Int. J. Med. Microbiol. 2021, 311, 151484. [Google Scholar] [CrossRef]
- Wotzka, S.Y.; Nguyen, B.D.; Hardt, W.-D. Salmonella Typhimurium Diarrhea Reveals Basic Principles of Enteropathogen Infection and Disease-Promoted DNA Exchange. Cell Host Microbe 2017, 21, 443–454. [Google Scholar] [CrossRef]
- Horrocks, V.; King, O.G.; Yip, A.Y.G.; Marques, I.M.; McDonald, J.A.K. Role of the Gut Microbiota in Nutrient Competition and Protection against Intestinal Pathogen Colonization. Microbiology 2023, 169, 001377. [Google Scholar] [CrossRef]
- Blondel, C.J.; Amaya, F.A.; Bustamante, P.; Santiviago, C.A.; Pezoa, D. Identification and Distribution of New Candidate T6SS Effectors Encoded in Salmonella Pathogenicity Island 6. Front. Microbiol. 2023, 14, 1252344. [Google Scholar] [CrossRef] [PubMed]
- Koczerka, M.; Lantier, I.; Morillon, M.; Deperne, J.; Clamagirand, C.D.; Virlogeux-Payant, I.; Grépinet, O. From Intestine to beyond: Salmonella Entry Factors Display Distinct Transcription Pattern upon Infection in Murine Models. Open Biol. 2024, 14, 230312. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Van Den Bogaart, G. Vacuolar Escape of Foodborne Bacterial Pathogens. J. Cell Sci. 2021, 134, jcs247221. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Gao, S.; Yuan, H.; Zuo, L.; Wu, C.; Huang, R.; Wu, S. Salmonella spvC Gene Inhibits Autophagy of Host Cells and Suppresses NLRP3 as Well as NLRC4. Front. Immunol. 2021, 12, 639019. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Winter, S.E. Salmonella Finds a Way: Metabolic Versatility of Salmonella Enterica Serovar Typhimurium in Diverse Host Environments. PLoS Pathog. 2020, 16, e1008540. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.D.; Cuenca, V.M.; Hartl, J.; Gül, E.; Bauer, R.; Meile, S.; Rüthi, J.; Margot, C.; Heeb, L.; Besser, F.; et al. Import of Aspartate and Malate by DcuABC Drives H2/Fumarate Respiration to Promote Initial Salmonella Gut-Lumen Colonization in Mice. Cell Host Microbe 2020, 27, 922–936.e6. [Google Scholar] [CrossRef] [PubMed]
- Eberl, C.; Weiss, A.S.; Jochum, L.M.; Durai Raj, A.C.; Ring, D.; Hussain, S.; Herp, S.; Meng, C.; Kleigrewe, K.; Gigl, M.; et al. E. Coli Enhance Colonization Resistance against Salmonella Typhimurium by Competing for Galactitol, a Context-Dependent Limiting Carbon Source. Cell Host Microbe 2021, 29, 1680–1692.e7. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.W.L.; Tsolis, R.M.; Bäumler, A.J. Salmonella versus the Microbiome. Microbiol. Mol. Biol. Rev. 2021, 85, e00027-19. [Google Scholar] [CrossRef]
- Grzymajło, K.; Dutkiewicz, A.; Czajkowska, J.; Carolak, E.; Aleksandrowicz, A.; Waszczuk, W. Salmonella Adhesion Is Decreased by Hypoxia Due to Adhesion and Motility Structure Crosstalk. Vet. Res. 2023, 54, 99. [Google Scholar] [CrossRef]
- Spragge, F.; Bakkeren, E.; Jahn, M.T.; Araujo, B.N.E.; Pearson, C.F.; Wang, X.; Pankhurst, L.; Cunrath, O.; Foster, K.R. Microbiome Diversity Protects against Pathogens by Nutrient Blocking. Science 2023, 382, eadj3502. [Google Scholar] [CrossRef]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef] [PubMed]
- Wotzka, S.Y.; Kreuzer, M.; Maier, L.; Arnoldini, M.; Nguyen, B.D.; Brachmann, A.O.; Berthold, D.L.; Zünd, M.; Hausmann, A.; Bakkeren, E.; et al. Escherichia Coli Limits Salmonella Typhimurium Infections after Diet Shifts and Fat-Mediated Microbiota Perturbation in Mice. Nat. Microbiol. 2019, 4, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, P.; Shao, W.; Yang, C.; Chen, L.; Zhu, A.; Pan, Z. Detection and Molecular Identification of Salmonella Pathogenic Islands and Virulence Plasmid Genes of Salmonella in Xuzhou Raw Meat Products. J. Food Prot. 2022, 85, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Hespanhol, J.T.; Nóbrega-Silva, L.; Bayer-Santos, E. Regulation of Type VI Secretion Systems at the Transcriptional, Posttranscriptional and Posttranslational Level. Microbiology 2023, 169, 001376. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Wang, S.; Zhao, J.-H.; Liu, S.-L. Salmonella Secretion Systems: Differential Roles in Pathogen-Host Interactions. Microbiol. Res. 2020, 241, 126591. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.E.; Gallegos-Monterrosa, R.; Coulthurst, S.J. Type VI Secretion System Effector Proteins: Effective Weapons for Bacterial Competitiveness. Cell. Microbiol. 2020, 22, e13241. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Pan, J.; Yang, Y.; Zhang, Z.; Cui, R.; Jia, S.; Wang, Z.; Yang, C.; Xu, L.; Dong, T.G.; et al. Contact-Independent Killing Mediated by a T6SS Effector with Intrinsic Cell-Entry Properties. Nat. Commun. 2021, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, Y.; Tarchichi, N.; Barakat, R.; Kawtharani, I.; Ghandour, R.; Ezzeddine, Z.; Ghssein, G. The Different Types of Metallophores Produced by Salmonella Enterica: A Review. Microbiol. Res. 2023, 14, 1457–1469. [Google Scholar] [CrossRef]
