Astragalus Polysaccharides and Metformin May Have Synergistic Effects on the Apoptosis and Ferroptosis of Lung Adenocarcinoma A549 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Viability Using APS, Metformin, and Combination Treatment
2.2. Apoptosis and Mitochondrial Membrane Potential Detection through Flow Cytometry Analysis
2.3. Ferroptosis Effect Determination
2.3.1. ROS Determination Assay
2.3.2. Reduced Glutathione (GSH) and Malondialdehyde (MDA) Levels
2.3.3. Protein Expression of Western Blotting Analysis and Protein Quantification
2.4. Protein Level Quantification
2.5. Statistical Analysis
3. Results
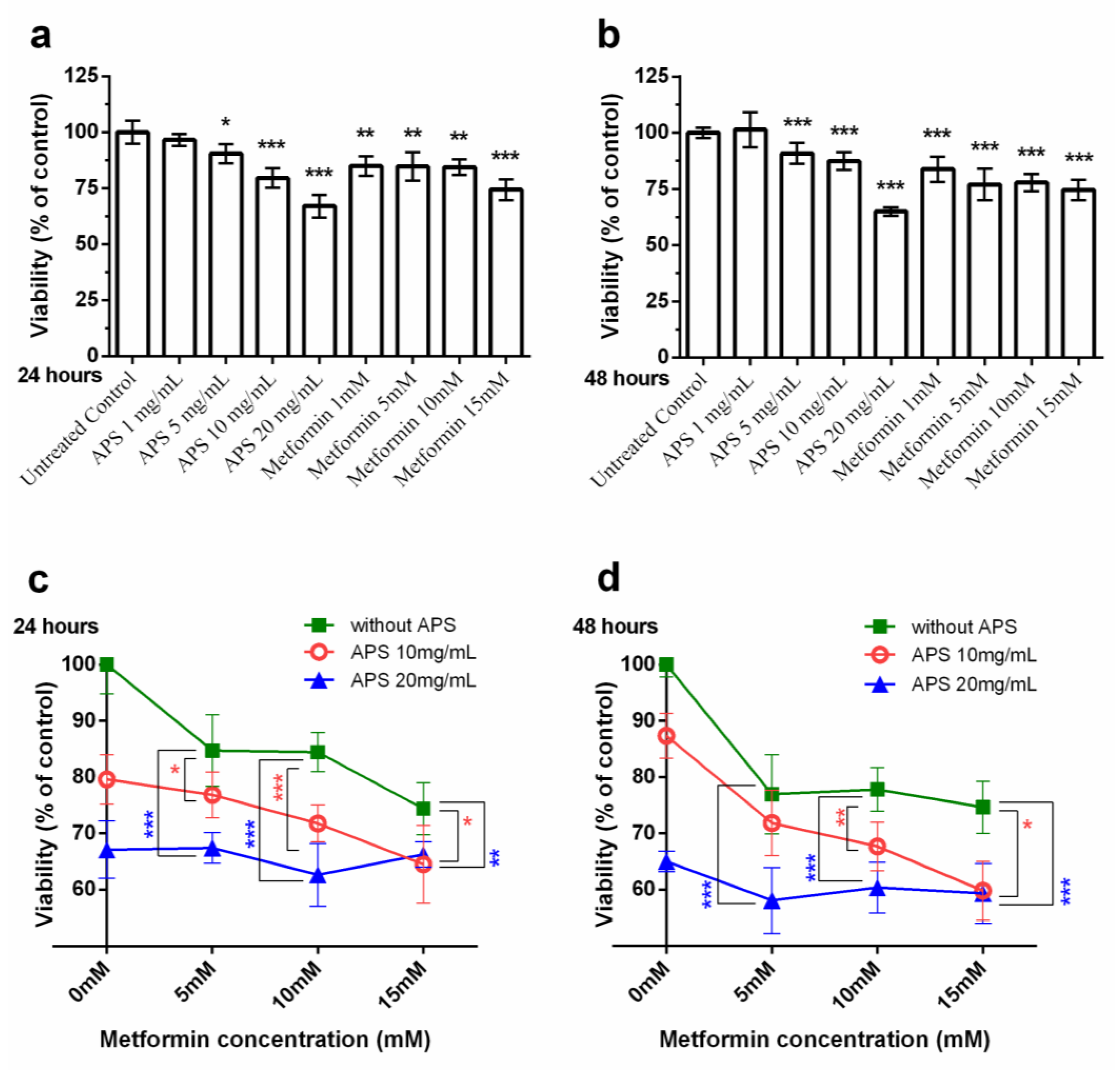
3.1. Cell Viability under the Treatment of APS, Metformin Alone/APS in Combination with Metformin
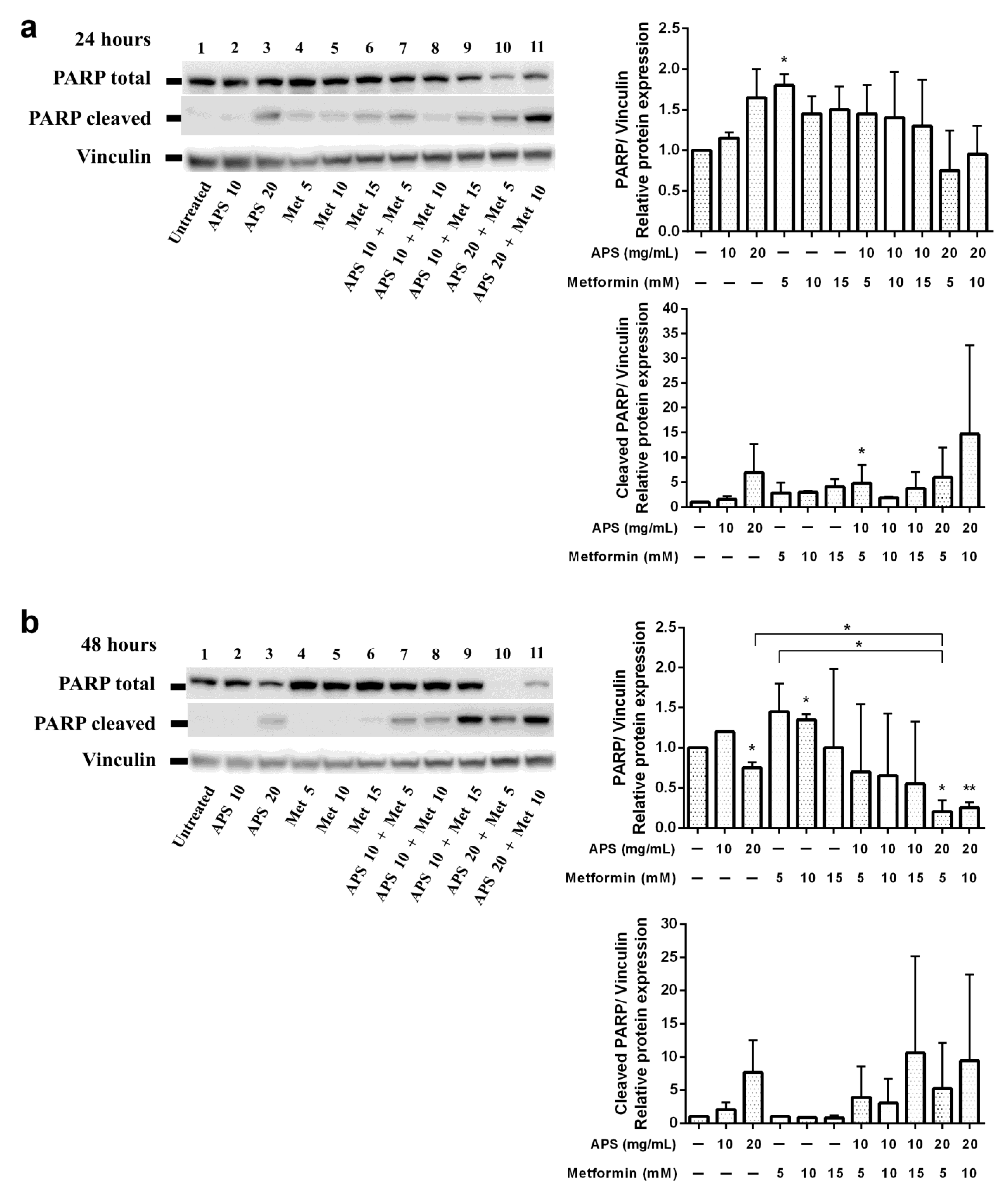
3.2. Apoptotic Effects of APS Combined with Metformin
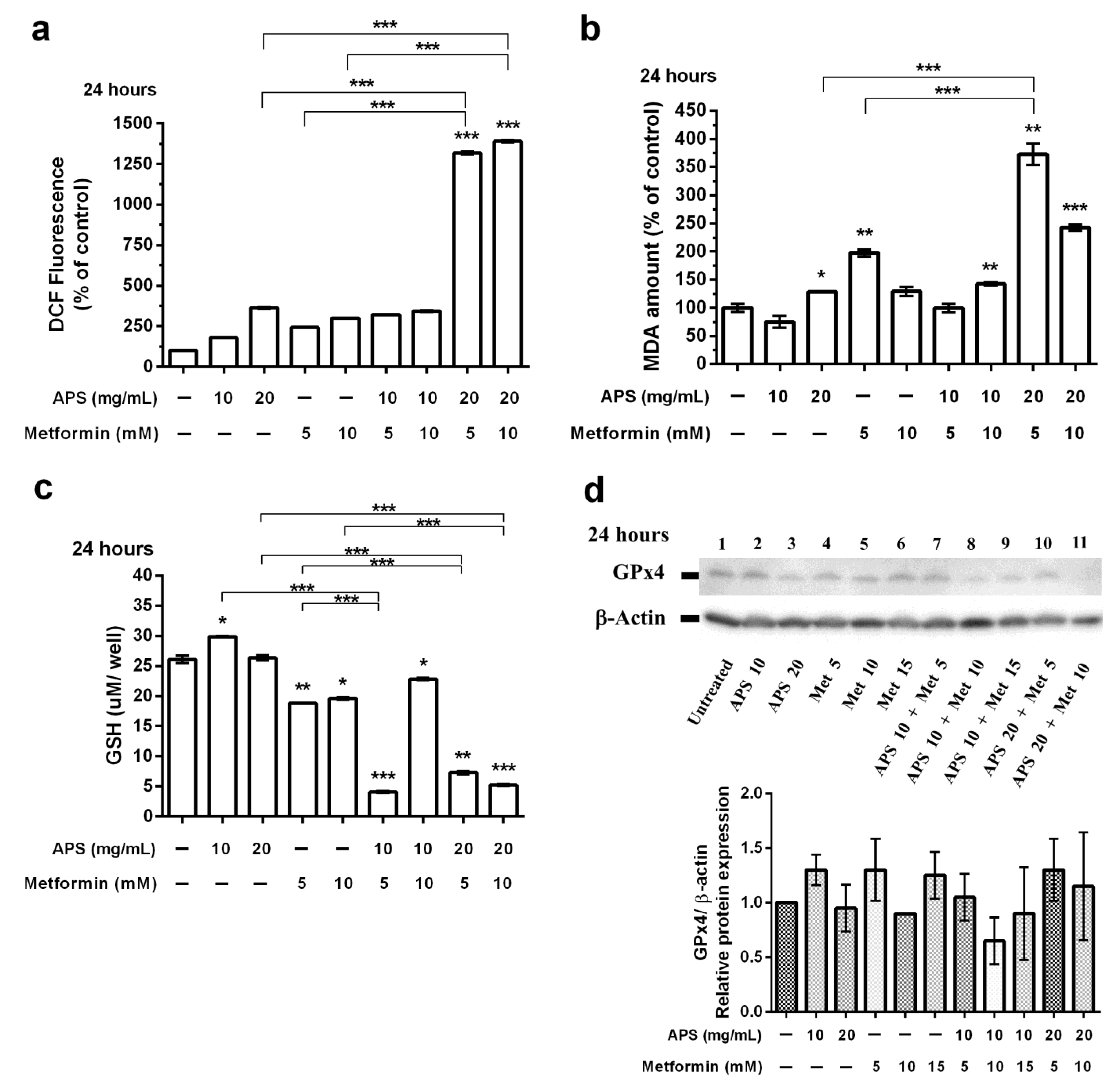
3.3. The Effects of APS, Metformin, and Their Combination on A549 Cell Ferroptosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, D.J. StatPearls: Lung Adenocarcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Byun, J.; Schwartz, A.G.; Lusk, C.; Wenzlaff, A.S.; de Andrade, M.; Mandal, D.; Gaba, C.; Yang, P.; You, M.; Kupert, E.Y.; et al. Genome-wide association study of familial lung cancer. Carcinogenesis 2018, 39, 1135–1140. [Google Scholar] [CrossRef]
- Guo, L.; Bai, S.P.; Zhao, L.; Wang, X.H. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med. Oncol. 2012, 29, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Chang, C.-C.; Chen, T.-L.; Yeh, C.-C.; Lin, J.-G.; Liu, C.-H.; Liao, C.-C. The long-term trend in utilization of traditional Chinese medicine and associated factors among older people in Taiwan. PLoS ONE 2024, 19, e0302658. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Cui, Y.; Zhao, L.; Sun, X.; Tang, W.; Cai, P. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci. Trends 2018, 12, 220–239. [Google Scholar] [CrossRef]
