Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings?
Abstract
1. Introduction
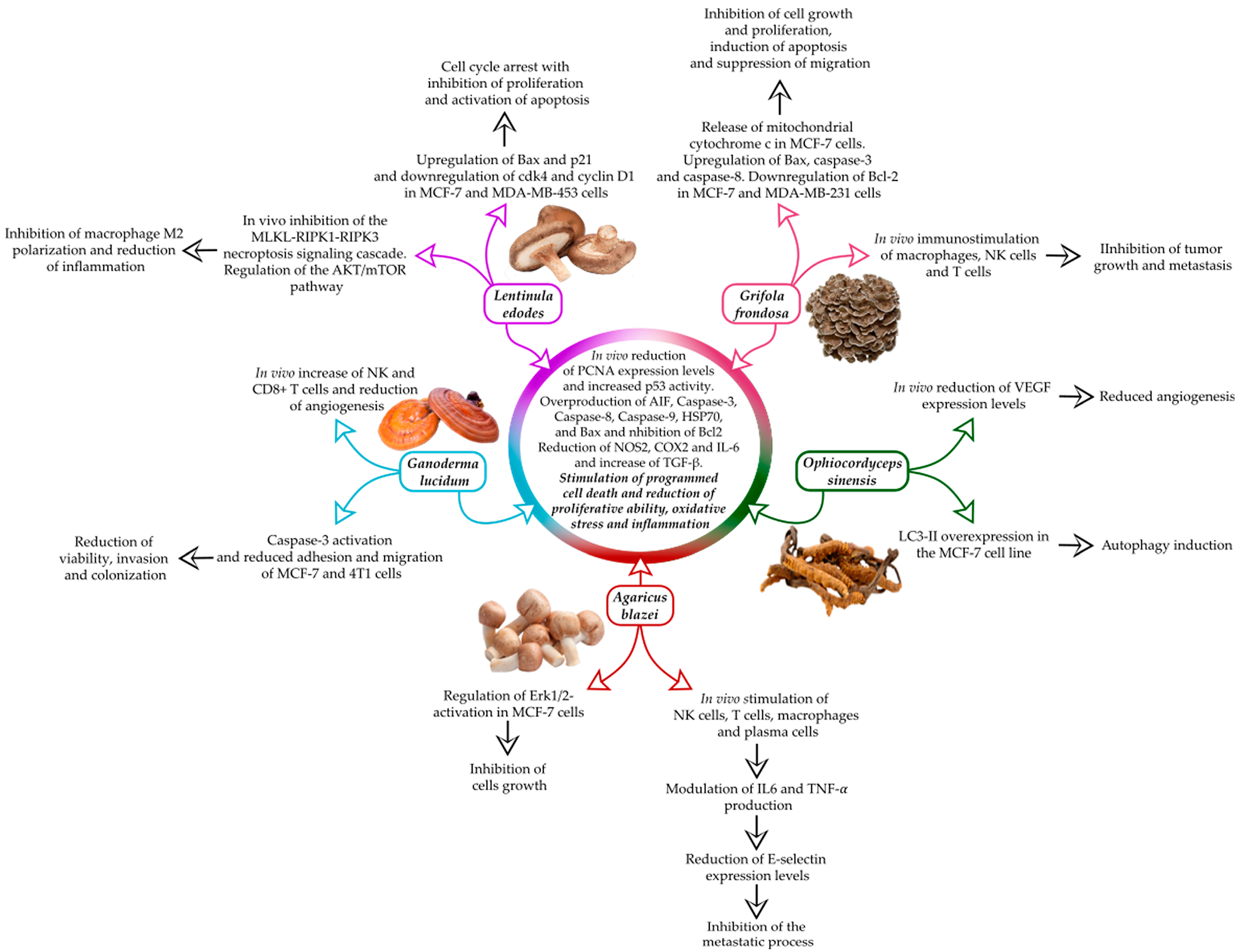
2. Agaricus blazei
3. Ophiocordyceps sinensis
4. Ganoderma lucidum
5. Grifola frondosa
6. Lentinula edodes
7. MMs and MBC Treatment: Limitations and Challenges
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Topcul, M. Triple Negative Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.; Boutsikos, P.; Papageorgis, P. Molecular Mechanisms and Emerging Therapeutic Targets of Triple-Negative Breast Cancer Metastasis. Front. Oncol. 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.-G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer Targets Ther. 2023, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Shah, H.; Yalamarty, S.S.K.; Ataide, J.A.; Torchilin, V.P. Recent Advances with Precision Medicine Treatment for Breast Cancer Including Triple-Negative Sub-Type. Cancers 2023, 15, 2204. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study Using 4t1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; De Luca, F.; Locatelli, C.A.; Ratto, D.; Di Iorio, C.; Savino, E.; Bottone, M.G.; Rossi, P. From a Medicinal Mushroom Blend a Direct Anticancer Effect on Triple-Negative Breast Cancer: A Preclinical Study on Lung Metastases. Molecules 2020, 25, 5400. [Google Scholar] [CrossRef]
- Dan, A.; Swain, R.; Belonce, S.; Jacobs, R.J. Therapeutic Effects of Medicinal Mushrooms on Gastric, Breast, and Colorectal Cancer: A Scoping Review. Cureus 2023, 15, e37574. [Google Scholar] [CrossRef]
- Park, H.-J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Lilburn, C. Medicinal Mushrooms (Specifically Ganoderma lucidum or Reishi) as an Adjuvant Treatment in Breast Cancer. Aust. J. Herb. Naturop. Med. 2022, 34, 27–33. [Google Scholar] [CrossRef]
- Atay, S.; Ak, H.; Kalmis, E.; Kayalar, H.; Aydin, H.H. Transcriptome-Wide Analysis Reveals the Molecular Mechanism of Tumoricidal Effects of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), on MCF-7 Breast Cancer Cells. Int. J. Med. Mushrooms 2021, 23, 91–106. [Google Scholar] [CrossRef]
- Mattila, P.; Suonpää, K.; Piironen, V. Functional Properties of Edible Mushrooms. Nutrition 2000, 16, 694–696. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discov. Today 2022, 27, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals 2022, 15, 176. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune Modulation From Five Major Mushrooms: Application to Integrative Oncology. Integr. Med. 2014, 13, 32–44. [Google Scholar]
