Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer
Abstract
1. Introduction
2. Data Collection Methodology
3. Withania somnifera
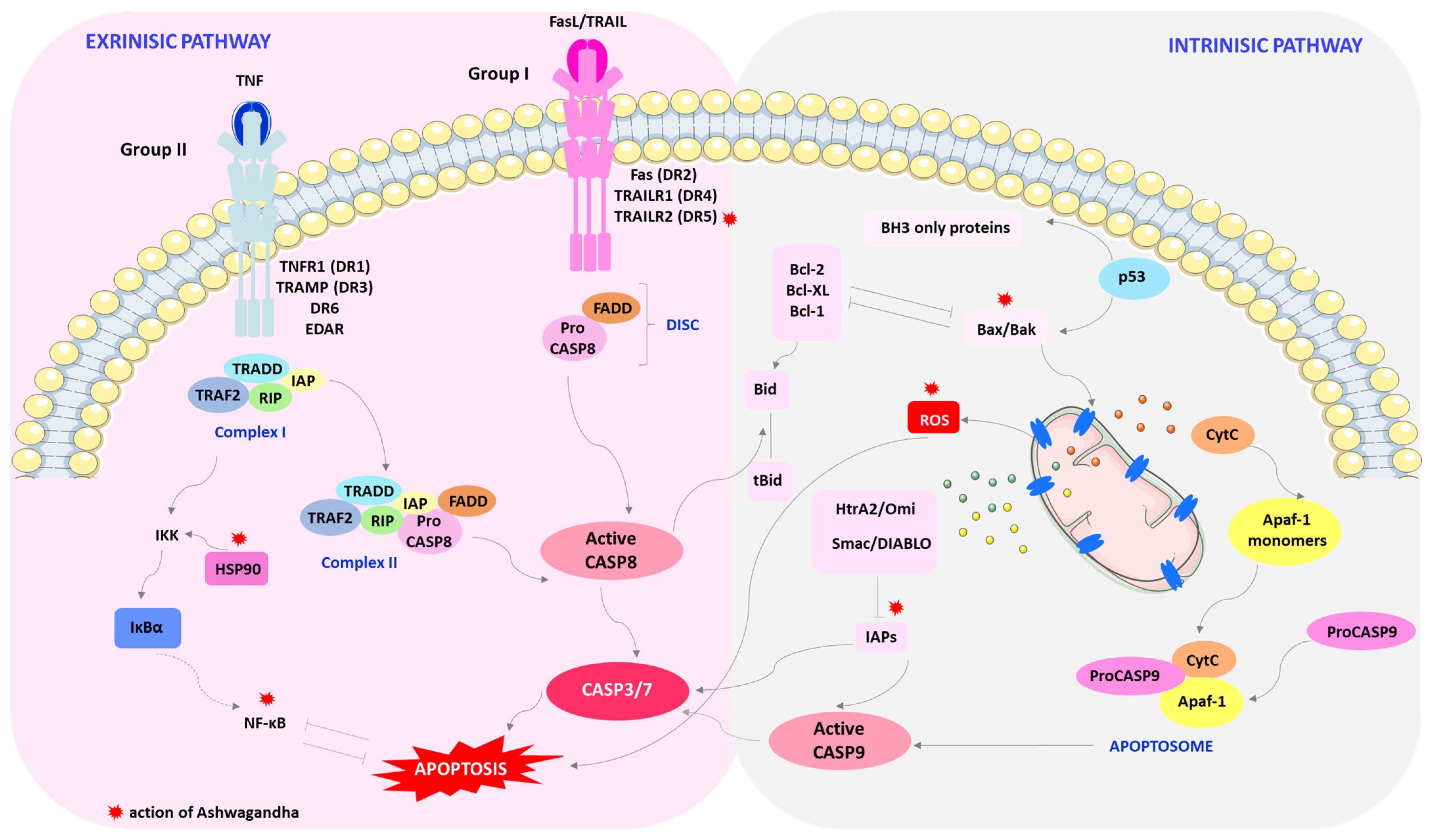
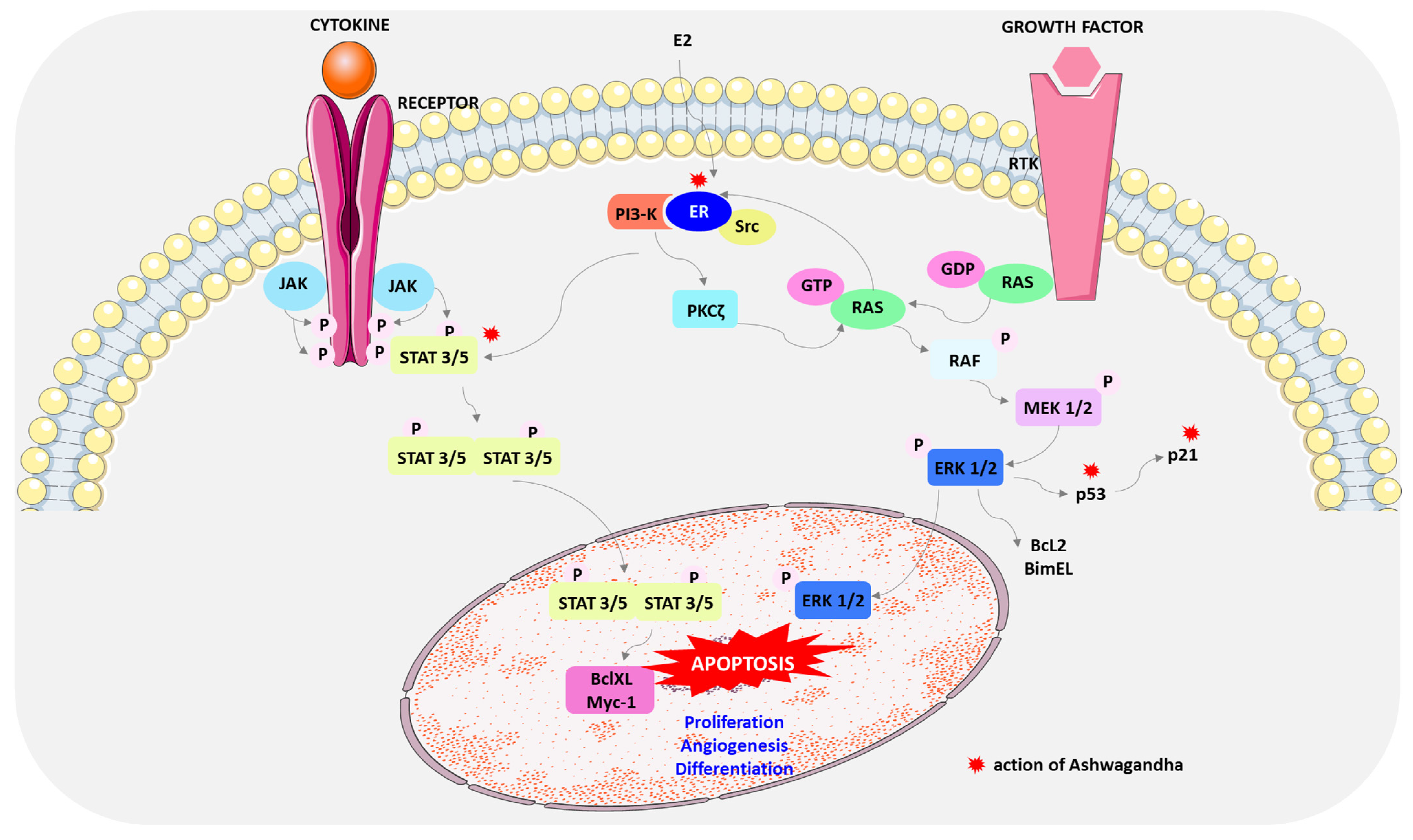
4. Mechanism of Breast Cancer Cell Death by Ashwagandha
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Jacques Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Dizon, D.S.; Kamal, A.H. Cancer statistics 2024: All hands on deck. CA Cancer J. Clin. 2024, 74, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Siddig, A.; Tengku Din, T.A.D.A.; Mohd Nafi, S.N.; Yahya, M.M.; Sulong, S.; Wan Abdul Rahman, W.F. The unique biology behind the early onset of breast cancer. Genes 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Carmen Criscitiello, C.; Corti, C. Breast cancer genetics: Diagnostics and treatment. Genes 2022, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Mohi ud din, N.; Ahmad Dar, R.; Rasool, M.; Assad, A. Breast cancer detection using deep learning: Datasets, methods, and challenges ahead. Comput. Biol. Med. 2022, 149, 106073. [Google Scholar] [CrossRef]
- Bhimani, C.; Matta, D.; Roth, R.G.; Liao, L.; Tinney, E.; Brill, K.; Germaine, P. Contrast-enhanced spectral mammography: Technique, indications, and clinical applications. Acad. Radiol. 2017, 24, 84–88. [Google Scholar] [CrossRef]
- Krzakowski, M.; Rutkowski, P.; Jassem, J.; Zaucha, R.; Fijuth, J.; Słuszniak, J.; Jarząb, B.; Zegarski, W.; Małkowski, B.; Kawecki, A.; et al. Recommendations on the application of positron emission tomography in oncology. Oncol. Clin. Pract. 2015, 11, 155–171. [Google Scholar]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.N.; Mesele, B.W.; Haile, D.C.; Sophia Khalayi, S. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Chander, H.; Munshi, A. Mechanisms of anti-tumor activity of Withania somnifera (Ashwagandha). Nutr. Cancer 2021, 73, 914–926. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health. Ashwagandha. Available online: https://www.nccih.nih.gov/health/ashwagandha#:~:text=What%20Have%20We%20Learned%3F,testosterone%20levels%20and%20sperm%20quality (accessed on 12 July 2024).