- Hsia, T.-C.; Li, C.-H.; Chen, K.-J.; Yang, Y.-H.; Yang, S.-T. Adjunctive Traditional Chinese Medicine Improves Survival in Patients With Advanced Lung Adenocarcinoma Treated with First-Line Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs): A Nationwide, Population-Based Cohort Study. Integr. Cancer Ther. 2019, 18, 1534735419827079. [Google Scholar] [CrossRef]
- Li, T.-M.; Yu, Y.-H.; Tsai, F.-J.; Cheng, C.-F.; Wu, Y.-C.; Ho, T.-J.; Liu, X.; Tsang, H.; Lin, T.-H.; Liao, C.-C.; et al. Characteristics of Chinese herbal medicine usage and its effect on survival of lung cancer patients in Taiwan. J. Ethnopharmacol. 2018, 213, 92–100. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Huang, H.-S.; Su, Y.-C.; Tu, C.-Y.; Hsia, T.-C.; Huang, S.-T. Conventional treatment integrated with Chinese herbal medicine improves the survival rate of patients with advanced non-small cell lung cancer. Complement. Ther. Med. 2018, 40, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2019, 9, 122. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Liu, K.; Li, M.; Jin, Y.; Gu, L.; Yu, X.-A.; Wang, S.; Wang, P.; Wang, B.; et al. A Rapid and Accurate UHPLC Method for Determination of Monosaccharides in Polysaccharides of Different Sources of Radix Astragali and Its Immune Activity Analysis. Molecules 2024, 29, 2287. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Sun, S.; Sun, X.; Ren, F.; Shi, Q.; Wang, S.; Zhang, W.; Li, X.; Zhang, J. Chemical analysis of Astragalus mongholicus polysaccharides and antioxidant activity of the polysaccharides. Carbohydr. Polym. 2010, 82, 636–640. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-D.; Li, J.-J.; Wang, L.; Wang, J.-H.; Dai, G.; Kang, B.; Mao, S.-M. Inhibitory effect of Astragalus polysaccharides on apoptosis of pancreatic beta-cells mediated by Fas in diabetes mellitus rats. Zhong Yao Cai 2011, 34, 1579–1582. [Google Scholar] [PubMed]