- Lyman, G.H.; Michels, S.L.; Reynolds, M.W.; Barron, R.; Tomic, K.S.; Yu, J. Risk of Mortality in Patients with Cancer Who Experience Febrile Neutropenia. Cancer 2010, 116, 5555–5563. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.D.; Roda, E.; Ratto, D.; Desiderio, A.; Venuti, M.T.; Ramieri, M.; Bottone, M.G.; Savino, E.; Rossi, P. Fighting Secondary Triple-Negative Breast Cancer in Cerebellum: A Powerful Aid from a Medicinal Mushrooms Blend. Biomed. Pharmacother. 2023, 159, 114262. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Vaz, J.A.; Vasconcelos, M.H.; Martins, A. Compounds from Wild Mushrooms with Antitumor Potential. Anticancer Agents Med. Chem. 2010, 10, 424–436. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Lin, Y.-C.; Rajendran, P.; Thigarajan, V.; Mathew, D.C.; Lin, K.-Y.; Way, T.-D.; Liao, J.-W.; Yang, H.-L. Antrodia Salmonea Suppresses Invasion and Metastasis in Triple-Negative Breast Cancer Cells by Reversing EMT through the NF-κB and Wnt/β-Catenin Signaling Pathway. Food Chem. Toxicol. 2019, 124, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, E.; Abidin, N.Z.; Abdullah, N. Cytotoxic Proteins from King Tuber Oyster Medicinal Mushroom, Pleurotus Tuber-Regium (Agaricomycetes), Sclerotium against Human MDA-MB-231 Breast Cancer Cells. Int. J. Med. Mushrooms 2022, 24, 27–40. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Mingoti, M.E.D.; Plissari, M.E.; Betti, G.; Roman Junior, W.A.; Luzardo, A.R.; Ignácio, Z.M. Agaricus blazei Murrill Mushroom: A Review on the Prevention and Treatment of Cancer. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I Clinical Study of the Dietary Supplement, Agaricus blazei Murill, in Cancer Patients in Remission. Evid. Based Complement. Altern. Med. 2011, 2011, 192381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Qi, Y. Adjuvant Treatment with Cordyceps sinensis for Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Ethnopharmacol. 2024, 327, 118044. [Google Scholar] [CrossRef]
- Gao, X.; Homayoonfal, M. Exploring the Anti-Cancer Potential of Ganoderma lucidum Polysaccharides (GLPs) and Their Versatile Role in Enhancing Drug Delivery Systems: A Multifaceted Approach to Combat Cancer. Cancer Cell Int. 2023, 23, 324. [Google Scholar] [CrossRef]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A Phase I/II Trial of a Polysaccharide Extract from Grifola Frondosa (Maitake Mushroom) in Breast Cancer Patients: Immunological Effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom Polysaccharide Lentinan for Treating Different Types of Cancers: A Review of 12 Years Clinical Studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef]