- Gül, E.; Bakkeren, E.; Salazar, G.; Steiger, Y.; Abi Younes, A.; Clerc, M.; Christen, P.; Fattinger, S.A.; Nguyen, B.D.; Kiefer, P.; et al. The Microbiota Conditions a Gut Milieu That Selects for Wild-Type Salmonella Typhimurium Virulence. PLoS Biol. 2023, 21, e3002253. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Z.; Liu, Z.; Li, D.; Liu, Z.; Dong, X.; Yan, S. Research Progress of Salmonella Pathogenicity Island. Int. J. Biol. Life Sci. 2023, 2, 7–11. [Google Scholar] [CrossRef]
- Velge, P.; Wiedemann, A.; Rosselin, M.; Abed, N.; Boumart, Z.; Chaussé, A.M.; Grépinet, O.; Namdari, F.; Roche, S.M.; Rossignol, A.; et al. Multiplicity of Salmonella Entry Mechanisms, a New Paradigm for Salmonella Pathogenesis. MicrobiologyOpen 2012, 1, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Mechanisms for the Invasion and Dissemination of Salmonella. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaussé, A.-M.; Roche, S.M.; Moroldo, M.; Hennequet-Antier, C.; Holbert, S.; Kempf, F.; Barilleau, E.; Trotereau, J.; Velge, P. Epithelial Cell Invasion by Salmonella Typhimurium Induces Modulation of Genes Controlled by Aryl Hydrocarbon Receptor Signaling and Involved in Extracellular Matrix Biogenesis. Virulence 2023, 14, 2158663. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, J.; Santos, J.C.; Stévenin, V.; Weiner, A.; Latour-Lambert, P.; Rechav, K.; Mallet, A.; Krijnse-Locker, J.; Elbaum, M.; Enninga, J. The Entry of Salmonella in a Distinct Tight Compartment Revealed at High Temporal and Ultrastructural Resolution. Cell. Microbiol. 2018, 20, e12816. [Google Scholar] [CrossRef]
- Fattinger, S.A.; Sellin, M.E.; Hardt, W.-D. Salmonella Effector Driven Invasion of the Gut Epithelium: Breaking in and Setting the House on Fire. Curr. Opin. Microbiol. 2021, 64, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, D.; Davidson, A.; Hume, P.J.; Koronakis, V. Salmonella Virulence Effector SopE and Host GEF ARNO Cooperate to Recruit and Activate WAVE to Trigger Bacterial Invasion. Cell Host Microbe 2012, 11, 129–139. [Google Scholar] [CrossRef]
- McGhie, E.J.; Hayward, R.D.; Koronakis, V. Control of Actin Turnover by a Salmonella Invasion Protein. Mol. Cell 2004, 13, 497–510. [Google Scholar] [CrossRef]
- Barilleau, E.; Védrine, M.; Koczerka, M.; Burlaud-Gaillard, J.; Kempf, F.; Grépinet, O.; Virlogeux-Payant, I.; Velge, P.; Wiedemann, A. Investigation of the Invasion Mechanism Mediated by the Outer Membrane Protein PagN of Salmonella Typhimurium. BMC Microbiol. 2021, 21, 153. [Google Scholar] [CrossRef]
- Rosselin, M.; Abed, N.; Virlogeux-Payant, I.; Bottreau, E.; Sizaret, P.-Y.; Velge, P.; Wiedemann, A. Heterogeneity of Type III Secretion System (T3SS)-1-Independent Entry Mechanisms Used by Salmonella Enteritidis to Invade Different Cell Types. Microbiology 2011, 157, 839–847. [Google Scholar] [CrossRef]
- Li, W.; Ren, Q.; Ni, T.; Zhao, Y.; Sang, Z.; Luo, R.; Li, Z.; Li, S. Strategies Adopted by Salmonella to Survive in Host: A Review. Arch. Microbiol. 2023, 205, 362. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, P.; Cheng, S.; Lu, Q.; Nowak, K.; Hopp, A.-K.; Li, L.; Shi, X.; Zhou, Z.; Gao, W.; et al. A Bacterial Effector Reveals the V-ATPase-ATG16L1 Axis That Initiates Xenophagy. Cell 2019, 178, 552–566.e20. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, F.S.; Thomas, M.; Sachse, M.; Santos, A.J.M.; Figueira, R.; Holden, D.W. The Salmonella Deubiquitinase SseL Inhibits Selective Autophagy of Cytosolic Aggregates. PLoS Pathog. 2012, 8, e1002743. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Z.; Jiang, A.-J.; Mao, A.-W.; Feng, Y.; Wang, W.; Li, J.; Zhang, X.; Xing, K.; Peng, X. The Salmonella Effectors SseF and SseG Inhibit Rab1A-Mediated Autophagy to Facilitate Intracellular Bacterial Survival and Replication. J. Biol. Chem. 2018, 293, 9662–9673. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Gao, S.; Wang, T.; Yan, J.; Xu, G.; Li, Y.; Niu, H.; Huang, R.; Wu, S. A Novel Contribution of spvB to Pathogenesis of Salmonella Typhimurium by Inhibiting Autophagy in Host Cells. Oncotarget 2016, 7, 8295–8309. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hu, S.; Huang, Y.; Zhang, Q.; Yi, X.; Pan, X.; Li, S. Arg-GlcNAcylation on TRADD by NleB and SseK1 Is Crucial for Bacterial Pathogenesis. Front. Cell Dev. Biol. 2020, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jones, R.M.; Neish, A.S. The Salmonella Effector AvrA Mediates Bacterial Intracellular Survival during Infection in Vivo: AvrA Facilitates Salmonella Intracellular Survival. Cell. Microbiol. 2012, 14, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, A.; Zhang, Y.; Cui, J.; Yang, L.; Li, J.; Zhang, G.; Wu, C.; Li, Z.; Wei, Y. Inflammasome Activation and Pyroptosis Mediate Coagulopathy and Inflammation in Salmonella Systemic Infection. Microbiol. Res. 2023, 275, 127460. [Google Scholar] [CrossRef]
- Gram, A.M.; Wright, J.A.; Pickering, R.J.; Lam, N.L.; Booty, L.M.; Webster, S.J.; Bryant, C.E. Salmonella Flagellin Activates NAIP/NLRC4 and Canonical NLRP3 Inflammasomes in Human Macrophages. J. Immunol. 2021, 206, 631–640. [Google Scholar] [CrossRef]