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by leaf extract of ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, B.; Rath, P.C.; Rao, A.R.; Singh, R.P. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid. Based Complement. Alternat. Med. 2005, 2, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.L.; Lee, J.; Hahm, E.-R.; Kim, S.-H.; Marcus, A.I.; Kumari, V.; Ji, X.; Yang, Z.; Vowell, C.L.; Wipf, P.; et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J. Biol. Chem. 2014, 289, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in integrative oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Rao, A.S.; Nandal, A.; Kumar, S.; Ganaie, S.A.; Narasihman, B. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J. Ethnopharmacol. 2021, 270, 113704. [Google Scholar] [CrossRef] [PubMed]
- Govindaram, L.K.; Al Bratty, M.; Alhazmi, H.A.; Kandasamy, R.; Thangavel, N.; Ibrahim, A.M.; Mariya, G.A.; Kumar, P. Formulation, biopharmaceutical evaluation and in-vitro screening of polyherbal phytosomes for breast cancer therapy. Drug Dev. Ind. Pharm. 2022, 48, 552–565. [Google Scholar] [CrossRef]
- Srivastava, A.N.; Ahmad, R.; Khan, M.A. Evaluation and comparison of the in vitro cytotoxic activity of Withania somnifera methanolic and ethanolic extracts against MDA-MB-231 and vero cell lines. Sci. Pharm. 2015, 84, 41–59. [Google Scholar] [CrossRef]
- Jawarneh, S.; Talib, W.H. Combination of Ashwagandha water extract and intermittent fasting as a therapy to overcome cisplatin resistance in breast cancer: An in vitro and in vivo study. Front. Nutr. 2022, 9, 863619. [Google Scholar] [CrossRef]
- Cavaleri, F.; Chattopadhyay, S.; Palsule, V.; Kar, P.K.; Chatterjee, R. Study of drug targets associated with oncogenesis and cancer cell survival and the therapeutic activity of engineered Ashwagandha extract having differential Withanolide constitutions. Integr. Cancer Ther. 2024, 23, 15347354231223499. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ranga, R.S.; Burikhanov, R.; Han, S.S.; Chendil, D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007, 67, 246–253. [Google Scholar] [CrossRef]
- Ahmad, R.; Fatima, A.; Srivastava, A.N.; Ali Khan, M. Evaluation of apoptotic activity of Withania coagulans methanolic extract against human breast cancer and Vero cell lines. J. Ayurveda Integr. Med. 2017, 8, 177–183. [Google Scholar] [CrossRef]
- Stan, S.D.; Hahm, E.R.; Warin, R.; Singh, S.V. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008, 68, 7661–7669. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Singh, R.; Shandilya, A.; Priyandoko, D.; Agrawal, V.; Bisaria, V.S.; Wadhwa, R.; Kaul, S.C.; Sundar, D. Ashwagandha derived withanone targets TPX2-aurora A complex: Computational and experimental evidence to its anticancer activity. PLoS ONE 2012, 7, e30890. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Lee, J.; Hahm, E.-R.; Singh, S.V. Peptidyl-prolyl cis/trans isomerase Pin1 regulates withaferin A-mediated cell cycle arrest in human breast cancer cells. Mol. Carcinog. 2018, 57, 936–946. [Google Scholar] [CrossRef]
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, e23354. [Google Scholar] [CrossRef]
- Gambhir, L.; Checker, R.; Sharma, D.; Thoh, M.; Patil, A.; Degani, M.; Vikram Gota, V.; Sandur, S.K. Thiol dependent NF-κB suppression and inhibition of T-cell mediated adaptive immune responses by a naturally occurring steroidal lactone Withaferin A. Toxicol. Appl. Pharmacol. 2015, 289, 297–312. [Google Scholar] [CrossRef]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Kaileh, M.; Vanden Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin a strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef]
- Lee, J.; Hahm, E.R.; Singh, S.V. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 2010, 31, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Lee, J.; Huang, Y.; Singh, S.V. Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol. Carcinog. 2011, 50, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Stan, S.D.; Zeng, Y.; Singh, S.V. Ayurvedic medicine constituent withaferin A causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer 2008, 60, 51–60. [Google Scholar] [CrossRef]
- Meher, K.; Paithankar, H.; Hosur, R.V.; Lopus, M. Ashwagandha-polyphenols-functionalized gold nanoparticles facilitate apoptosis by perturbing microtubule assembly dynamics in breast cancer cells. J. Drug Deliv. Sci. Technol. 2022, 70, 103225. [Google Scholar] [CrossRef]