- Huang, W.-C.; Kuo, K.-T.; Bamodu, O.A.; Lin, Y.-K.; Wang, C.-H.; Lee, K.-Y.; Wang, L.-S.; Yeh, C.-T.; Tsai, J.-T. Astragalus polysaccharide (PG2) Ameliorates Cancer Symptom Clusters, as well as Improves Quality of Life in Patients with Metastatic Disease, through Modulation of the Inflammatory Cascade. Cancers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, J.; Li, J.; Huang, Y.; Wu, Y. Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult. Sci. 2021, 100, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.E.; Li, H.D.; Yan, M.; Cai, H.L.; Tan, Q.Y.; Zhang, W.Y. Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J. Gastroenterol. 2012, 18, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, H.; Wang, K.; Zhuang, J.; Chu, F.; Gao, C.; Liu, L.; Feng, F.; Zhou, C.; Zhang, W.; et al. Identifying the Antiproliferative Effect of Astragalus Polysaccharides on Breast Cancer: Coupling Network Pharmacology with Targetable Screening From the Cancer Genome Atlas. Front. Oncol. 2019, 9, 368. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C-Mater. 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Huang, W.-H.; Liao, W.-R.; Sun, R.-X. Astragalus polysaccharide induces the apoptosis of human hepatocellular carcinoma cells by decreasing the expression of Notch1. Int. J. Mol. Med. 2016, 38, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, Y.-J.; Liu, Z.-H.; Chen, Y.-W.; Liang, Q.-K.; Li, Y.; Ming, H.-X. Inhibitory Effect of Astragalus Polysaccharide on Premetastatic Niche of Lung Cancer through the S1PR1-STAT3 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2023, 2023, 4010797. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; He, D.; Tan, W.; Lv, F.; Liang, B.; Xia, T.; Li, J. Astragalus Polysaccharide Promotes Adriamycin-Induced Apoptosis in Gastric Cancer Cells. Cancer Manag. Res. 2020, 12, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; See, C.; Shu, X.-J.; Broffman, M.; Kramer, A.; Fan, W.-Y.; Gao, J.; Lieb, W.; Shieh, K.; Colford, J.M., Jr. Astragalus-Based Chinese Herbs and Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer: Meta-Analysis of Randomized Trials. J. Clin. Oncol. 2006, 24, 419–430. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Cheng, K.-C.; Mgbeahuruike, M.O.; Lin, Y.-H.; Wu, C.-Y.; Wang, H.-M.D.; Yen, C.-H.; Chiu, C.-C.; Sheu, S.-J. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef] [PubMed]
