Downregulation of Splicing Factor PTBP1 Curtails FBXO5 Expression to Promote Cellular Senescence in Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatics Database Sources
2.2. Cell Culture and Transfection
2.3. RNA Isolation and Quantitative Real-Time PCR Analysis
2.4. Western Blot Analysis
2.5. SA-β-Gal Staining
2.6. Cell Proliferation and Colony Formation Assay
2.7. Cell Migration Assay
2.8. Cell Cycle Flow Cytometric Analysis
2.9. mRNA Stability Assay
2.10. RNA-seq and Bioinformatics Analysis Methods
2.11. Statistical Analysis
3. Results
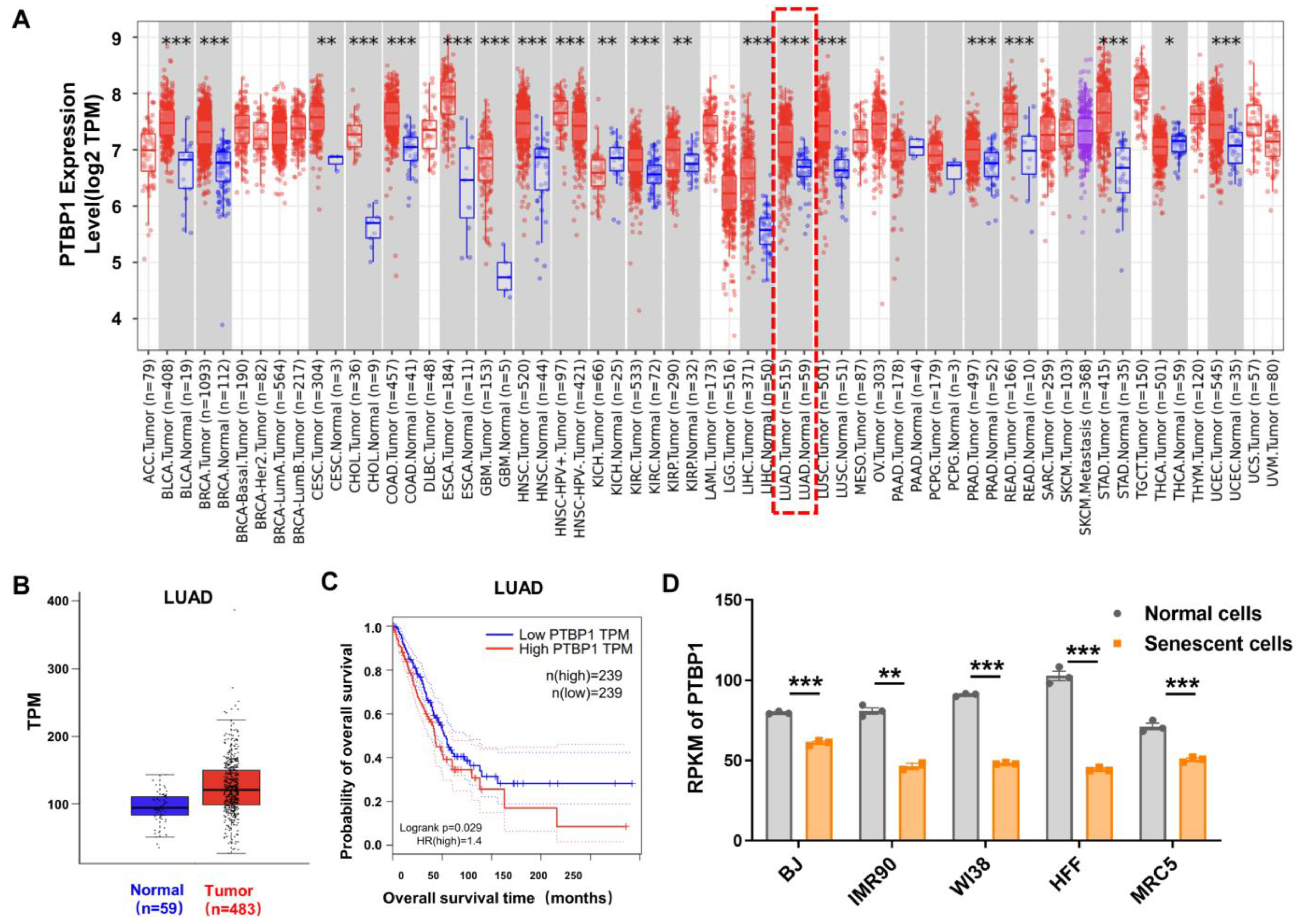
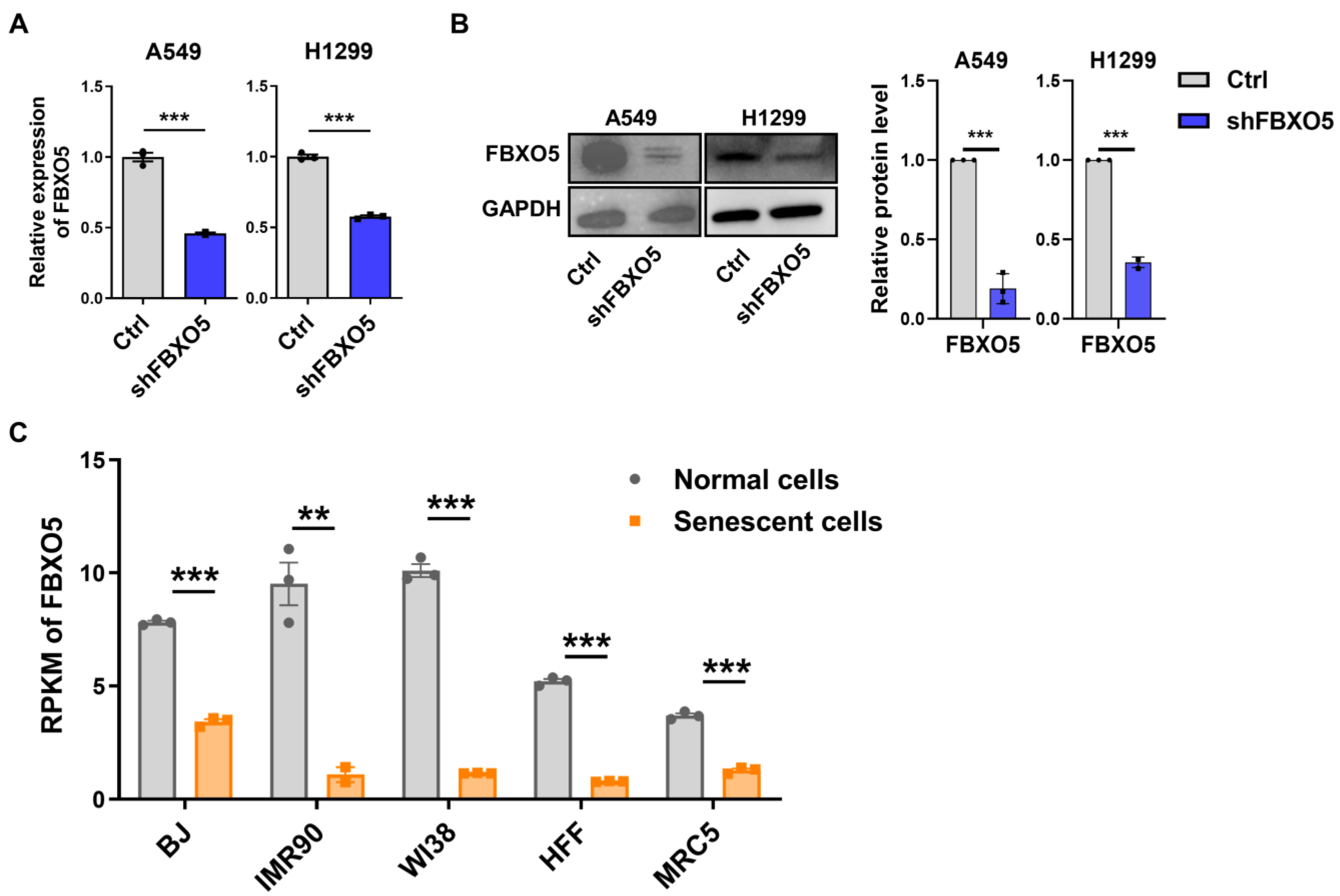
3.1. Opposite Levels of PTBP1 in LUAD and Cellular Senescence
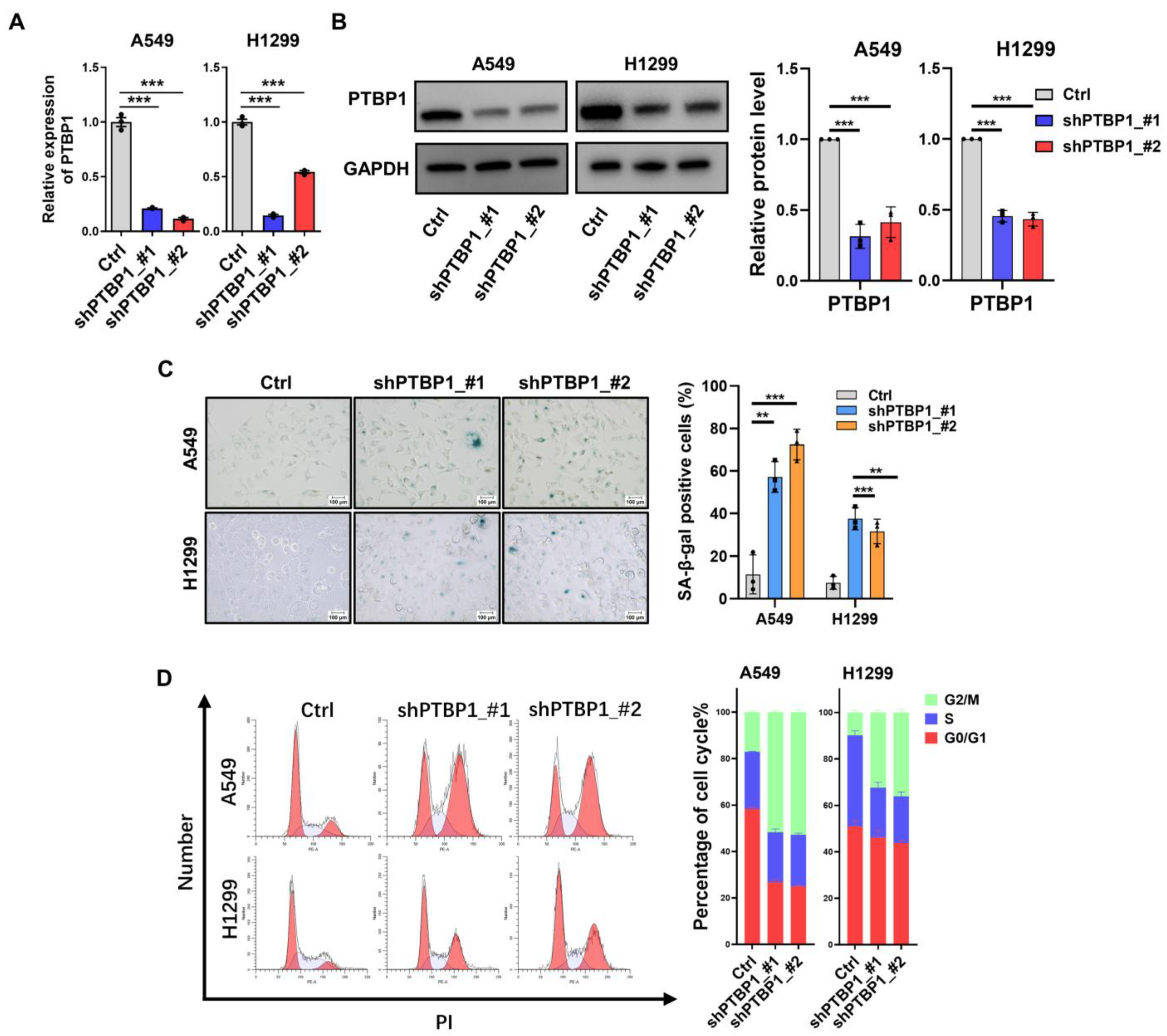
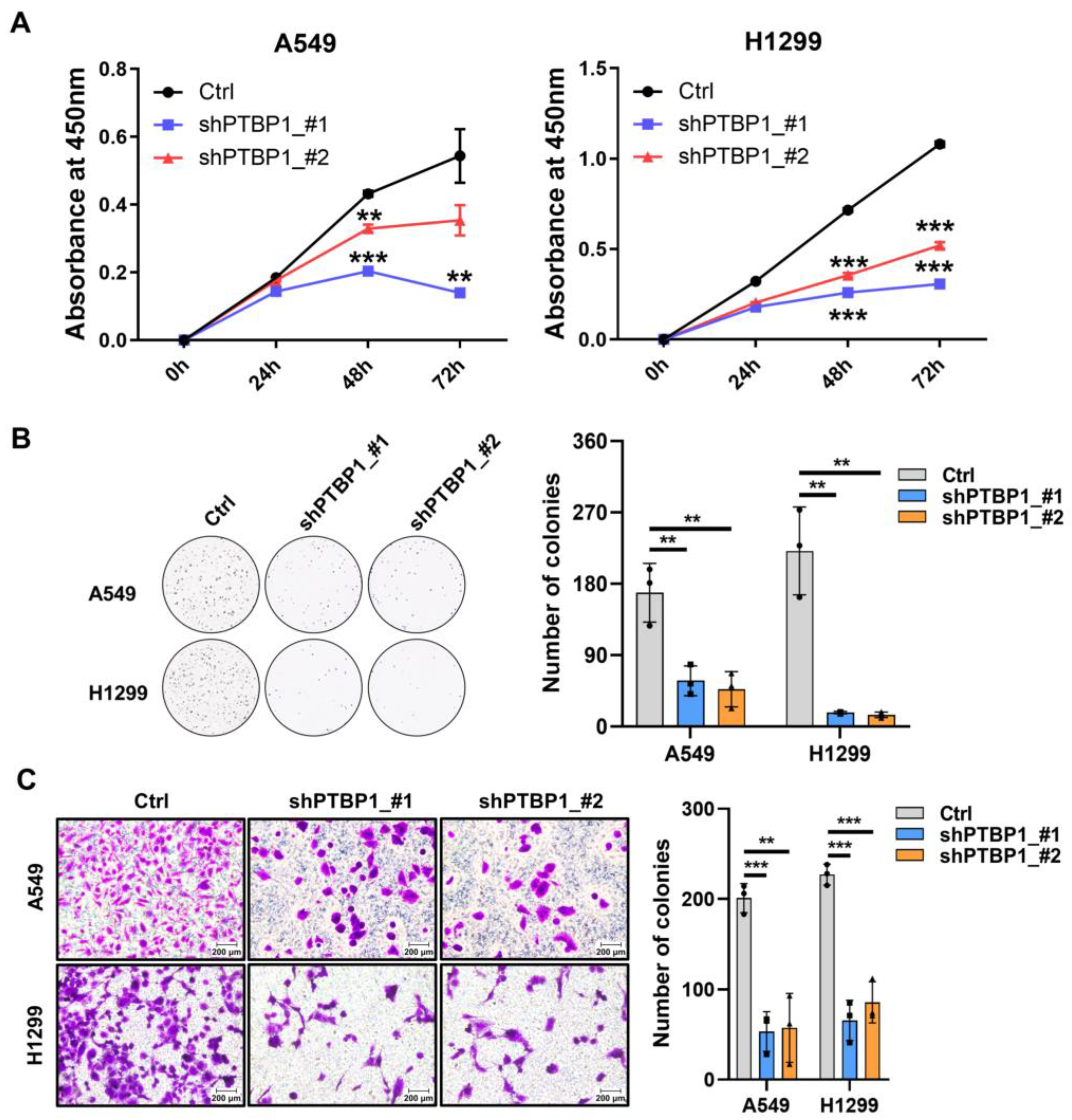
3.2. Downregulation of PTBP1 Induces Senescence in LUAD Cells
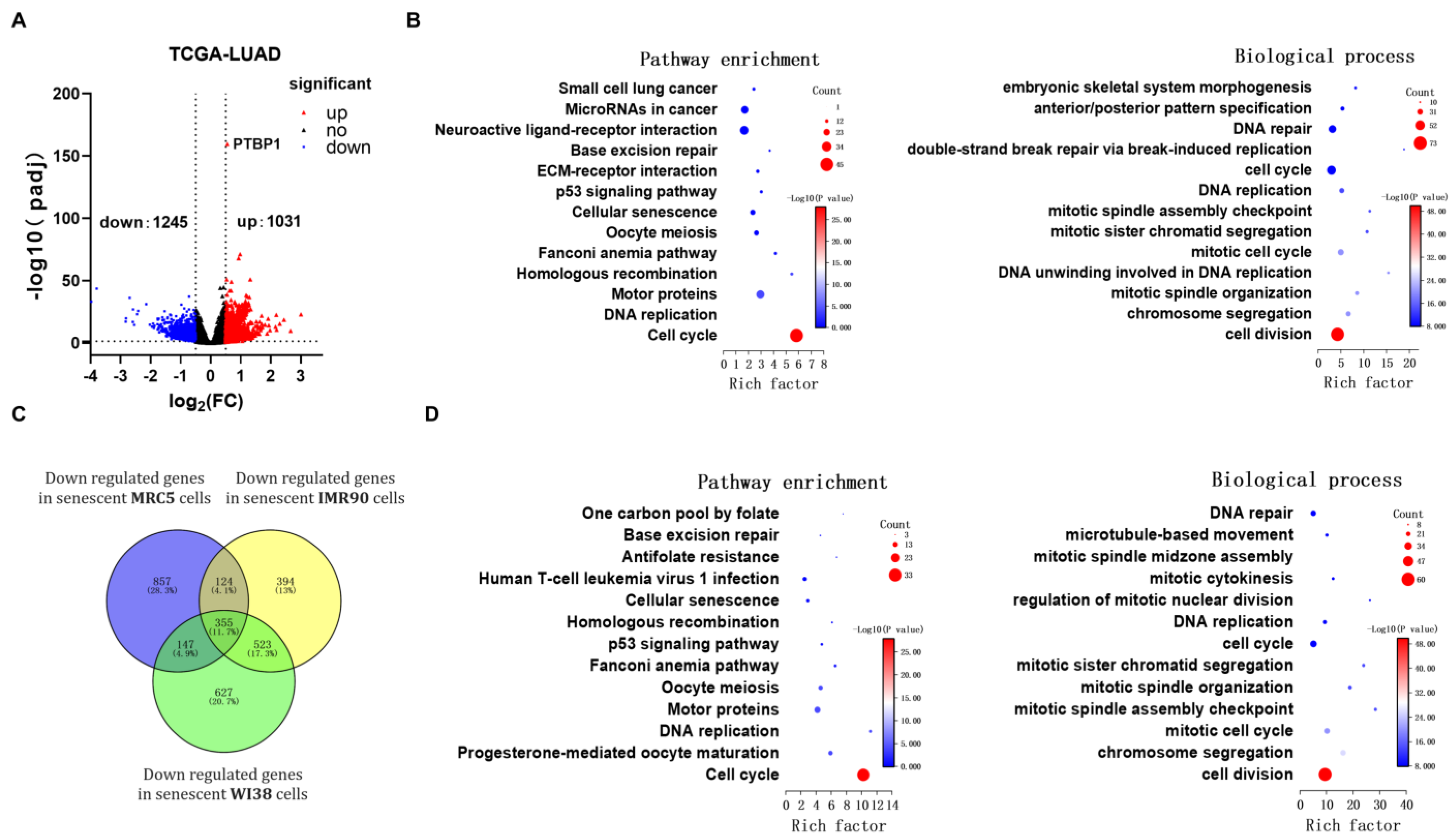
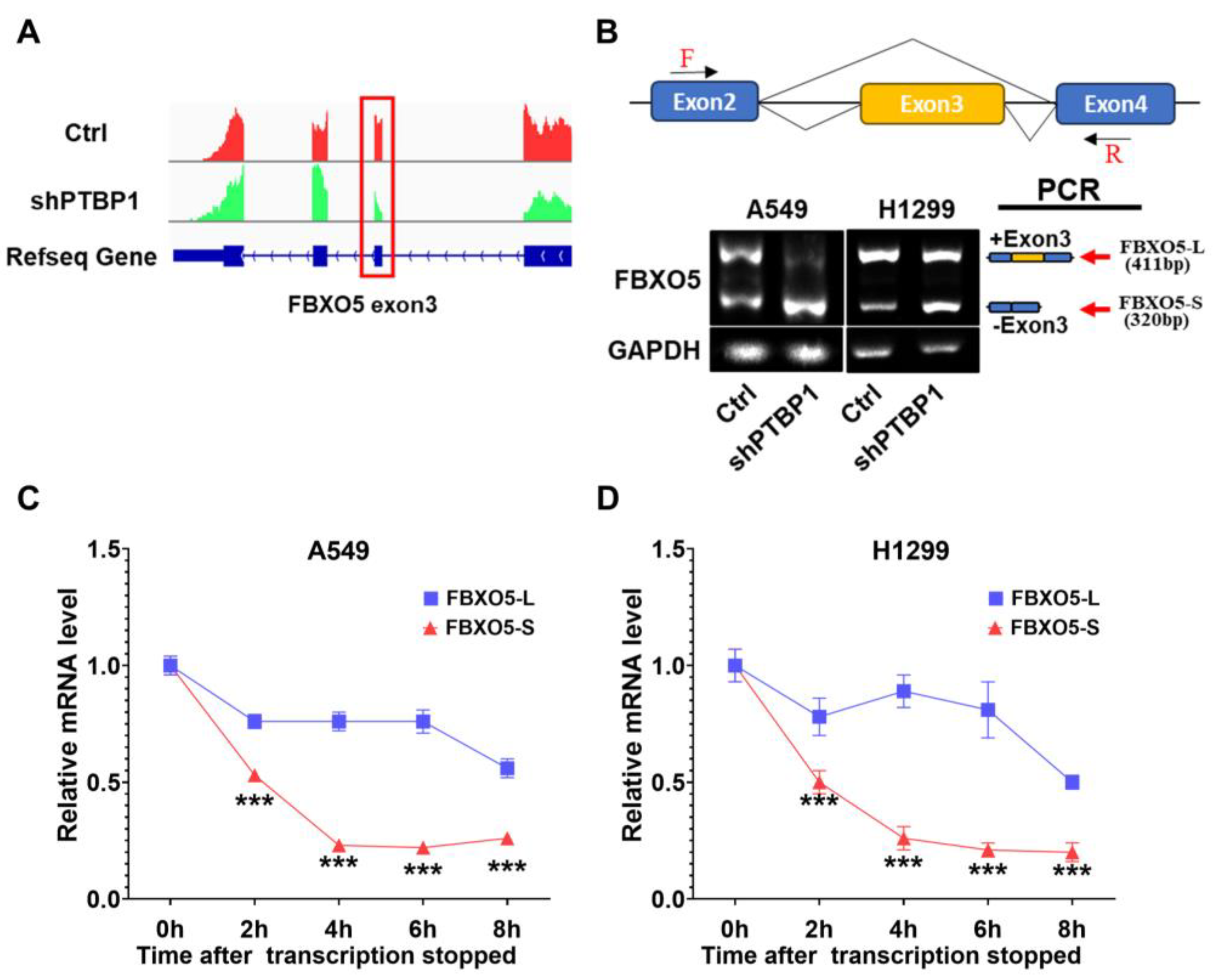
3.3. PTBP1 Regulates Expression of Cell Cycle Related Gene FBXO5 Via Alternative Splicing
3.4. PTBP1 Mediates Exon 3 Skipping of FBXO5 Pre-mRNA in LUAD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Soreq, L.; UK Brain Expression Consortium; North American Brain Expression Consortium; Rose, J.; Soreq, E.; Hardy, J.; Trabzuni, D.; Cookson, M.R.; Smith, C.; Ryten, M.; et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017, 18, 557–570. [Google Scholar]
- Li, X.; Ma, L. From Biological Aging to Functional Decline: Insights into Chronic Inflammation and Intrinsic Capacity. Ageing Res. Rev. 2024, 93, 102175. [Google Scholar] [CrossRef]