- Nagashima, Y.; Yoshino, S.; Yamamoto, S.; Maeda, N.; Azumi, T.; Komoike, Y.; Okuno, K.; Iwasa, T.; Tsurutani, J.; Nakagawa, K.; et al. Lentinula Edodes Mycelia Extract plus Adjuvant Chemotherapy for Breast Cancer Patients: Results of a Randomized Study on Host Quality of Life and Immune Function Improvement. Mol. Clin. Oncol. 2017, 7, 359–366. [Google Scholar] [CrossRef][Green Version]
- Ayeka, P.A. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evid. Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef]
- Rózsa, S.; Măniuțiu, D.-N.; Poșta, G.; Gocan, T.-M.; Andreica, I.; Bogdan, I.; Rózsa, M.; Lazăr, V. Influence of the Culture Substrate on the Agaricus blazei Murrill Mushrooms Vitamins Content. Plants 2019, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Kan, S.-F.; Shu, C.-H.; Lu, T.-J.; Sun-Hwang, L.; Wang, P.S. Inhibitory Mechanisms of Agaricus blazei Murill on the Growth of Prostate Cancer In Vitro and In Vivo. J. Nutr. Biochem. 2009, 20, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Beppu, H.; Akiyama, H.; Wakamatsu, K.; Ito, S.; Kawamoto, Y.; Shimpo, K.; Sumiya, T.; Koike, T.; Matsui, T. Agaritine Purified from Agaricus blazei Murrill Exerts Anti-Tumor Activity against Leukemic Cells. Biochim. Biophys. Acta 2010, 1800, 669–673. [Google Scholar] [CrossRef]
- Hetland, G.; Johnson, E.; Lyberg, T.; Bernardshaw, S.; Tryggestad, A.M.A.; Grinde, B. Effects of the Medicinal Mushroom Agaricus blazei Murill on Immunity, Infection and Cancer. Scand. J. Immunol. 2008, 68, 363–370. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom Extracts and Compounds with Suppressive Action on Breast Cancer: Evidence from Studies Using Cultured Cancer Cells, Tumor-Bearing Animals, and Clinical Trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural Characterization and Immunostimulatory Activity of a Glucan from Natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Winkler, D. Yartsa gunbu (Cordyceps sinensis) and the Fungal Commodification of Tibet’s Rural Economy. Econ. Bot. 2008, 62, 291–305. [Google Scholar] [CrossRef]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The Scientific Rediscovery of an Ancient Chinese Herbal Medicine: Cordyceps sinensis Part I. J. Altern. Complement. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef]
- Holliday, J. On the Trail of the Yak Ancient Cordyceps in the Modern World. 2011. Available online: https://www.researchgate.net/publication/237378518_On_the_Trail_of_The_Yak_Ancient_Cordyceps_in_the_Modern_World (accessed on 3 June 2024).