- Brodsky, I.E. JAK-Ing into M1/M2 Polarization SteErs Salmonella-Containing Macrophages Away from Immune Attack to Promote Bacterial Persistence. Cell Host Microbe 2020, 27, 3–5. [Google Scholar] [CrossRef]
- Panagi, I.; Jennings, E.; Zeng, J.; Günster, R.A.; Stones, C.D.; Mak, H.; Jin, E.; Stapels, D.A.C.; Subari, N.Z.; Pham, T.H.M.; et al. Salmonella Effector SteE Converts the Mammalian Serine/Threonine Kinase GSK3 into a Tyrosine Kinase to Direct Macrophage Polarization. Cell Host Microbe 2020, 27, 41–53.e6. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Ma, S.; Yan, X.; Ma, S.; Sun, H.; Sun, Y.; Jiang, L. Lactate Promotes Salmonella Intracellular Replication and Systemic Infection via Driving Macrophage M2 Polarization. Microbiol. Spectr. 2023, 11, e02253-23. [Google Scholar] [CrossRef] [PubMed]
- Alix, E.; Godlee, C.; Cerny, O.; Blundell, S.; Tocci, R.; Matthews, S.; Liu, M.; Pruneda, J.N.; Swatek, K.N.; Komander, D.; et al. The Tumour Suppressor TMEM127 Is a Nedd4-Family E3 Ligase Adaptor Required by Salmonella SteD to Ubiquitinate and Degrade MHC Class II Molecules. Cell Host Microbe 2020, 28, 54–68.e7. [Google Scholar] [CrossRef] [PubMed]
- Cerny, O.; Godlee, C.; Tocci, R.; Cross, N.E.; Shi, H.; Williamson, J.C.; Alix, E.; Lehner, P.J.; Holden, D.W. CD97 Stabilises the Immunological Synapse between Dendritic Cells and T Cells and Is Targeted for Degradation by the Salmonella Effector SteD. PLoS Pathog. 2021, 17, e1009771. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Alejo, N.; Santos-Argumedo, L. Innate Defects of the IL-12/IFN-γ Axis in Susceptibility to Infections by Mycobacteria and Salmonella. J. Interferon Cytokine Res. 2014, 34, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.M.; Han, X.; Allaire, J.M.; Stahl, M.; Rauch, I.; Knodler, L.A.; Vallance, B.A. Intestinal Restriction of Salmonella Typhimurium Requires Caspase-1 and Caspase-11 Epithelial Intrinsic Inflammasomes. PLoS Pathog. 2020, 16, e1008498. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Roa, C.; Salazar, G.A.; Noguera, L.P.; Salazar-Echegarai, F.J.; Vallejos, O.P.; Suazo, I.D.; Schultz, B.M.; Coronado-Arrázola, I.; Kalergis, A.M.; Bueno, S.M. Pathogenicity Island Excision during an Infection by Salmonella Enterica Serovar Enteritidis Is Required for Crossing the Intestinal Epithelial Barrier in Mice to Cause Systemic Infection. PLoS Pathog. 2019, 15, e1008152. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, H.-L.; Weng, C.-C.; Janapatla, R.P.; Chen, C.-L.; Chiu, C.-H. Activation of Apoptosis by Salmonella Pathogenicity Island-1 Effectors through Both Intrinsic and Extrinsic Pathways in Salmonella-Infected Macrophages. J. Microbiol. Immunol. Infect. 2021, 54, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Li, Q.; Li, X.; Park, C.G.; He, Y.; Zhang, Y.; Wu, B.; Xue, Y.; Yang, K.; Lv, Y.; et al. Salmonella Enterica Serovar Typhimurium Interacts with CD209 Receptors To Promote Host Dissemination and Infection. Infect. Immun. 2019, 87, e00100-19. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. A Breathtaking Feat: To Compete with the Gut Microbiota, Salmonella Drives Its Host to Provide a Respiratory Electron Acceptor. Gut Microbes 2011, 2, 58–60. [Google Scholar] [CrossRef]
- Behnsen, J.; Perez-Lopez, A.; Nuccio, S.-P.; Raffatellu, M. Exploiting Host Immunity: The Salmonella Paradigm. Trends Immunol. 2015, 36, 112–120. [Google Scholar] [CrossRef]
- Gillis, C.C.; Hughes, E.R.; Spiga, L.; Winter, M.G.; Zhu, W.; Furtado De Carvalho, T.; Chanin, R.B.; Behrendt, C.L.; Hooper, L.V.; Santos, R.L.; et al. Dysbiosis-Associated Change in Host Metabolism Generates Lactate to Support Salmonella Growth. Cell Host Microbe 2018, 23, 54–64.e6. [Google Scholar] [CrossRef] [PubMed]
- Shelton, C.D.; Yoo, W.; Shealy, N.G.; Torres, T.P.; Zieba, J.K.; Calcutt, M.W.; Foegeding, N.J.; Kim, D.; Kim, J.; Ryu, S.; et al. Salmonella Enterica Serovar Typhimurium Uses Anaerobic Respiration to Overcome Propionate-Mediated Colonization Resistance. Cell Rep. 2022, 38, 110180. [Google Scholar] [CrossRef] [PubMed]
- Takaya, A.; Yamamoto, T.; Tokoyoda, K. Humoral Immunity vs. Salmonella. Front. Immunol. 2020, 10, 3155. [Google Scholar] [CrossRef] [PubMed]