- Montégut, L.; López-Otín, C.; Kroemer, G. Aging and Cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef]
- van Deursen, J.M. The Role of Senescent Cells in Ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A Biomarker That Identifies Senescent Human Cells in Culture and in Aging Skin in Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking Senescence: Context-Dependent Effects of SASP in Cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Cho, K.A.; Ryu, S.J.; Oh, Y.S.; Park, J.H.; Lee, J.W.; Kim, H.-P.; Kim, K.T.; Jang, I.S.; Park, S.C. Morphological Adjustment of Senescent Cells by Modulating Caveolin-1 Status. J. Biol. Chem. 2004, 279, 42270–42278. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takahashi, A.; Motoi, N.; Yoshimoto, S.; Tajima, T.; Yamakoshi, K.; Hirao, A.; Yanagi, S.; Fukami, K.; Ishikawa, Y.; et al. Intrinsic Cooperation between p16INK4a and p21Waf1/Cip1 in the Onset of Cellular Senescence and Tumor Suppression In Vivo. Cancer Res. 2010, 70, 9381–9390. [Google Scholar] [CrossRef]
- Ohtani, N.; Yamakoshi, K.; Takahashi, A.; Hara, E. The p16INK4a-RB Pathway: Molecular Link between Cellular Senescence and Tumor Suppression. J. Med. Investig. 2004, 51, 146–153. [Google Scholar] [CrossRef]
- Beauséjour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of Human Cellular Senescence: Roles of the P53 and P16 Pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef]
