Abstract
The objective of this meta-analysis was to evaluate the association between maternal and fetal genetic variants and the risk of preeclampsia, a pregnancy-related condition that affects women. Despite the unclear role of these genetic factors in the development of preeclampsia, this analysis aimed to provide insights into the potential contributing factors. An electronic search of online databases was conducted to identify relevant studies. Stata SE software was used for the meta-analysis. A random-effects model was used to establish the association between the genetic variants and preeclampsia risk. Egger’s test was utilized to evaluate publication bias. Ten observational studies were selected from databases that met the inclusion criteria and included seven genes and twenty polymorphisms to analyze preeclampsia susceptibility influenced by the genetic background of both the mother and fetus. Our meta-analysis revealed that both the maternal and fetal polymorphisms, FLT1 rs4769613, were significantly associated with the risk of preeclampsia. However, the association between the maternal ACE rs4646994 polymorphism and preeclampsia risk was not statistically significant. Nevertheless, a significant association was observed between the fetal ACE rs4646994 polymorphism and preeclampsia in a dominant genetic model. In this study, the associations between maternal and fetal polymorphisms in ERAP2, VEGF, VDR, REN, and MMP were not statistically significant. According to the available evidence, maternal and fetal polymorphisms can impact the likelihood of developing preeclampsia. Additional research is required to fully understand the underlying mechanisms connecting maternal and fetal polymorphisms to preeclampsia, and to formulate recommendations for screening pregnant women based on these genetic variations.
1. Introduction
Preeclampsia (PE), an obstetric complication characterized by new-onset hypertension, frequently manifests after the 20th week of gestation [1]. It represents the most severe pregnancy complication, resulting in cesarean section, prematurity, chronic heart failure, and/or maternal kidney failure [2,3]. PE is usually categorized into two types according to the gestational age at which it initiates: Type I PE, or placental PE, which starts before 34 weeks of gestation, and Type II PE, or maternal PE, which begins after 34 weeks of gestation. Type I PE, caused by abnormal placental development in early gestation, progresses rapidly and can result in fetal growth restriction, leading to various complications later in life [4,5,6].
Several studies have reported that preeclampsia (PE) is a complex disorder that arises from the interplay between genetic, immunological, and environmental factors. This condition can be attributed to abnormal changes in the remodeling of spiral arteries, placental ischemia, and oxidative stress and progress in two stages. The first stage involves abnormal placentation, while the second stage involves dysregulation of anti-angiogenic factors and angiogenic imbalance, ultimately leading to PE clinical syndrome during the second and third trimesters of pregnancy [2,4,5,7].
The pathogenesis of preeclampsia (PE) remains unclear; however, the placenta is believed to be at the center of the etiology map [2,5,8,9,10]. Researchers have highlighted sFLT1 in the pathogenesis of preeclampsia and its role in the clinical syndrome of PE owing to its high protein levels of sFLT1 in maternal blood experiments and high sFLT1 mRNA expression in preeclamptic placentas [2,5]. At the molecular level, miRNAs are thought to be associated with the mechanisms and prediction of PE [8,9,11]. PE is likely an immune maladaptation that causes malnormal placentation by disrupting the establishment of immune tolerance, including TNFα, IFN-γ, IL-1, IL-4, IL-10, IL-27, HLA-G, TGF-β, E, HLA-C2, and KIR [8]. Molecular pathways that participate in the pathology of PE include the prolactin signaling pathway, peptide hormone metabolism, glycoprotein hormones, hormone ligand-binding receptors, peptide hormone biosynthesis, steroid hormone metabolism, steroid synthesis, the AMP-activated protein kinase (AMPK) signaling pathway, and the FoxO signaling pathway [11,12,13,14,15].
Preeclampsia is strongly associated with various molecular pathways. Researchers worldwide have conducted numerous studies on single-nucleotide polymorphisms (SNPs) to understand the genetic basis of preeclampsia. These studies have focused on genetic variations in anti-inflammatory mediators, vascular- and angiogenesis-related genes, histocompatibility-related genes, genes involved in metabolic changes during pregnancy, detoxification, DNA repair, and apoptosis [16,17]. Various SNPs have been associated with preeclampsia, including FLT1 rs4769613 [8], VEGF rs3025039, and rs2010963 [18,19], VDR rs1544410 polymorphism [20,21], ERAPT1 rs30187, [8,22], TGF-β1 rs1800469 [23], prothrombin G20210A SNP [24], SOD2 A16V polymorphism [25], eNOS c.894 T [26], XPC rs2228000 [27], and the C1431T variant of PPARγ [28]. However, some SNPs that have been explored may not be associated with preeclampsia risk, such as TLR4 rs4986790 and rs4986791 [29] or ROS1 rs9489124 [30].