- Diard, M.; Bakkeren, E.; Cornuault, J.K.; Moor, K.; Hausmann, A.; Sellin, M.E.; Loverdo, C.; Aertsen, A.; Ackermann, M.; De Paepe, M.; et al. Inflammation Boosts Bacteriophage Transfer between Salmonella Spp. Science 2017, 355, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Männe, C.; Takaya, A.; Yamasaki, Y.; Mursell, M.; Hojyo, S.; Wu, T.-Y.; Sarkander, J.; McGrath, M.A.; Cornelis, R.; Hahne, S.; et al. Salmonella SiiE Prevents an Efficient Humoral Immune Memory by Interfering with IgG + Plasma Cell Persistence in the Bone Marrow. Proc. Natl. Acad. Sci. USA 2019, 116, 7425–7430. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Wotzka, S.Y.; Toska, A.; Diard, M.; Hapfelmeier, S.; Slack, E. Peracetic Acid Treatment Generates Potent Inactivated Oral Vaccines from a Broad Range of Culturable Bacterial Species. Front. Immunol. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Diard, M.; Sellin, M.E.; Felmy, B.; Wotzka, S.Y.; Toska, A.; Bakkeren, E.; Arnoldini, M.; Bansept, F.; Co, A.D.; et al. High-Avidity IgA Protects the Intestine by Enchaining Growing Bacteria. Nature 2017, 544, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent Bacterial Infections and Persister Cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Ertelt, J.M.; Rowe, J.H.; Way, S.S. Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Salmonella Infection. PLoS Pathog. 2010, 6, e1001043. [Google Scholar] [CrossRef]
- Stapels, D.A.C.; Hill, P.W.S.; Westermann, A.J.; Fisher, R.A.; Thurston, T.L.; Saliba, A.-E.; Blommestein, I.; Vogel, J.; Helaine, S. Salmonella Persisters Undermine Host Immune Defenses during Antibiotic Treatment. Science 2018, 362, 1156–1160. [Google Scholar] [CrossRef]
- Luk, C.H.; Valenzuela, C.; Gil, M.; Swistak, L.; Bomme, P.; Chang, Y.-Y.; Mallet, A.; Enninga, J. Salmonella Enters a Dormant State within Human Epithelial Cells for Persistent Infection. PLoS Pathog. 2021, 17, e1009550. [Google Scholar] [CrossRef]
- Fierer, J. Invasive Non-Typhoidal Salmonella (iNTS) Infections. Clin. Infect. Dis. 2022, 75, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Maurya, S.; Chadrasekhar, H.; Srikanth, C.V. Molecular Determinants of Peaceful Coexistence versus Invasiveness of Non-Typhoidal Salmonella: Implications in Long-Term Side-Effects. Mol. Aspects Med. 2021, 81, 100997. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Chauhan, V.; Chaudhary, J.; Chauhan, N. An Extensive Review on the Exploration of Non-Typhoidal Salmonella and Its Associated Infections. J. Pure Appl. Microbiol. 2023, 17, 112–126. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, Y.; Han, M.S. Nontyphoidal Salmonella Meningitis in an Immunocompetent Child. Pediatr. Infect. Vaccine 2022, 29, 54. [Google Scholar] [CrossRef]
- Taliha, M.D.; Balti, E.; Maillart, E.; Leemans, S.; Taghavi, M.; Carausu, S.-A.; Sanoussi, S.; Mahadeb, B.; Clevenbergh, P. Invasive Non-Typhoidal Salmonella Infection Complicated by Metastatic Infections: Report of Three Cases. IDCases 2022, 28, e01498. [Google Scholar] [CrossRef]
- Ansari, F.A.; Gafoor, S.; Aftab, M.; Nlandu, Z. An Unusual Case of Non-Typhoidal Salmonella Bacteremia Causing Life-Threatening Aortitis. Cureus 2024, 16, e54645. [Google Scholar] [CrossRef] [PubMed]
- Alhamadh, M.S.; Alanazi, R.B.; Alhowaish, T.S.; Alhabeeb, A.Y.; Algarni, S.T.; Wadaan, O.M.; Suliman, I.; Al-Ghamdi, M.G. Refractory Salmonella Prosthetic Valve Endocarditis Complicated by Splenic Infarction and Aortic Pseudoaneurysm in a Patient with Double Prosthetic Valves: A Case Report. Diagnostics 2022, 12, 1982. [Google Scholar] [CrossRef]
- Winicki, N.M.; Desai, D.; Desai, A.; Perswani, P.; Smadi, K.A.; Doyle, K.; Gandhi, H.; Sethi, P.S.; Mukherjee, A. ‘From Gut to Heart’: A Rare Case of Salmonella Typhimurium Bacteremia and Native Valve Endocarditis. IDCases 2023, 32, e01787. [Google Scholar] [CrossRef]
- Ismail, S.; Thomas, M.; Razok, A.; Akbar, R.; Abid, F.B.; Wilson, G. Salmonella-Induced Pulmonary and Pericardial Abscesses in a Patient Presenting with Subacute Cough. IDCases 2022, 27, e01430. [Google Scholar] [CrossRef]
- Boguniewicz, J.; Rubiano Landinez, A.; Kaplan, S.L.; Lamb, G.S. Comparison of Musculoskeletal Infections Due to Nontyphoidal Salmonella Species and Staphylococcus Aureus in Immunocompetent Children. Pediatr. Infect. Dis. J. 2019, 38, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, C.; Shi, T.; Wu, B.; Chen, Y.; Li, W.; Fang, X.; Zhang, W. Salmonella Osteomyelitis in Adults: A Systematic Review. Orthop. Surg. 2021, 13, 1135–1140. [Google Scholar] [CrossRef]
- Katzouraki, G.; Vasiliadis, E.S.; Marougklianis, V.; Evangelopoulos, D.S.; Pneumaticos, S.G. A Systematic Review of the Diagnosis and Treatment of Non-Typhoid Salmonella Spondylodiscitis in Immunocompetent Children. Children 2022, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Altaf, A.; Tunio, N.; Tunio, S.; Zafar, M.R.; Bajwa, N. Salmonella Urinary Tract Infection and Bacteremia Following Non-Typhoidal Salmonella Gastroenteritis: An Unusual Presentation. Cureus 2020, 12, e12194. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; Pelt, W.V.; Neefjes, J. Increased Colon Cancer Risk after Severe Salmonella Infection. PLoS ONE 2018, 13, e0189721. [Google Scholar] [CrossRef] [PubMed]
- Shanker, E.B.; Sun, J. Salmonella Infection Acts as an Environmental Risk Factor for Human Colon Cancer. Cell Insight 2023, 2, 100125. [Google Scholar] [CrossRef] [PubMed]
- Van Elsland, D.M.; Duijster, J.W.; Zhang, J.; Stévenin, V.; Zhang, Y.; Zha, L.; Xia, Y.; Franz, E.; Sun, J.; Mughini-Gras, L.; et al. Repetitive Non-Typhoidal Salmonella Exposure Is an Environmental Risk Factor for Colon Cancer and Tumor Growth. Cell Rep. Med. 2022, 3, 100852. [Google Scholar] [CrossRef] [PubMed]