- Zhang, J.; Manley, J.L. Misregulation of Pre-mRNA Alternative Splicing in Cancer. Cancer Discov. 2013, 3, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Shirai, C.L.; Ley, J.N.; White, B.S.; Kim, S.; Tibbitts, J.; Shao, J.; Ndonwi, M.; Wadugu, B.; Duncavage, E.J.; Okeyo-Owuor, T.; et al. Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell 2015, 27, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Basile, K.J.; Le, K.; Hartsough, E.J.; Aplin, A.E. Inhibition of Mutant BRAF Splice Variant Signaling by Next-Generation, Selective RAF Inhibitors. Pigment. Cell Melanoma Res. 2014, 27, 479–484. [Google Scholar] [CrossRef]
- Dutertre, M.; Sanchez, G.; De Cian, M.-C.; Barbier, J.; Dardenne, E.; Gratadou, L.; Dujardin, G.; Le Jossic-Corcos, C.; Corcos, L.; Auboeuf, D. Cotranscriptional Exon Skipping in the Genotoxic Stress Response. Nat. Struct. Mol. Biol. 2010, 17, 1358–1366. [Google Scholar] [CrossRef]
- House, A.E.; Lynch, K.W. Regulation of Alternative Splicing: More than Just the ABCs. J. Biol. Chem. 2008, 283, 1217–1221. [Google Scholar] [CrossRef]
- Braunschweig, U.; Gueroussov, S.; Plocik, A.M.; Graveley, B.R.; Blencowe, B.J. Dynamic Integration of Splicing within Gene Regulatory Pathways. Cell 2013, 152, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Min, S.; Jeoun, U.; Sim, M.S.; Jung, G.H.; Hong, S.M.; Jee, B.A.; Woo, H.G.; Lee, C.; Yoon, G. Global Spliceosome Activity Regulates Entry into Cellular Senescence. FASEB J. 2021, 35, e21204. [Google Scholar] [CrossRef]
- Holly, A.C.; Melzer, D.; Pilling, L.C.; Fellows, A.C.; Tanaka, T.; Ferrucci, L.; Harries, L.W. Changes in Splicing Factor Expression Are Associated with Advancing Age in Man. Mech. Ageing Dev. 2013, 134, 356–366. [Google Scholar] [CrossRef]