- Yang, H.; Shi, G.; Dou, Q.P. The tumor proteasome is a primary target for the natural anticancer compound withaferin A isolated from “Indian winter cherry”. Mol. Pharmacol. 2007, 71, 426–437. [Google Scholar] [CrossRef]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Lee, J.; Hahm, E.-R.; Marcus, A.I.; Singh, S.V. Withaferin A inhibits experimental epithelial-mesenchymal transition in MCF-10a cells and suppresses vimentin protein level in vivo in breast tumors. Mol. Carcinog. 2015, 54, 417–429. [Google Scholar] [CrossRef]
- Yang, Z.; Garcia, A.; Xu, S.; Powell, D.R.; Vertino, P.M.; Singh, S.; Marcus, A.I. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS ONE 2013, 8, e75069. [Google Scholar] [CrossRef] [PubMed]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.; et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 2011, 129, 2744–2755. [Google Scholar] [CrossRef]
- Szarc vel Szic, K.; Op de Beeck, K.; Ratman, D.; Wouters, A.; Beck, I.M.; Declerck, K.; Heyninck, K.; Fransen, E.; Bracke, M.; De Bosscher, K.; et al. Pharmacological levels of withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS ONE 2014, 9, e87850. [Google Scholar] [CrossRef]
- Gao, R.; Shah, N.; Lee, J.-S.; Katiyar, S.P.; Li, L.; Oh, E.; Sundar, D.; Yun, C.-O.; Wadhwa, R.; Kaul, S.C. Withanone-rich combination of Ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol. Cancer Ther. 2014, 13, 2930–2940. [Google Scholar] [CrossRef]
- Lee, J.; Sehrawat, A.; Singh, S.V. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res. Treat. 2012, 136, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Nabi, M.; Tabassum, N.; Afzal, S.; Ayoub, M. An updated review on phytochemistry and molecular targets of Withania somnifera (L.) Dunal (Ashwagandha). Front. Pharmacol. 2023, 14, 1049334. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Farah, M.A.; Al-Anazi, K.M.; Basha, S.H.; Bai, F.; Lee, J.; Al-Hemaid, F.M.A.; Mahmoud, A.H.; Hailan, W.A.Q. In silico elucidation of the plausible inhibitory potential of Withaferin A of Withania somnifera medicinal herb against Breast cancer targeting estrogen receptor. Curr. Pharm. Biotechnol. 2020, 21, 842–851. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Kim, S.-H.; Singh, K.B.; Singh, K.; Singh, S.V. A comprehensive review and perspective on anticancer mechanisms of withaferin A in breast cancer. Cancer Prev. Res. 2020, 13, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Vashi, R.; Patel, B.M.; Goyal, R.K. Keeping abreast about ashwagandha in breast cancer. J. Ethnopharmacol. 2021, 269, 113759. [Google Scholar] [CrossRef] [PubMed]
- Mallipeddi, H.; Thyagarajan, A.; Sahu, R.P. Implications of Withaferin-A for triple-negative breast cancer chemoprevention. Biomed. Pharmacother. 2021, 134, 111124. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Saggam, A.; Guruprasad, K.P.; Tillu, G.; Patwardhan, B.; Satyamoorthy, K. Molecular mechanisms of Asparagus racemosus willd. and Withania somnifera (L.) Dunal as chemotherapeutic adjuvants for breast cancer treatment. J. Ethnopharmacol. 2024, 331, 118261. [Google Scholar] [CrossRef] [PubMed]
- Sivasankarapillai, V.S.; Nair, R.M.K.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. Int. 2020, 27, 26025–26035. [Google Scholar] [CrossRef]
- Rahman, M.M.; Huixin Wu, H.; Tollefsbol, T.O. A novel combinatorial approach using sulforaphane- and withaferin A-rich extracts for prevention of estrogen receptor-negative breast cancer through epigenetic and gut microbial mechanisms. Sci. Rep. 2024, 14, 12091. [Google Scholar] [CrossRef]
- Ahmad, M.; Dar, N.J. Withania somnifera: Ethnobotany, Pharmacology, and Therapeutic Functions. In Sustained Energy for Enhanced Human Functions and Activity; Academic Press: Cambridge, MA, USA, 2017; pp. 137–154. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Winters, M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern. Med. Rev. 2006, 11, 269–277. [Google Scholar] [PubMed]
- Bhat, J.A.; Akther, T.; Najar, R.A.; Rasool, F.; Hamid, A. Withania somnifera (L.) Dunal (Ashwagandha); current understanding and future prospect as a potential drug candidate. Front. Pharmacol. 2022, 13, 1029123. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Ayatollahi, S.A.; Kobarfard, F.; Staniak, M.; Stępień, A.; Czopek, K.; Sen, S.; Acharya, K.; Matthews, K.R.; et al. Chemical composition, biological activity, and health-promoting effects of Withania somnifera for pharma-food industry applications. J. Food Qual. 2021, 2021, 8985179. [Google Scholar] [CrossRef]
- Gaurav, H.; Yadav, D.; Maurya, A.; Yadav, H.; Yadav, R.; Shukla, A.C.; Sharma, M.; Gupta, V.K.; Palazon, J. Biodiversity, biochemical profiling, and pharmaco-commercial applications of Withania somnifera: A Review. Molecules 2023, 28, 1208. [Google Scholar] [CrossRef] [PubMed]