- Pillay, T.D.; Hettiarachchi, S.U.; Gan, J.; Diaz-Del-Olmo, I.; Yu, X.-J.; Muench, J.H.; Thurston, T.L.M.; Pearson, J.S. Speaking the Host Language: How Salmonella Effector Proteins Manipulate the Host: This Article Is Part of the Bacterial Cell Envelopes Collection. Microbiology 2023, 169, 001342. [Google Scholar] [CrossRef]
- Lu, R.; Bosland, M.; Xia, Y.; Zhang, Y.; Kato, I.; Sun, J. Presence of Salmonella AvrA in Colorectal Tumor and Its Precursor Lesions in Mouse Intestine and Human Specimens. Oncotarget 2017, 8, 55104–55115. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Y.; Lin, Z.; Lu, R.; Xia, Y.; Meng, C.; Pan, Z.; Xu, X.; Jiao, X.; Sun, J. Salmonella Enteritidis Effector AvrA Suppresses Autophagy by Reducing Beclin-1 Protein. Front. Immunol. 2020, 11, 686. [Google Scholar] [CrossRef]
- Zha, L.; Garrett, S.; Sun, J. Salmonella Infection in Chronic Inflammation and Gastrointestinal Cancer. Diseases 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current Knowledge and Perspectives of Potential Impacts of Salmonella Enterica on the Profile of the Gut Microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.; Barreto, S.G.; Sahoo, B.; Chandrani, P.; Ramadwar, M.R.; Shrikhande, S.V.; Dutt, A. Non-Typhoidal Salmonella DNA Traces in Gallbladder Cancer. Infect. Agent. Cancer 2016, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, A.; Pal, D.; Kumar, A. Salmonella Typhi Induced Oncogenesis in Gallbladder Cancer: Co-Relation and Progression. Adv. Cancer Biol. Metastasis 2022, 4, 100032. [Google Scholar] [CrossRef]
- D’Afonseca, V.; Arencibia, A.D.; Echeverría-Vega, A.; Cerpa, L.; Cayún, J.P.; Varela, N.M.; Salazar, M.; Quiñones, L.A. Identification of Altered Genes in Gallbladder Cancer as Potential Driver Mutations for Diagnostic and Prognostic Purposes: A Computational Approach. Cancer Inform. 2020, 19, 117693512092215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Huda, F.; Naithani, M.; Kumar Singh, S.; Kumar, N.; Basu, S. Role of Gut Microbiome and Enteric Bacteria in Gallbladder Cancer. In Immunology of the GI Tract—Recent Advances; Rodrigo, L., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Kushwaha, M.; Nukala, V.; Singh, A.K.; Makharia, G.K.; Mohan, A.; Kumar, A.; Dalal, N. Emerging Implications of Bacterial Biofilm in Cancer Biology: Recent Updates and Major Perspectives. Gut Microbes Rep. 2024, 1, 1–20. [Google Scholar] [CrossRef]
- Ajene, A.N.; Walker, C.L.F.; Black, R.E. Enteric Pathogens and Reactive Arthritis: A Systematic Review of Campylobacter, Salmonella and Shigella-Associated Reactive Arthritis. J. Health Popul. Nutr. 2013, 31, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Pogreba-Brown, K.; Austhof, E.; Tang, X.; Trejo, M.J.; Owusu-Dommey, A.; Boyd, K.; Armstrong, A.; Schaefer, K.; Bazaco, M.C.; Batz, M.; et al. Enteric Pathogens and Reactive Arthritis: Systematic Review and Meta-Analyses of Pathogen-Associated Reactive Arthritis. Foodborne Pathog. Dis. 2021, 18, 627–639. [Google Scholar] [CrossRef]
- Jayaprakash, T.; Leela, K.V.; Sundaram, A.; Rajendran, C.P.; Aparna, R. Prevalence of Human Leukocyte Antigen B27 Positivity and Microbiological Profiles of Patients with Reactive Arthritis—A Cross Sectional Study. J. Pure Appl. Microbiol. 2021, 15, 382–389. [Google Scholar] [CrossRef]
- Antoniou, A.N.; Lenart, I.; Kriston-Vizi, J.; Iwawaki, T.; Turmaine, M.; McHugh, K.; Ali, S.; Blake, N.; Bowness, P.; Bajaj-Elliott, M.; et al. Salmonella Exploits HLA-B27 and Host Unfolded Protein Responses to Promote Intracellular Replication. Ann. Rheum. Dis. 2019, 78, 74–82. [Google Scholar] [CrossRef]
- Hayes, K.M.; Hayes, R.J.P.; Turk, M.A.; Pope, J.E. Evolving Patterns of Reactive Arthritis. Clin. Rheumatol. 2019, 38, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Sharip, A.; Kunz, J. Understanding the Pathogenesis of Spondyloarthritis. Biomolecules 2020, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Pasternak, J.A.; Medeiros, N.J.; Nicastro, L.K.; Tursi, S.A.; Hansen, E.G.; Krochak, R.; Sokaribo, A.S.; MacKenzie, K.D.; Palmer, M.B.; et al. In Vivo Synthesis of Bacterial Amyloid Curli Contributes to Joint Inflammation during S. Typhimurium Infection. PLoS Pathog. 2020, 16, e1008591. [Google Scholar] [CrossRef] [PubMed]
- Olovo, C.V.; Wiredu Ocansey, D.K.; Ji, Y.; Huang, X.; Xu, M. Bacterial Membrane Vesicles in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Gut Microbes 2024, 16, 2341670. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Li, C.-P.; Sun, C.-K.; Cho, D.-Y.; Tsai, F.-J.; Yip, H.-T.; Chang, R.; Hung, Y.-M. Increased Risk of Inflammatory Bowel Disease Among Patients With Nontyphoidal Salmonella Infections: A Population-Based Cohort Study. Inflamm. Bowel Dis. 2024, izae053. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Pottebaum, B.; Piryanka, K.; Calcagno, C.; Yang, S. S2770 Recurrent Salmonella Associated With Relapse of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2022, 117 (Suppl. 10), e1816–e1817. [Google Scholar] [CrossRef]