- Lee, B.P.; Pilling, L.C.; Emond, F.; Flurkey, K.; Harrison, D.E.; Yuan, R.; Peters, L.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D.; et al. Changes in the Expression of Splicing Factor Transcripts and Variations in Alternative Splicing Are Associated with Lifespan in Mice and Humans. Aging Cell 2016, 15, 903–913. [Google Scholar] [CrossRef]
- Tabrez, S.S.; Sharma, R.D.; Jain, V.; Siddiqui, A.A.; Mukhopadhyay, A. Differential Alternative Splicing Coupled to Nonsense-Mediated Decay of mRNA Ensures Dietary Restriction-Induced Longevity. Nat. Commun. 2017, 8, 306. [Google Scholar] [CrossRef]
- Georgilis, A.; Klotz, S.; Hanley, C.J.; Herranz, N.; Weirich, B.; Morancho, B.; Leote, A.C.; D’Artista, L.; Gallage, S.; Seehawer, M.; et al. PTBP1-Mediated Alternative Splicing Regulates the Inflammatory Secretome and the Pro-Tumorigenic Effects of Senescent Cells. Cancer Cell 2018, 34, 85. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Zhang, H.; Wang, K.; Liu, X.; Zhao, Y.; Guo, Z.; Zhang, N.; Zhou, Y.; Gao, M.; Gu, F.; et al. The Alternative Splicing of Intersectin 1 Regulated by PTBP1 Promotes Human Glioma Progression. Cell Death Dis. 2022, 13, 835. [Google Scholar] [CrossRef]
- Cho, C.-Y.; Chung, S.-Y.; Lin, S.; Huang, J.-S.; Chen, Y.-L.; Jiang, S.-S.; Cheng, L.-C.; Kuo, T.-H.; Lay, J.-D.; Yang, Y.-Y.; et al. PTBP1-Mediated Regulation of AXL mRNA Stability Plays a Role in Lung Tumorigenesis. Sci. Rep. 2019, 9, 16922. [Google Scholar] [CrossRef]
- Ge, Z.; Quek, B.L.; Beemon, K.L.; Hogg, J.R. Polypyrimidine Tract Binding Protein 1 Protects mRNAs from Recognition by the Nonsense-Mediated mRNA Decay Pathway. Elife 2016, 5, e11155. [Google Scholar] [CrossRef]