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The Chemical Constituents and Pharmacological Actions of Cordyceps sinensis. Evid. Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis Polysaccharide Inhibits Colon Cancer Cells Growth by Inducing Apoptosis and Autophagy Flux Blockage via mTOR Signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.-J.; Zhou, X.-W. Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism-A Review. Molecules 2022, 27, 6576. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, J.; Gu, B.; Xiao, Y.; Chen, R.; Liu, X.; Xie, X.; Cao, L. Extracts of Cordyceps sinensis Inhibit Breast Cancer Cell Metastasis via Down-Regulation of Metastasis-Related Cytokines Expression. J. Ethnopharmacol. 2018, 214, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Zhu, J.; Bi, S.; Luo, Y.; Yu, R.; Huang, W.; Song, L. Cordyceps Militaris Fraction Inhibits Angiogenesis of Hepatocellular Carcinoma In Vitro and In Vivo. Pharmacogn. Mag. 2020, 16, 169–176. [Google Scholar] [CrossRef]
- Shaffique, S.; Kang, S.-M.; Kim, A.-Y.; Imran, M.; Aaqil Khan, M.; Lee, I.-J. Current Knowledge of Medicinal Mushrooms Related to Anti-Oxidant Properties. Sustainability 2021, 13, 7948. [Google Scholar] [CrossRef]
- Mohsin, M.; Negi, P.; Ahmed, Z. Determination of the Antioxidant Activity and Polyphenol Contents of Wild Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (W.Curt. Fr.) P. Karst. (Higher Basidiomycetes) from Central Himalayan Hills of India. Int. J. Med. Mushrooms 2011, 13, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-H.; Xiao, D.-M.; Chen, D.-X.; Xiao, Y.; Liang, Z.-Q.; Zhong, J.-J. Polysaccharides from the Medicinal Mushroom Cordyceps Taii Show Antioxidant and Immunoenhancing Activities in a D-Galactose-Induced Aging Mouse Model. Evid. Based Complement. Altern. Med. 2012, 2012, 273435. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, W.; Gao, H.; Chan, E.; Lan, J.; Li, X.; Zhou, S. Antimicrobial Activity of the Medicinal Mushroom Ganoderma. Food Rev. Int. 2005, 21, 211–229. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S.; Ho, C.K. The Anti-Tumor Effect of Ganoderma lucidum Is Mediated by Cytokines Released from Activated Macrophages and T Lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Hsu, M.-J.; Lee, S.-S.; Lee, S.T.; Lin, W.-W. Signaling Mechanisms of Enhanced Neutrophil Phagocytosis and Chemotaxis by the Polysaccharide Purified from Ganoderma lucidum. Br. J. Pharmacol. 2003, 139, 289–298. [Google Scholar] [CrossRef]
- Lee, J.M.; Kwon, H.; Jeong, H.; Lee, J.W.; Lee, S.Y.; Baek, S.J.; Surh, Y.J. Inhibition of Lipid Peroxidation and Oxidative DNA Damage by Ganoderma lucidum. Phytother. Res. 2001, 15, 245–249. [Google Scholar] [CrossRef]
- Zhong, C.; Li, Y.; Li, W.; Lian, S.; Li, Y.; Wu, C.; Zhang, K.; Zhou, G.; Wang, W.; Xu, H.; et al. Ganoderma lucidum Extract Promotes Tumor Cell Pyroptosis and Inhibits Metastasis in Breast Cancer. Food Chem. Toxicol. 2023, 174, 113654. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Acevedo, R.R.; Otero-Franqui, E.; Cubano, L.A.; Dharmawardhane, S.F. Ganoderma lucidum (Reishi) Inhibits Cancer Cell Growth and Expression of Key Molecules in Inflammatory Breast Cancer. Nutr. Cancer 2011, 63, 1085–1094. [Google Scholar] [CrossRef]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-Glucans from Grifola Frondosa and Ganoderma lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V. Maitake Mushroom Extracts Ameliorate Progressive Hypertension and Other Chronic Metabolic Perturbations in Aging Female Rats. Int. J. Med. Sci. 2010, 7, 169–180. [Google Scholar] [CrossRef][Green Version]