- Schultz, B.M.; Paduro, C.A.; Salazar, G.A.; Salazar-Echegarai, F.J.; Sebastián, V.P.; Riedel, C.A.; Kalergis, A.M.; Alvarez-Lobos, M.; Bueno, S.M. A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Xu, B.; Wang, C.; Xiao, Y.; Jin, Y. Bacterial Membrane Vesicles in Inflammatory Bowel Disease. Life Sci. 2022, 306, 120803. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Rudensky, E.; Martin, P.; Torres, V.; Cadwell, K. Heterozygosity for Crohn’s Disease Risk Allele of Atg16L1 Protects from Salmonella Infection. J. Immunol. 2023, 210 (Suppl. 1), 160.03. [Google Scholar] [CrossRef]
- Kirti, N.; Krishna, S.; Shukla, D. Salmonella Infections: An Update, Detection and Control Strategies. In Salmonella—Current Trends and Perspectives in Detection and Control; Huang, C., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Wattiau, P.; Boland, C.; Bertrand, S. Methodologies for Salmonella Enterica Subsp. Enterica Subtyping: Gold Standards and Alternatives. Appl. Environ. Microbiol. 2011, 77, 7877–7885. [Google Scholar] [CrossRef]
- Kuijpers, L.M.F.; Chung, P.; Peeters, M.; Phoba, M.-F.; Kham, C.; Barbé, B.; Lunguya, O.; Jacobs, J. Diagnostic Accuracy of Antigen-Based Immunochromatographic Rapid Diagnostic Tests for the Detection of Salmonella in Blood Culture Broth. PLoS ONE 2018, 13, e0194024. [Google Scholar] [CrossRef] [PubMed]
- Manore, C.; Graham, T.; Carr, A.; Feryn, A.; Jakhar, S.; Mukundan, H.; Highlander, H.C. Modeling and Cost Benefit Analysis to Guide Deployment of POC Diagnostics for Non-Typhoidal Salmonella Infections with Antimicrobial Resistance. Sci. Rep. 2019, 9, 11245. [Google Scholar] [CrossRef] [PubMed]
- Chirambo, A.C.; Nyirenda, T.S.; Jambo, N.; Msefula, C.; Kamng’ona, A.; Molina, S.; Mandala, W.L.; Heyderman, R.S.; Iturizza-Gomara, M.; Henrion, M.Y.R.; et al. Performance of Molecular Methods for the Detection of Salmonella in Human Stool Specimens. Wellcome Open Res. 2021, 5, 237. [Google Scholar] [CrossRef]
- Lertsethtakarn, P.; Silapong, S.; Sakpaisal, P.; Serichantalergs, O.; Ruamsap, N.; Lurchachaiwong, W.; Anuras, S.; Platts-Mills, J.A.; Liu, J.; Houpt, E.R.; et al. Travelers’ Diarrhea in Thailand: A Quantitative Analysis Using TaqMan® Array Card. Clin. Infect. Dis. 2018, 67, 120–127. [Google Scholar] [CrossRef]
- O’Boyle, H.; Kirpalani, A.; Weiss, L.; Hames, N.; Li, R.; Leong, T.; Gonzalez, M.; Shane, A.L.; Charvat, C. Management and Outcomes of Salmonella Gastroenteritis in the Era of Rapid Molecular Testing. Hosp. Pediatr. 2022, 12, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, J.; Shi, Z.; Cao, A.; Fang, W.; Yan, D.; Wang, Q.; Li, Y. Molecular Methods for Identification and Quantification of Foodborne Pathogens. Molecules 2022, 27, 8262. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Golabi, M.; Than, T.L.Q.; Wolff, A.; Bang, D.D. Point-of-Care Diagnosis of Invasive Non-Typhoidal Salmonella Enterica in Bloodstream Infections Using Immunomagnetic Capture and Loop-Mediated Isothermal Amplification. New Biotechnol. 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Banerji, S.; Simon, S.; Tille, A.; Fruth, A.; Flieger, A. Genome-Based Salmonella Serotyping as the New Gold Standard. Sci. Rep. 2020, 10, 4333. [Google Scholar] [CrossRef]
- Inns, T.; Ashton, P.M.; Herrera-Leon, S.; Lighthill, J.; Foulkes, S.; Jombart, T.; Rehman, Y.; Fox, A.; Dallman, T.; De Pinna, E.; et al. Prospective Use of Whole Genome Sequencing (WGS) Detected a Multi-Country Outbreak of Salmonella Enteritidis. Epidemiol. Infect. 2017, 145, 289–298. [Google Scholar] [CrossRef]
- Leekitcharoenphon, P.; Nielsen, E.M.; Kaas, R.S.; Lund, O.; Aarestrup, F.M. Evaluation of Whole Genome Sequencing for Outbreak Detection of Salmonella Enterica. PLoS ONE 2014, 9, e87991. [Google Scholar] [CrossRef]
- Uelze, L.; Becker, N.; Borowiak, M.; Busch, U.; Dangel, A.; Deneke, C.; Fischer, J.; Flieger, A.; Hepner, S.; Huber, I.; et al. Toward an Integrated Genome-Based Surveillance of Salmonella Enterica in Germany. Front. Microbiol. 2021, 12, 626941. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases Evidence-Based Guidelines for the Management of Acute Gastroenteritis in Children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef]
- Chen, J.; Wan, C.-M.; Gong, S.-T.; Fang, F.; Sun, M.; Qian, Y.; Huang, Y.; Wang, B.-X.; Xu, C.-D.; Ye, L.-Y.; et al. Chinese Clinical Practice Guidelines for Acute Infectious Diarrhea in Children. World J. Pediatr. 2018, 14, 429–436. [Google Scholar] [CrossRef]
- Li, W.; Han, H.; Liu, J.; Ke, B.; Zhan, L.; Yang, X.; Tan, D.; Yu, B.; Huo, X.; Ma, X.; et al. Antimicrobial Resistance Profiles of Salmonella Isolates from Human Diarrhea Cases in China: An Eight-Year Surveilance Study. One Health Adv. 2023, 1, 2. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, S.; Kuang, D.; Hsu, C.-H.; Yin, L.; Luo, Y.; Chen, Z.; Xu, X.; Strain, E.; McDermott, P.; et al. Geography Shapes the Genomics and Antimicrobial Resistance of Salmonella Enterica Serovar Enteritidis Isolated from Humans. Sci. Rep. 2023, 13, 1331. [Google Scholar] [CrossRef]