- Liu, L.; Liu, K.; Yan, Y.; Chu, Z.; Tang, Y.; Tang, C. Two Transcripts of FBXO5 Promote Migration and Osteogenic Differentiation of Human Periodontal Ligament Mesenchymal Stem Cells. Biomed. Res. Int. 2018, 2018, 7849294. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Joung, J.-G.; Zheng, Y.; Chen, Y.; Liu, B.; Shao, Y.; Xiang, J.Z.; Fei, Z.; Giovannoni, J.J. High-Throughput Illumina Strand-Specific RNA Sequencing Library Preparation. Cold Spring Harb. Protoc. 2011, 2011, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, G.; Yu, D.; Huang, W.; Cheng, C.; Liao, S.; Wu, Q.; Zhang, Y. Transcriptome Analysis Reveals the Complexity of Alternative Splicing Regulation in the Fungus Verticillium Dahliae. BMC Genom. 2017, 18, 130. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and Flexible Detection of Differential Alternative Splicing from Replicate RNA-Seq Data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Reimann, J.D.R.; Jackson, P.K. Emi1 Is Required for Cytostatic Factor Arrest in Vertebrate Eggs. Nature 2002, 416, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Margottin-Goguet, F.; Hsu, J.Y.; Loktev, A.; Hsieh, H.M.; Reimann, J.D.R.; Jackson, P.K. Prophase Destruction of Emi1 by the SCF(betaTrCP/Slimb) Ubiquitin Ligase Activates the Anaphase Promoting Complex to Allow Progression beyond Prometaphase. Dev. Cell 2003, 4, 813–826. [Google Scholar] [CrossRef]