- Veeramachaneni, R.; Yu, W.; Newton, J.M.; O Kemnade, J.; Skinner, H.D.; Sikora, A.G.; Sandulache, V.C. Metformin generates profound alterations in systemic and tumor immunity with associated antitumor effects. J. Immunother. Cancer 2021, 9, e002773. [Google Scholar] [CrossRef]
- Tsogas, F.K.; Majerczyk, D.; Hart, P.C. Possible Role of Metformin as an Immune Modulator in the Tumor Microenvironment of Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 867. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Y.-S.; Wang, L.-C.; Huang, J.-B. Advances in metformin-based metabolic therapy for non-small cell lung cancer (Review). Oncol. Rep. 2022, 47, 55. [Google Scholar] [CrossRef]
- Lee, B.B.; Kim, Y.; Kim, D.; Cho, E.Y.; Han, J.; Kim, H.K.; Shim, Y.M.; Kim, D.H. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J. Cell Mol. Med. 2019, 23, 2872–2889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Lan, Y.-W.; Tsai, Y.-T.; Chen, Y.-C.; Staniczek, T.; Tsou, Y.-A.; Yen, C.-C.; Chen, C.-M. Additive Antiproliferative and Antiangiogenic Effects of Metformin and Pemetrexed in a Non-Small-Cell Lung Cancer Xenograft Model. Front. Cell Dev. Biol. 2021, 9, 688062. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, T.; Yang, S.; Sheng, H.; Tang, X.; Bao, F.; Wang, Y.; Lin, X.; Yu, W.; Cheng, F.; et al. Metformin reverses chemoresistance in non-small cell lung cancer via accelerating ubiquitination-mediated degradation of Nrf2. Transl. Lung Cancer Res. 2020, 9, 2337–2355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Han, J.; Mei, H.; Yu, D.; Ding, Q.; Zhang, T.; Wu, G.; Peng, G.; Lin, Z. Metformin Attenuates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Radiat. Res. 2017, 188, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yakisich, J.S.; Azad, N.; Kaushik, V.; Iyer, A.K.V. The Biguanides Metformin and Buformin in Combination with 2-Deoxy-glucose or WZB-117 Inhibit the Viability of Highly Resistant Human Lung Cancer Cells. Stem Cells Int. 2019, 2019, 6254269. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jeong, S.-M.; Shin, D.W.; Cho, M.; Cho, J.H.; Kim, J. The Associations of Aspirin, Statins, and Metformin With Lung Cancer Risk and Related Mortality: A Time-Dependent Analysis of Population-Based Nationally Representative Data. J. Thorac. Oncol. 2021, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, F.; Liu, J.; Xu, J.; Wu, Q.; Li, X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, X.; Wang, L.; Yang, B.; Cai, S. Metformin Adjunct With Antineoplastic Agents for the Treatment of Lung Cancer: A Meta-Analysis of Randomized Controlled Trials and Observational Cohort Studies. Front. Pharmacol. 2021, 12, 639016. [Google Scholar] [CrossRef]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.; Coster, J.; Yang, A.X.; et al. Addition of Metformin to Concurrent Chemoradiation in Patients With Locally Advanced Non-Small Cell Lung Cancer: The NRG-LU001 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef]
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in Combination With Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: The OCOG-ALMERA Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1333–1341. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, Y.-H.; Lin, Y.-S.; Chang, G.-H.; Tsai, M.-S.; Hsu, C.-M.; Yeh, R.-A.; Shu, L.-H.; Cheng, Y.-C.; Liu, H.-T. Dihydroisotanshinone I induced ferroptosis and apoptosis of lung cancer cells. Biomed. Pharmacother. 2021, 139, 111585. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Shen, Y.-C.; Wu, C.-Y.; Tsai, Y.-Y.; Yang, Y.-H.; Lin, Y.-Y.; Kuan, F.-C.; Lu, C.-N.; Chang, G.-H.; Tsai, M.-S.; et al. Danshen Improves Survival of Patients with Breast Cancer and Dihydroisotanshinone I Induces Ferroptosis and Apoptosis of Breast Cancer Cells. Front. Pharmacol. 2019, 10, 1226. [Google Scholar] [CrossRef]