- Hengkrawit, K.; Tangjade, C. Prevalence and Trends in Antimicrobial Susceptibility Patterns of Multi-Drug-Resistance Non-Typhoidal Salmonella in Central Thailand, 2012–2019. Infect. Drug Resist. 2022, 15, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Singh, K.; Kodgire, P. Biochemical and Molecular Mechanisms of Antibiotic Resistance in Salmonella Spp. Res. Microbiol. 2023, 174, 103985. [Google Scholar] [CrossRef]
- Yang, C.; Xiang, Y.; Qiu, S. Resistance in Enteric Shigella and Nontyphoidal Salmonella: Emerging Concepts. Curr. Opin. Infect. Dis. 2023, 36, 360–365. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Wang, Y.-W.; Chen, B.-H.; Hong, Y.-P.; Teng, R.-H.; Liu, P.-Y.; Chiou, C.-S. Carbapenem Resistance in Extensively Drug-Resistant Salmonella Enterica Serovar Agona and AmpC β-Lactamase-Producing S. Infantis. Microbiol. Spectr. 2023, 11, e02922-23. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO Guidelines on Probiotics: 10 Years Later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Pothuraju, R.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Roy, H.K.; Bouvet, M.; Batra, S.K. Mucins, Gut Microbiota, and Postbiotics Role in Colorectal Cancer. Gut Microbes 2021, 13, 1974795. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.M.S.; Nascimento Da Silva, L.C.; Carmo, M.S. Probiotics, Prebiotics, and Synbiotics in Childhood Diarrhea. Braz. J. Med. Biol. Res. 2024, 57, e13205. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Higgins, S.E.; Vicente, J.L.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Temporal Effects of Lactic Acid Bacteria Probiotic Culture on Salmonella in Neonatal Broilers. Poult. Sci. 2007, 86, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Menconi, A.; Wolfenden, A.D.; Shivaramaiah, S.; Terraes, J.C.; Urbano, T.; Kuttel, J.; Kremer, C.; Hargis, B.M.; Tellez, G. Effect of Lactic Acid Bacteria Probiotic Culture for the Treatment of Salmonella Enterica Serovar Heidelberg in Neonatal Broiler Chickens and Turkey Poults. Poult. Sci. 2011, 90, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.E.; Higgins, J.P.; Wolfenden, A.D.; Henderson, S.N.; Torres-Rodriguez, A.; Tellez, G.; Hargis, B. Evaluation of a Lactobacillus-Based Probiotic Culture for the Reduction of Salmonella Enteritidis in Neonatal Broiler Chicks. Poult. Sci. 2008, 87, 27–31. [Google Scholar] [CrossRef]
- El Hage, R.; El Hage, J.; Snini, S.P.; Ammoun, I.; Touma, J.; Rachid, R.; Mathieu, F.; Sabatier, J.-M.; Abi Khattar, Z.; El Rayess, Y. The Detection of Potential Native Probiotics Lactobacillus Spp. against Salmonella Enteritidis, Salmonella Infantis and Salmonella Kentucky ST198 of Lebanese Chicken Origin. Antibiotics 2022, 11, 1147. [Google Scholar] [CrossRef]
- Fadare, O.S.; Singh, V.; Enabulele, O.I.; Shittu, O.H.; Pradhan, D. In Vitro Evaluation of the Synbiotic Effect of Probiotic Lactobacillus Strains and Garlic Extract against Salmonella Species. LWT 2022, 153, 112439. [Google Scholar] [CrossRef]
- Szajewska, H.; Berni Canani, R.; Domellöf, M.; Guarino, A.; Hojsak, I.; Indrio, F.; Lo Vecchio, A.; Mihatsch, W.A.; Mosca, A.; Orel, R.; et al. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 232–247. [Google Scholar] [CrossRef]
- Muñoz, M.; Castaño, G.E.; Esquivel Suman, R.; Alvarado, M. Septicemia Por Bacillus Clausii Posterior al Uso de Probióticos. Una Complicación Para Tener Presente. Andes Pediatr. 2023, 94, 379. [Google Scholar] [CrossRef]
- Gondwe, E.N.; Molyneux, M.E.; Goodall, M.; Graham, S.M.; Mastroeni, P.; Drayson, M.T.; MacLennan, C.A. Importance of Antibody and Complement for Oxidative Burst and Killing of Invasive Nontyphoidal Salmonella by Blood Cells in Africans. Proc. Natl. Acad. Sci. USA 2010, 107, 3070–3075. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, P.D.; Canals, R.; Ramasamy, M.N. The iNTS-GMMA Vaccine: A Promising Step in Non-Typhoidal Salmonella Vaccine Development. Expert Rev. Vaccines 2023, 22, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative Immunogenicity and Efficacy of Equivalent Outer Membrane Vesicle and Glycoconjugate Vaccines against Nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef]
- Fiorino, F.; Pettini, E.; Koeberling, O.; Ciabattini, A.; Pozzi, G.; Martin, L.B.; Medaglini, D. Long-Term Anti-Bacterial Immunity against Systemic Infection by Salmonella Enterica Serovar Typhimurium Elicited by a GMMA-Based Vaccine. Vaccines 2021, 9, 495. [Google Scholar] [CrossRef]