- Wang, Z.-N.; Liu, D.; Yin, B.; Ju, W.-Y.; Qiu, H.-Z.; Xiao, Y.; Chen, Y.-J.; Peng, X.-Z.; Lu, C.-M. High Expression of PTBP1 Promote Invasion of Colorectal Cancer by Alternative Splicing of Cortactin. Oncotarget 2017, 8, 36185–36202. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Arslan, A.D.; Ho, T.-T.; Yuan, C.; Stampfer, M.R.; Beck, W.T. Involvement of Polypyrimidine Tract-Binding Protein (PTBP1) in Maintaining Breast Cancer Cell Growth and Malignant Properties. Oncogenesis 2014, 3, e84. [Google Scholar] [CrossRef]
- He, X.; Yuan, C.; Yang, J. Regulation and Functional Significance of CDC42 Alternative Splicing in Ovarian Cancer. Oncotarget 2015, 6, 29651–29663. [Google Scholar] [CrossRef]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a Model of Parkinson’s Disease with in Situ Converted Nigral Neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef]
- Yano, K.; Takahashi, R.-U.; Shiotani, B.; Abe, J.; Shidooka, T.; Sudo, Y.; Yamamoto, Y.; Kan, S.; Sakagami, H.; Tahara, H. PRPF19 Regulates P53-Dependent Cellular Senescence by Modulating Alternative Splicing of MDM4 mRNA. J. Biol. Chem. 2021, 297, 100882. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Horikawa, I.; Ajiro, M.; Robles, A.I.; Fujita, K.; Mondal, A.M.; Stauffer, J.K.; Zheng, Z.-M.; Harris, C.C. Downregulation of Splicing Factor SRSF3 Induces P53β, an Alternatively Spliced Isoform of P53 That Promotes Cellular Senescence. Oncogene 2013, 32, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Robu, M.E.; Zhang, Y.; Rhodes, J. Rereplication in Emi1-Deficient Zebrafish Embryos Occurs through a Cdh1-Mediated Pathway. PLoS ONE 2012, 7, e47658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, B.; Qu, W.; Cao, Y.; Li, J.; Zhao, H. Systematic