- Uddin, Q.; Samiulla, L.; Singh, V.K.; Jamil, S.S. Phytochemical and pharmacological profile of Withania somnifera Dunal: A Review. J. Appl. Pharm. Sci. 2012, 2, 170–175. [Google Scholar]
- Visweswari, G.; Christopher, R.; Rajendra, W. Phytochemical screening of active secondary metabolites present in Withania somnifera root: Role in traditional medicine. Int. J. Pharm. Sci. Res. 2013, 4, 2770–2776. [Google Scholar] [CrossRef]
- Bhatia, A.; Bharti, S.K.; Tewari, S.K.; Sidhu, O.P.; Roy, R. Metabolic profiling for studying chemotype variations in Withania somnifera (L.) Dunal fruits using GC–MS and NMR spectroscopy. Phytochemistry 2013, 93, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Hossain, M.; Mottalib, M.A.; Sulaiman, S.A.; Gan, S.H.; Khalil, M.I. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement. Altern. Med. 2012, 12, 175. [Google Scholar] [CrossRef]
- Ozeer, F.Z.; Nagandran, S.; Wu, Y.S.; Wong, L.S.; Stephen, A.; Lee, M.F.; Kijsomporn, J.; Guad, R.M.; Batumalaie, K.; Oyewusi, H.A.; et al. A comprehensive review of phytochemicals of Withania somnifera (L.) Dunal (Solanaceae) as antiviral therapeutics. Discov. Appl. Sci. 2024, 6, 187. [Google Scholar] [CrossRef]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N.A. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef]
- Bahira, S.; Moharana, T.; Sahu, R.R.; Raut, S.; Behera, S. In vitro study of the antibacterial activities of Withania somnifera leaf extract against human pathogenic bacteria. Int. J. Pharm. Sci. Rev. Res. 2019, 56, 139–145. [Google Scholar]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effect of Ashwagandha: A preliminary study in vitro. Pharmacogn. J. 2012, 4, 47–49. [Google Scholar] [CrossRef]
- Pandey, A.; Bani, S.; Dutt, P.; Satti, N.K.; Suri, K.A.; Qazi, G.N. Multifunctional neuroprotective effect of withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rauf, A.; Akhter, S.; Islam, M.N.; Emran, T.B.; Mitra, S.; Khan, I.N.; Mubarak, M.S. Role of withaferin A and its derivatives in the management of Alzheimer’s disease: Recent trends and future perspectives. Molecules 2021, 26, 3696. [Google Scholar] [CrossRef]
- Singh, M.; Ramassamy, C. In vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J. Nutr. Sci. 2017, 6, e54. [Google Scholar] [CrossRef]
- Wongtrakul, J.; Thongtan, T.; Kumrapich, B.; Saisawang, C.; Ketterman, A.J. Neuroprotective effects of Withania somnifera in the SH-SY5Y Parkinson cell model. Heliyon 2021, 7, e08172. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J. Med. Food 2009, 12, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Kumar, V.; Kaznacheyeva, E.V.; Jana, N.R. Withaferin A induces heat shock response and ameliorates disease progression in a mouse model of Huntington’s disease. Mol. Neurobiol. 2021, 58, 3992–4006. [Google Scholar] [CrossRef] [PubMed]
- Anju, T.R.; Smijin, S.; Jobin, M.; Paulose, C.S. Altered muscarinic receptor expression in the cerebral cortex of epileptic rats: Restorative role of Withania somnifera. Biochem. Cell Biol. 2018, 96, 433–440. [Google Scholar] [CrossRef]
- SoRelle, J.A.; Itoh, T.; Peng, H.; Kanak, M.A.; Sugimoto, K.; Matsumoto, S.; Levy, M.S.; Lawrence, M.C.; Naziruddin, B. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia 2013, 56, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Auddy, B.; Hazra, J.; Mitra, A.; Abedon, B. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: A double-blind, randomized, placebo-controlled study. J. Am. Neutraceut. Assoc. 2008, 11, 50–56. [Google Scholar]
- Majeed, M.; Nagabhushanam, K.; Mundkur, L. A standardized Ashwagandha root extract alleviates stress, anxiety, and improves quality of life in healthy adults by modulating stress hormones: Results from a randomized, double-blind, placebo-controlled study. Medicine 2023, 102, 41. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.K.; Peasah-Darkwah, G.; Dhasmana, A.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Withania somnifera: Progress towards a pharmaceutical agent for immunomodulation and cancer therapeutics. Pharmaceutics 2022, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Singh, S.V.; Kensler, T.W. Withania somnifera: From prevention to treatment of cancer. Mol. Nutr. Food Res. 2016, 60, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Poxviral strategies to overcome host cell ppoptosis. Pathogens 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.-e.