- Lieber, M.; Todaro, G.; Smith, B.; Szakal, A.; Nelson-Rees, W. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.-J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Ke, Y.; Zeng, Y.-F.; Zhang, Y.-W.; Yu, H.-J. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Barrios-Bernal, P.; Hernandez-Pedro, N.; Orozco-Morales, M.; Viedma-Rodríguez, R.; Lucio-Lozada, J.; Avila-Moreno, F.; Cardona, A.F.; Rosell, R.; Arrieta, O. Metformin Enhances TKI-Afatinib Cytotoxic Effect, Causing Downregulation of Glycolysis, Epithelial–Mesenchymal Transition, and EGFR-Signaling Pathway Activation in Lung Cancer Cells. Pharmaceuticals 2022, 15, 381. [Google Scholar] [CrossRef]
- Granja, S.; Marchiq, I.; Le Floch, R.; Moura, C.S.; Baltazar, F.; Pouysségur, J. Disruption of BASIGIN decreases lactic acid export and sensitizes non-small cell lung cancer to biguanides independently of the LKB1 status. Oncotarget 2015, 6, 6708–6721. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-Y.; Wang, T.-C.; Kuo, Y.-J.; Shih, W.-T.; Yang, P.-R.; Hsu, C.-M.; Lin, Y.-S.; Kuo, R.-S.; Wu, C.-Y. Astragalus Polysaccharides and Metformin May Have Synergistic Effects on the Apoptosis and Ferroptosis of Lung Adenocarcinoma A549 Cells. Curr. Issues Mol. Biol. 2024, 46, 7782-7794. https://doi.org/10.3390/cimb46080461
Lee I-Y, Wang T-C, Kuo Y-J, Shih W-T, Yang P-R, Hsu C-M, Lin Y-S, Kuo R-S, Wu C-Y. Astragalus Polysaccharides and Metformin May Have Synergistic Effects on the Apoptosis and Ferroptosis of Lung Adenocarcinoma A549 Cells. Current Issues in Molecular Biology. 2024; 46(8):7782-7794. https://doi.org/10.3390/cimb46080461
Chicago/Turabian StyleLee, I-Yun, Ting-Chung Wang, Yu-Jen Kuo, Wei-Tai Shih, Pei-Rung Yang, Cheng-Ming Hsu, Yu-Shih Lin, Ren-Shyang Kuo, and Ching-Yuan Wu. 2024. "Astragalus Polysaccharides and Metformin May Have Synergistic Effects on the Apoptosis and Ferroptosis of Lung Adenocarcinoma A549 Cells" Current Issues in Molecular Biology 46, no. 8: 7782-7794. https://doi.org/10.3390/cimb46080461