Analysis of the Expression and Prognosis Relevance of FBXO Family Reveals the Significance of FBXO1 in Human Breast Cancer. Cancer Cell Int. 2021, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhang, J.; Zhang, J.; Shi, H.; Ni, R. Enhanced Expression of Early Mitotic Inhibitor-1 Predicts a Poor Prognosis in Esophageal Squamous Cell Carcinoma Patients. Oncol. Lett. 2016, 12, 114–120. [Google Scholar] [CrossRef]
- Min, K.-W.; Park, M.H.; Hong, S.R.; Lee, H.; Kwon, S.Y.; Hong, S.H.; Joo, H.J.; Park, I.A.; An, H.J.; Suh, K.S.; et al. Clear Cell Carcinomas of the Ovary: A Multi-Institutional Study of 129 Cases in Korea with Prognostic Significance of Emi1 and Galectin-3. Int. J. Gynecol. Pathol. 2013, 32, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qu, X.; Liu, S.; Yang, X.; Bie, F.; Wang, Y.; Huang, C.; Du, J. Identification of Aberrantly Expressed F-Box Proteins in Squamous-Cell Lung Carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Lehman, N.L.; Tibshirani, R.; Hsu, J.Y.; Natkunam, Y.; Harris, B.T.; West, R.B.; Masek, M.A.; Montgomery, K.; van de Rijn, M.; Jackson, P.K. Oncogenic Regulators and Substrates of the Anaphase Promoting Complex/Cyclosome Are Frequently Overexpressed in Malignant Tumors. Am. J. Pathol. 2007, 170, 1793–1805. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]

| Target | Primer Sequence |
|---|---|
| shPTBP1_#1 | 5′-CTCAACGTCAAGTACAACAAT-3′ |
| shPTBP1_#2 | 5′-AGCAAACGGAAATGACAGCAA-3′ |
| shFBXO5 | 5′-CCAGACCAATATCCCAACAAA-3′ |
| Target | Primer Sequence |
|---|---|