Scientists have increasingly focused on the association between maternal, fetal, and paternal gene variants, especially SNPs, as well as the development of preeclampsia (PE). However, several studies have found no evidence to support the notion that SNPs play a role in PE [31,32,33,34]. Additionally, there have been no reports linking GSTP1 Val105, eNOS 298Asp, and LPL -93G polymorphisms to the three genotypes of mothers, fathers, and neonates with PE [35]. Maternal and offspring VEGF polymorphisms can increase the risk of maternal PE in Romanian and Han Chinese pregnant women through the VEGF-T936 allele and VEGF-A rs2010963 polymorphism [36,37]. The placental FLT1 rs4769613 C allele was identified as a risk factor in two studies based on GWAS data [38,39], and sFlt-1 is a soluble splice variant of the full-length membrane receptor VEGFR-1.
Prior investigations have been constrained to exploring the association between genetic variations in mothers and preeclampsia. No meta-analysis has assessed the association between genetic variations in offspring and the occurrence of preeclampsia. The current study was conducted to examine the association between genetic alterations in pregnant women and their children and the initiation of preeclampsia. It has been postulated that genetic variations in children could influence the occurrence of maternal preeclampsia.
2. Materials and Methods
2.1. Search Strategy and Identification of Relevant Studies
A thorough exploration was carried out utilizing computer-based methods across a variety of online databases, including PubMed, Embase, Web of Science, and Google Scholar, to pinpoint pertinent research. The search criteria encompassed terms such as ‘Preeclampsia’ or ‘Pre-eclampsia’ and ‘Polymorphisms’ or ‘Genetic Polymorphism’ or ‘Single nucleotide polymorphism’ or ‘SNP’ or ‘variant’ or ‘genotype’ or ‘mutation’. Subsequently, the identified articles underwent individual scrutiny, with relevant research chosen for inclusion in the meta-analysis.
2.2. Criteria for Inclusion and Exclusion
Studies were selected based on the following criteria: (a) they examined the association between genetic variations and susceptibility to PE; (b) they included the genotype and allele frequencies of both cases and controls for the analyzed polymorphisms; (c) they presented original data; (d) they employed a case–control, cohort, or cross-sectional study design; and (e) they were published in English. Studies were excluded if they (a) used a different study design than case–control, cohort, or cross-sectional; (b) did not contain original data or were not relevant to the current analysis; and (c) lacked genotypic distribution or allele frequency data.
2.3. Data Extraction
The extracted data elements included the first author’s name, publication year, ethnicity, genotyping method, single-nucleotide polymorphism (SNP), early- or late-onset stratification groups, number of case and control samples, maternal and fetal genotype distribution, and maternal and fetal allele frequencies in cases and controls. The genotype distribution of each gene was assessed for compliance with the Hardy–Weinberg equilibrium. The data extraction process was carried out separately by three authors (PTNV, QALT, and TNPT) and subsequently validated by the corresponding author.
2.4. Risk of Bias Assessment
The quality of the inclusion of trials was assessed using the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) [40]. Two authors (PTNV, QALT) independently assessed the risk of bias in including studies. Disagreement was resolved by consensus.
2.5. Statistical Analysis
A meta-analysis was conducted using Stata SE version 13.1 software (StataCorp, College Station, TX, USA). A random-effects model was employed to summarize the relationship between each SNP and preeclampsia susceptibility. The Higgins I2 metric was used to measure heterogeneity among the included studies, with an I2 value of 0% indicating no observed heterogeneity and a value above 50% indicating substantial heterogeneity. The strength of the association was assessed using adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Pooled ORs were calculated using the allele genetic (additive), dominant, and recessive models. Funnel plots and Egger’s tests were performed to evaluate publication bias, with a significance level of 0.05.