-S. Understanding apoptosis and apoptotic pathways targeted cancer herapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-dos-Santos, Â.; Cell’s Fate, A. An overview of the molecular biology and genetics of apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Schlereth, K.; Charles, J.P.; Bretz, A.C.; Stiewe, T. Life or death: p53-induced apoptosis requires DNA binding cooperativity. Cell Cycle 2010, 15, 4068–4076. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Shukla, Y. Death receptors: Targets for cancer therapy. Exp. Cell Res. 2010, 316, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.W.; Seo, J.; Jeong, M.; Lee, S.; Song, J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012, 45, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hawke, N.; Baldwin, A.S. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006, 13, 738–747. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecul ar basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, 78–103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting apoptosis, a promising way to treat breast cancer with natural products: A comprehensive review. Front. Pharmacol. 2022, 12, 801662. [Google Scholar] [CrossRef]
- Dar, P.A.; Mir, S.A.; Bhat, J.A.; Hamid, A.; Singh, L.R.; Malik, F.; Dar, T.A. Ananti-cancerous protein fraction from Withania somnifera induces ROS dependent mitochondria-mediated apoptosis in human MDA-MB-231 breast cancer cells. Int. J. Biol. Macromol. 2019, 135, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Priyandoko, D.; Shah, N.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by Ashwagandha leaf extract and its component Withanone involves ROS signaling. PLoS ONE 2010, 5, e13536. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, A.; Samanta, S.K.; Hahm, E.R.; St Croix, C.; Watkins, S.; Singh, S.V. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion 2019, 47, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Singh, S.V. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr. Cancer Drug Targets 2013, 13, 640–650. [Google Scholar] [CrossRef]
- Jamalzadeh, L.; Ghafoori, H.; Aghamaali, M.; Sariri, R. Induction of apoptosis in human breast cancer MCF-7 cells by a semi-synthetic derivative of artemisinin: A caspase-related mechanism. Iran. J. Biotechnol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression. Cancer Lett. 2013, 334, 101–108. [Google Scholar] [CrossRef]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. The role of TRAIL in apoptosis and immunosurveillance in cancer. Cancers 2023, 15, 2752. [Google Scholar] [CrossRef] [PubMed]
- Ralff, M.D.; El-Deiry, W.S. TRAIL pathway targeting therapeutics. Expert. Rev. Precis. Med. Drug Dev. 2018, 3, 197–204. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Snajdauf, M.; Havlova, K.; Vachtenheim, J., Jr.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Smrz, D.; Strizova, Z. The TRAIL in the treatment of human cancer: An update on clinical trials. Front. Mol. Biosci. 2021, 8, 628332. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Endo, Y.; Dine, J.L.; Lipkowitz, S. Targeting TRAIL death receptors in triple-negative breast cancers: Challenges and strategies for cancer therap. Cells 2022, 11, 3717. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, A.; Kuppusamy, P.; Singh, S.V.; Sharma, D.; Saxena, N.K. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014, 74, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Martin, T.A.; Davies, E.L.; Ruge, F.; Yu, H.; Zhang, Y.; Teng, X.; Jiang, W.G. The clinical implications of RSK1-3 in human breast cancer. Anticancer Res. 2016, 36, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mukerji, R.; Samadi, A.K.; Cohen, M.S. Down-regulation of estrogen receptor-alpha and rearranged during transfection tyrosine kinase is associated with withaferin a-induced apoptosis in MCF-7 breast cancer cells. BMC Compl. Altern. Med. 2011, 11, 84. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signalling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Powers, M.V.; Workman, P. Targeting of multiple signaling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr. Relat. Cancer 2006, 13, 125–135. [Google Scholar] [CrossRef]
- Wang, H.-C.; Tsai, Y.-L.; Wu, Y.-C.; Chang, F.-R.; Liu, M.-H.; Chen, W.-Y.; Wu, C.-C. Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90. PLoS ONE 2012, 27, 1984–2009. [Google Scholar] [CrossRef]