- Hanumunthadu, B.; Kanji, N.; Owino, N.; Ferreira Da Silva, C.; Robinson, H.; White, R.; Ferruzzi, P.; Nakakana, U.; Canals, R.; Pollard, A.J.; et al. Salmonella Vaccine Study in Oxford (SALVO) Trial: Protocol for an Observer-Participant Blind Randomised Placebo-Controlled Trial of the iNTS-GMMA Vaccine within a European Cohort. BMJ Open 2023, 13, e072938. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Lu, Y.-J.; Malley, R. Overview of the Nontyphoidal and Paratyphoidal Salmonella Vaccine Pipeline: Current Status and Future Prospects. Clin. Infect. Dis. 2020, 71 (Suppl. 2), S151–S154. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.J.; Jang, A.-Y.; Song, J.Y.; Ahn, K.B.; Han, S.H.; Bang, S.J.; Jung, H.K.; Hur, J.; Seo, H.S. Development of Live Attenuated Salmonella Typhimurium Vaccine Strain Using Radiation Mutation Enhancement Technology (R-MET). Front. Immunol. 2022, 13, 931052. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Moscoso, M.; Fuentes-Valverde, V.; Rodicio, M.R.; Herrera-León, S.; Bou, G. A Highly-Safe Live Auxotrophic Vaccine Protecting against Disease Caused by Non-Typhoidal Salmonella Typhimurium in Mice. J. Microbiol. Immunol. Infect. 2023, 56, 324–336. [Google Scholar] [CrossRef]
- Angelakopoulos, H.; Hohmann, E.L. Pilot Study of phoP/phoQ -Deleted Salmonella Enterica Serovar Typhimurium Expressing Helicobacter Pylori Urease in Adult Volunteers. Infect. Immun. 2000, 68, 2135–2141. [Google Scholar] [CrossRef]
- Hindle, Z.; Chatfield, S.N.; Phillimore, J.; Bentley, M.; Johnson, J.; Cosgrove, C.A.; Ghaem-Maghami, M.; Sexton, A.; Khan, M.; Brennan, F.R.; et al. Characterization of Salmonella Enterica Derivatives Harboring Defined aroC and Salmonella Pathogenicity Island 2 Type III Secretion System (ssaV ) Mutations by Immunization of Healthy Volunteers. Infect. Immun. 2002, 70, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Haldar, R.; Dhar, A.; Ganguli, D.; Chakraborty, S.; Pal, A.; Banik, G.; Miyoshi, S.; Das, S. A Candidate Glycoconjugate Vaccine Induces Protective Antibodies in the Serum and Intestinal Secretions, Antibody Recall Response and Memory T Cells and Protects against Both Typhoidal and Non-Typhoidal Salmonella Serovars. Front. Immunol. 2024, 14, 1304170. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Wang, J.Y.; Boyd, M.A.; Tulapurkar, M.E.; Ramachandran, G.; Tennant, S.M.; Pasetti, M.; Galen, J.E.; Levine, M.M. Sustained Protection in Mice Immunized with Fractional Doses of Salmonella Enteritidis Core and O Polysaccharide-Flagellin Glycoconjugates. PLoS ONE 2013, 8, e64680. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Curtis, B.; Toema, D.; Tennant, S.M.; Levine, M.M.; Pasetti, M.F.; Simon, R. Immunogenicity and Efficacy Following Sequential Parenterally-Administered Doses of Salmonella Enteritidis COPS:FliC Glycoconjugates in Infant and Adult Mice. PLoS Negl. Trop. Dis. 2018, 12, e0006522. [Google Scholar] [CrossRef]
- Malley, R.; Lu, Y.-J.; Sebastian, S.; Zhang, F.; Willer, D.O. Multiple Antigen Presenting System (MAPS): State of the Art and Potential Applications. Expert Rev. Vaccines 2024, 23, 196–204. [Google Scholar] [CrossRef]
- Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. [Google Scholar] [CrossRef]
| Antibiotic | Europe | USA | China | Molecular Mechanism of Resistance | Year of Antimicrobial Resistance Assessment |
|---|---|---|---|---|---|
| Ampicilin | 25.2% | 6.6% | 73.4% | β-lactamases | Europe 2020–2021 [6] |
| USA 2004–2016 [24] | |||||
| China 2014–2021 [150] | |||||
| Third-generation cephalosporin | 1.1% | 3% | 20% | genes encoding ESBL blaTEM blaSHV blaCMY blaCTX-M blaOXA | Europe 2020–2021 [6] USA 2004–2016 [24] China 2014–2021 [150] |
| Fluroquinolones | 14.9% | 3% | 16.2% | dual mutations in the gyrA gene single mutation in the parC gene rarely mutations in the gyrB and parE genes PMQR 1 efflux pumps | Europe 2020–2021 [6] USA 2004–2016 [24] China 2014–2021 [150] |
| MDR | 22.6% | 10.3% | 40–81% | Europe 2020–2021 [6] | |
| USA 2004–2016 [24] | |||||
| China 2012–2019 [151,152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sima, C.M.; Buzilă, E.R.; Trofin, F.; Păduraru, D.; Luncă, C.; Duhaniuc, A.; Dorneanu, O.S.; Nastase, E.V. Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment. Curr. Issues Mol. Biol. 2024, 46, 7447-7472. https://doi.org/10.3390/cimb46070442
Sima CM, Buzilă ER, Trofin F, Păduraru D, Luncă C, Duhaniuc A, Dorneanu OS, Nastase EV. Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment. Current Issues in Molecular Biology. 2024; 46(7):7447-7472. https://doi.org/10.3390/cimb46070442
Chicago/Turabian StyleSima, Cristina Mihaela, Elena Roxana Buzilă, Felicia Trofin, Diana Păduraru, Cătălina Luncă, Alexandru Duhaniuc, Olivia Simona Dorneanu, and Eduard Vasile Nastase. 2024. "Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment" Current Issues in Molecular Biology 46, no. 7: 7447-7472. https://doi.org/10.3390/cimb46070442
APA StyleSima, C. M., Buzilă, E. R., Trofin, F., Păduraru, D., Luncă, C., Duhaniuc, A., Dorneanu, O. S., & Nastase, E. V. (2024). Emerging Strategies against Non-Typhoidal Salmonella: From Pathogenesis to Treatment. Current Issues in Molecular Biology, 46(7), 7447-7472. https://doi.org/10.3390/cimb46070442