3. Results
3.1. Characterization of Eligible Studies
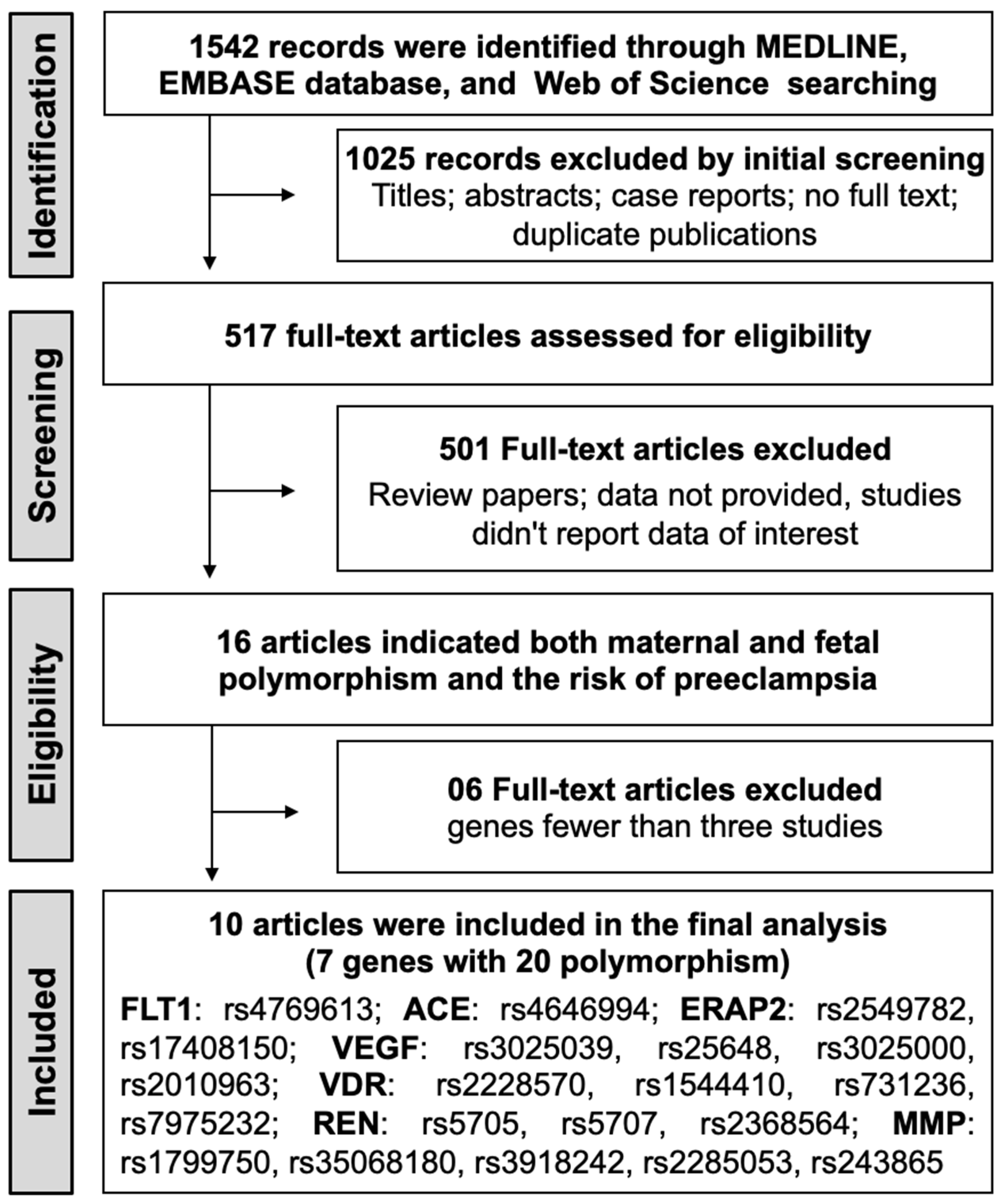
The initial search identified 1542 potential records from electronic databases, and 517 full-text articles were evaluated for eligibility. Among these, 15 articles provided data on both maternal and fetal polymorphisms in relation to preeclampsia risk. Genes implicated in a minimum of three studies were selected, resulting in the exclusion of five articles. Consequently, 10 observational studies that satisfied the inclusion criteria were included in the meta-analysis [32,36,37,38,39,41,42,43,44,45]. The detailed steps of the literature selection process are shown in Figure 1.
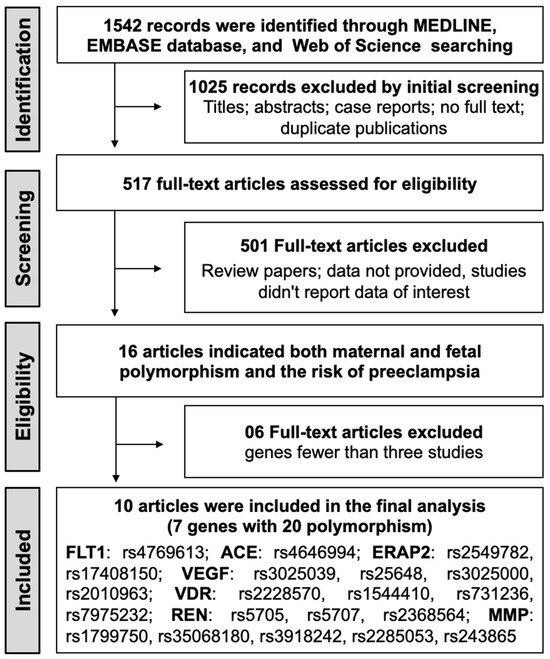
Figure 1.