- Bar-Natan, M.; Nelson, E.A.; Xiang, M.; Frank, D.A. STAT signaling in the pathogenesis and treatment of myeloid malignancies. JAKSTAT 2012, 1, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Blaskovich, M.A.; Forinash, K.D.; Sebti, S.M. Withacnistin inhibits recruitment of STAT3 and STAT5 to growth factor and cytokine receptors and induces regression of breast tumours. Br. J. Cancer 2014, 26, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Arlinghaus, R.B. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005, 65, 2532–2536. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in oncogenesis. Biomedicines 2020, 8, 316. [Google Scholar] [CrossRef]
- Igelmann, S.; Neubauer, H.A.; Ferbeyre, G. STAT3 and STAT5 activation in solid cancers. Cancers 2019, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Lee, J.; Singh, S.V. Role of mitogen-activated protein kinases and mcl-1 in apoptosis induction by withaferin a in human breast cancer cells. Mol. Carcinog. 2014, 53, 907–916. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef]
- Jiang, M.; Wen, F.; Cao, J.; Li, P.; She, J.; Chu, Z. Genome-wide exploration of the molecular evolution and regulatory network of mitogen-activated protein kinase cascades upon multiple stresses in Brachypodium distachyon. BMC Genom. 2015, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.F.; Braun, B.S.; Shannon, K.M. Targeting oncogenic Ras signaling in hematologic malignancies. Blood 2012, 120, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, X.; Geng, M.; Huang, M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B 2018, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Castoria, G.; Migliaccio, A.; Di Domenico, M.; Lombardi, M.; de Falco, A.; Varricchio, L.; Bilancio, A.; Barone, M.V.; Auricchio, F. Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells. Mol. Cell Biol. 2004, 24, 7643–7653. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Choi, Y. Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep. 2018, 51, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Totta, P.; Ogawa, S.; Cardillo, I.; Inoue, S.; Leone, S.; Trentalance, A.; Muramatsu, M.; Marino, M. Survival versus apoptotic 17β-estradiol effect: Role of ERα and ERβ activated non-genomic signaling. J. Cell Physiol. 2005, 203, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Björnström, L.; Sjöberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lee, E.J.; Madison, L.D.; Lazennec, G. Expression of estrogen receptor b in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004, 566, 169–172. [Google Scholar] [CrossRef]
- Paruthiyil, S.; Parmar, H.; Kerekatte, V.; Cunha, G.R.; Firestone, G.L.; Leitman, D.C. Estrogen receptor b inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004, 64, 423–428. [Google Scholar] [CrossRef]
- Sayeed, A.; Konduri, S.D.; Liu, W.; Bansal, S.; Li, F.; Das, G.M. Estrogen receptor alpha inhibits p53-mediated transcriptional repression: Implications for the regulation of apoptosis. Cancer Res. 2007, 67, 7746–7755. [Google Scholar] [CrossRef] [PubMed]
- Esseghir, S.; Todd, S.K.; Hunt, T.; Poulsom, R.; Plaza-Menacho, I.; Reis-Filho, J.S.; Isacke, C.M. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor upregulation in breast cancer. Cancer Res. 2007, 67, 11732–11741. [Google Scholar] [CrossRef]
- Janku, F.; McConkeya, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, N.; Siddharth, S.; Nagalingam, A.; Walker, A.; Woo, J.; Gyorffy, B.; Gabrielsona, E.; Saxena, N.K.; Sharma, D. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis 2019, 40, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; De, S.; Mukherjee, S.; Das, S.; Ghosh, A.N.; Sengupta, S.B. Withaferin A induced impaired autophagy and unfolded protein response in human breast cancer cell-lines MCF-7 and MDA-MB-231. Toxicol. Vitr. 2017, 44, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; De, S.; Das, S.; Mukherjee, S.; Bandyopadhyay, S. Withaferin A induces ROS-mediated paraptosis in human breast cancer cell-lines MCF-7 and MDA-MB-231. PLoS ONE 2016, 11, e168488. [Google Scholar] [CrossRef]
- Biswal, B.M.; Sulaiman, A.M.; Ismail, H.C.; Zakaria, H.; Jalil Abdul, M.I.; Muhammad, K.I. AOS14 Phase II clinical study of combination chemotherapy with herb Withania somnifera (ashwagandha) in breast cancer. Eur. J. Cancer 2012, 48, 8–9. [Google Scholar] [CrossRef]
- Biswal, B.M.; Sulaiman, S.A.; Ismail, H.C.; Zakaria, H.; Musa, K.I. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr. Cancer Ther. 2013, 12, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Afewerky, H.K.; Ayodeji, A.E.; Tiamiyu, B.B.; Orege, J.I.; Okeke, E.S.; Oyejobi, A.O.; Bate, P.N.N.; Adeyemi, S.B. Critical review of the Withania somnifera (L.) Dunal: Ethnobotany, pharmacological efficacy, and commercialization significance in Africa. Bull. Natl. Res. Cent. 2021, 45, 176. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S.; Avula, B.; Khan, I.A.; Jonasson, J.C.; Ghabril, M.; Hayashi, P.H.; Navarro, V. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020, 40, 825–829. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J. Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: A systematic review of human trialsexternal link disclaimer. J. Herb. Med. 2021, 28, 100434. [Google Scholar] [CrossRef]