| RT-qPCR | |
| GAPDH | Forward: CTGGGCTACACTGAGCACC |
| Reverse: AAGTGGTCGTTGAGGGCAATG | |
| PTBP1 | Forward: AGCGCGTGAAGATCCTGTTC |
| Reverse: CAGGGGTGAGTTGCCGTAG | |
| FBXO5 | Forward: CAGCGAACTCTTTCGAAGGGGACTC |
| Reverse: GTGAATTACAGCGAATACAGGCTTTGAGGC | |
| FBXO5-S | Forward: GCACAACTCAGTGACATGGACTTAATCAAGAAAAC |
| Reverse: CTGCTGATTTCTGAACAGAAGCCAGTGG | |
| FBXO5-L | Forward: CCAGTTGTACAGTAAAGCAATACAAAGAGTTACCGAAAAC |
| Reverse: CTGCTGATTTCTGAACAGAAGCCAGTGG | |
| RT-PCR | |
| GAPDH | Forward: CTGGGCTACACTGAGCACC |
| Reverse: AAGTGGTCGTTGAGGGCAATG | |
| FBXO5exon 2-exon 4 | Forward: CAGCGAACTCTTTCGAAGGGGACTC |
| Reverse: GGTGAATTACAGCGAATACAGGCTTTGAGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sun, X.; Lv, Y.; Wei, G.; Ni, T.; Qin, W.; Jin, H.; Jia, Q. Downregulation of Splicing Factor PTBP1 Curtails FBXO5 Expression to Promote Cellular Senescence in Lung Adenocarcinoma. Curr. Issues Mol. Biol. 2024, 46, 7730-7744. https://doi.org/10.3390/cimb46070458
Li H, Sun X, Lv Y, Wei G, Ni T, Qin W, Jin H, Jia Q. Downregulation of Splicing Factor PTBP1 Curtails FBXO5 Expression to Promote Cellular Senescence in Lung Adenocarcinoma. Current Issues in Molecular Biology. 2024; 46(7):7730-7744. https://doi.org/10.3390/cimb46070458
Chicago/Turabian StyleLi, Haoyu, Xiaoxiao Sun, Yuanyuan Lv, Gang Wei, Ting Ni, Wenxin Qin, Haojie Jin, and Qi Jia. 2024. "Downregulation of Splicing Factor PTBP1 Curtails FBXO5 Expression to Promote Cellular Senescence in Lung Adenocarcinoma" Current Issues in Molecular Biology 46, no. 7: 7730-7744. https://doi.org/10.3390/cimb46070458