- Frenkel, M.; Abrams, D.I.; Ladas, E.J.; Deng, G.; Hardy, M.; Capodice, J.L.; Winegardner, M.F.; Gubili, J.K.; Yeung, K.S.; Kussmann, H.; et al. Integrating Dietary Supplements into Cancer Care. Integr. Cancer Ther. 2013, 12, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola Frondosa) D-Fraction on the Activation of NK Cells in Cancer Patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, D.; Meng, Q.; Guo, W.; Chen, Q.; Zhang, Y. [Corrigendum] Grifola Frondosa Polysaccharides Induce Breast Cancer Cell Apoptosis via the Mitochondrial-Dependent Apoptotic Pathway. Int. J. Mol. Med. 2022, 50, 136. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and Antimetastatic Activity of Maitake D-Fraction in Triple-Negative Breast Cancer Cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef]
- Masuda, Y.; Inoue, M.; Miyata, A.; Mizuno, S.; Nanba, H. Maitake β-Glucan Enhances Therapeutic Effect and Reduces Myelosupression and Nephrotoxicity of Cisplatin in Mice. Int. Immunopharmacol. 2009, 9, 620–626. [Google Scholar] [CrossRef]
- Israilides, C.; Kletsas, D.; Arapoglou, D.; Philippoussis, A.; Pratsinis, H.; Ebringerová, A.; Hríbalová, V.; Harding, S.E. In Vitro Cytostatic and Immunomodulatory Properties of the Medicinal Mushroom Lentinula Edodes. Phytomedicine 2008, 15, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Kong, F.; Lin, J.; Gao, Y. Induction of Immunomodulating Cytokines by a New Polysaccharide-Peptide Complex from Culture Mycelia of Lentinus edodes. Immunopharmacology 1998, 40, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cai, W.; Ren, Z.; Jia, L.; Zhang, J. Antioxidant and Hepatoprotective Effects of Acidic-Hydrolysis Residue Polysaccharides from Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) in Mice. Int. J. Med. Mushrooms 2021, 23, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ishikawa, S.; Matsui, Y.; Tamesada, M.; Harashima, N.; Harada, M. Oral Ingestion of Lentinula Edodes Mycelia Extract Inhibits B16 Melanoma Growth via Mitigation of Regulatory T Cell-Mediated Immunosuppression. Cancer Sci. 2011, 102, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsui, Y.; Ishikawa, S.; Kawanishi, T.; Harada, M. Oral Ingestion of Lentinula Edodes Mycelia Extract Can Restore the Antitumor T Cell Response of Mice Inoculated with Colon-26 Cells into the Subserosal Space of the Cecum. Oncol. Rep. 2012, 27, 325–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alagbaoso, C.A.; Mizuno, M. Polysaccharides from Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) Suppress pMLKL-Mediated Necroptotic Cell Death and Colitis in Mice. Int. J. Med. Mushrooms 2021, 23, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Patel, K.; Belgamwar, V.; Wadher, K. Functional Polysaccharide Lentinan: Role in Anti-Cancer Therapies and Management of Carcinomas. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100045. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, Q.; Feng, J.; Zhang, X.; Li, T.; Liu, S.; Chen, Y.; Li, X.; Wu, Q.; Xue, Y.; et al. β-Glucan Produced by Lentinus edodes Suppresses Breast Cancer Progression via the Inhibition of Macrophage M2 Polarization by Integrating Autophagy and Inflammatory Signals. Immun. Inflamm. Dis. 2023, 11, e876. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Cheng, L.P.; Zhou, L.M.; Cheung, Y.T.; Zuo, Z. Herb-Drug Interactions between the Medicinal Mushrooms Lingzhi and Yunzhi and Cytotoxic Anticancer Drugs: A Systematic Review. Chin. Med. 2020, 15, 75. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Narayanan, S.; de Mores, A.R.; Cohen, L.; Anwar, M.M.; Lazar, F.; Hicklen, R.; Lopez, G.; Yang, P.; Bruera, E. Medicinal Mushroom Supplements in Cancer: A Systematic Review of Clinical Studies. Curr. Oncol. Rep. 2023, 25, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-S.; Kim, D.-J.; Chae, G.-T.; Lee, J.-M.; Bae, S.-M.; Sin, J.-I.; Kim, Y.-W.; Namkoong, S.-E.; Lee, I.P. Natural Killer Cell Activity and Quality of Life Were Improved by Consumption of a Mushroom Extract, Agaricus blazei Murill Kyowa, in Gynecological Cancer Patients Undergoing Chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Uno, K. Efficacy of Orally Administered Lentinula Edodes Mycelia Extract for Advanced Gastrointestinal Cancer Patients Undergoing Cancer Chemotherapy: A Pilot Study. Asian Pac. J. Cancer Prev. 2011, 12, 1671–1674. [Google Scholar] [PubMed]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of Oral Administration of Lentinula Eododes Mycelia Extract for Breast Cancer Patients Undergoing Postoperative Hormone Therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of Host Quality of Life and Immune Function in Breast Cancer Patients Treated with Combination of Adjuvant Chemotherapy and Oral Administration of Lentinula Edodes Mycelia Extract. OncoTargets Ther. 2013, 6, 853. [Google Scholar] [CrossRef] [PubMed]
- Comen, E.A.; Bowman, R.L.; Kleppe, M. Underlying Causes and Therapeutic Targeting of the Inflammatory Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, Q.; Wei, Y.; Yuan, H. Secondary Metabolites from the Cultures of Medicinal Mushroom Vanderbylia Robiniophila and Their Tyrosinase Inhibitory Activities. J. Fungi 2023, 9, 702. [Google Scholar] [CrossRef]
| Medicinal Mushroom | Bioactive Compound | Mechanism of Action | Outcome |
|---|---|---|---|
| Lentinula edodes | Lentinan, L. edodes mycelia (LEM) and β-glucans |
|
|
| Grifola frondosa | Maitake D-Fraction, ascorbic acid, flavonoids, α-tocopherol residues, β-glucans, flavonoids and fatty acids |
|
|
| Ganoderma lucidum | β-glucan polysaccharides and aminopolysaccharides |
|
|
| Ophiocordyceps sinensis | O. sinensis-derived polysaccharide (CSP-1 and CSP-2), APS, ergosterol, cordycepin, EPSF |
|
|
| Agaricus blazei | Proteoglycans, highly branched β1,3-/1,6-glucans, provitamin D2 and agaritine |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, F.; Roda, E.; Rossi, P.; Bottone, M.G. Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings? Curr. Issues Mol. Biol. 2024, 46, 7577-7591. https://doi.org/10.3390/cimb46070450
De Luca F, Roda E, Rossi P, Bottone MG. Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings? Current Issues in Molecular Biology. 2024; 46(7):7577-7591. https://doi.org/10.3390/cimb46070450
Chicago/Turabian StyleDe Luca, Fabrizio, Elisa Roda, Paola Rossi, and Maria Grazia Bottone. 2024. "Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings?" Current Issues in Molecular Biology 46, no. 7: 7577-7591. https://doi.org/10.3390/cimb46070450
APA StyleDe Luca, F., Roda, E., Rossi, P., & Bottone, M. G. (2024). Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings? Current Issues in Molecular Biology, 46(7), 7577-7591. https://doi.org/10.3390/cimb46070450