- Gannon, J.M.; Forrest, P.E.; Roy Chengappa, K.N. Subtle changes in thyroid indices during a placebo-controlled study of an extract of Withania somnifera in persons with bipolar disorder. J. Ayurveda Integr. Med. 2014, 5, 241–245. [Google Scholar] [CrossRef]
- Smith, S.J.; Lopresti, A.L.; Teo, S.Y.M.; Fairchild, T.J. Examining the effects of herbs on testosterone concentrations in men: A systematic review. Adv. Nutr. 2021, 12, 744–765. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Smith, S.J. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of Ashwagandha (Withania somnifera) in aging, overweight males. Am. J. Mens. Health 2019, 13, 1557988319835985. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.L.; Norhayati, M.N.; Husniati Yaacob, L.; Abdul Rahman, R. Effect of ashwagandha (Withania somnifera) extract on sleep: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0257843. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of ashwagandha root extract: A randomized, placebo-controlled, study in healthy volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J. Diet Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef]

| Phytochemicals/ Concentration | Cell Lines | Mechanism of Action of Ashwagandha | Reference |
|---|---|---|---|
| WA/2.5 μM | MDA-MB-23 MCF-7 MDA-MB-231 | ROS-mediated apoptotic induction due to inhibition of mitochondrial respiration. | [28] |
| WA/3.0 mg/mL | MCF-7 MDA-MB-231 | ROS-mediated apoptotic induction. Dysregulation of Bax/Bcl-2, loss of mitochondrial membrane potential and caspase-3 activation. | [97] |
| Extract/6 μg/mL; WA/1 μM, witanonem/25 μg/mL | MCF-7 | ROS-mediated apoptotic induction. DNA damage, mitochondrial structure, and membrane potential. | [98] |
| WA/2 μM | MCF-7 i SUM159 | Changes the assembly of complex III. Inhibition of mitochondrial dynamics. | [99] |
| WA/IC50 < 2 μM | MCF-7 MDA-MB-231 | Regulation of apoptosis involving FOXO3a and Bim. Induction of Bim-s and Bim-L in MCF-7 cells. Induction of Bim-s and Bim-EL isoforms in MDA-MB-231 cells. | [25] |
| WA/2.5 and 5 μM | MDA-MB-231 MCF-7 | Decrease in the expression of XIAP, cIAP-2 and survivin proteins, apoptosis inhibitors. | [102] |
| WFA/5 μM | MCF7 MDA-MB-231 | DR5 upregulation. Increased nuclear accumulation of Elk1 and CHOP. | [108] |
| WA/5 μM, 4β-Hydroxywithanolide (HW)/20 μM, Anomanolide A (AA)/20 μM, Peruvianolide H (PH)/20 μM) | MDA-MB-231 MCF-7 | Inhibition of Hsp90. | [107] |
| WA/3 μM | MDA-MB-468 | Inhibition of STAT3 and STAT5 recruitment. | [117] |
| WA/2.5 μM | MCF-7 | Inhibition of ERK and p38 MAPK. | [121] |
| MCF-7 T47D | Inhibition of estrogen receptor α expression. | [33] | |
| WA/2.5, 5 μM | MCF-7 | [110] | |
| WA/5 μM | MCF7, MDA-MB-231, MDA-MB-468, T47D, SUM149, SUM159, SKBR3 | Blocking the flow of autophagy and lysosomal proteolytic activity. | [136] |
| WA/4 µM | MCF-7 MDA-MB-231 | Inhibition of the proteasome degradation system and disruption of autophagy. | [137] |
| MCF-7 MDA-MB-231 | ROS-mediated paraptosis induction. | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejska, R.; Tafelska-Kaczmarek, A.; Pawluk, M.; Sergot, K.; Pisarska, L.; Woźniak, A.; Pawluk, H. Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer. Curr. Issues Mol. Biol. 2024, 46, 7668-7685. https://doi.org/10.3390/cimb46070454
Kołodziejska R, Tafelska-Kaczmarek A, Pawluk M, Sergot K, Pisarska L, Woźniak A, Pawluk H. Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer. Current Issues in Molecular Biology. 2024; 46(7):7668-7685. https://doi.org/10.3390/cimb46070454
Chicago/Turabian StyleKołodziejska, Renata, Agnieszka Tafelska-Kaczmarek, Mateusz Pawluk, Krzysztof Sergot, Lucyna Pisarska, Alina Woźniak, and Hanna Pawluk. 2024. "Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer" Current Issues in Molecular Biology 46, no. 7: 7668-7685. https://doi.org/10.3390/cimb46070454
APA StyleKołodziejska, R., Tafelska-Kaczmarek, A., Pawluk, M., Sergot, K., Pisarska, L., Woźniak, A., & Pawluk, H. (2024). Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer. Current Issues in Molecular Biology, 46(7), 7668-7685. https://doi.org/10.3390/cimb46070454