PRISMA flow chart of the study selection process for the meta-analysis.
The information presented in Table 1 is a summary of the characteristics of studies published independently over 22 years from 1999 to 2020. These studies investigated the relationship between 20 polymorphisms in 7 genes and the risk of preeclampsia in 16,025 maternal and fetal cases and 2,994,233 maternal and fetal controls.

Table 1.
Characteristics and Distribution of Studies Examining the Association between Maternal and Fetal Genetic Variants and Preeclampsia.
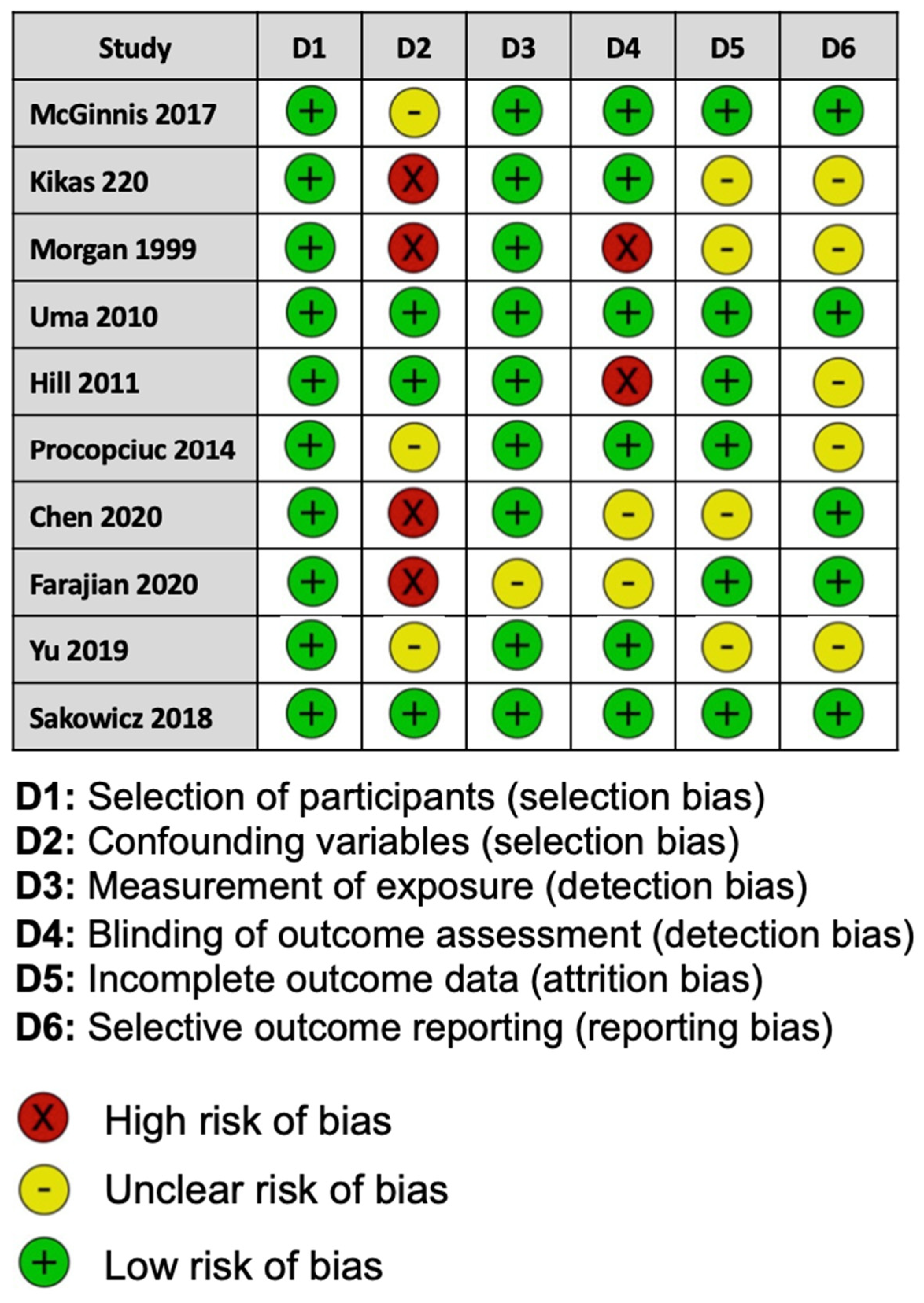
For the risk of bias for each study assessed, two of the ten studies included were considered to be at a low risk of bias, and three studies showed a high risk of bias. Five studies reported methods that raised concerns about the risk of bias (Figure 2).
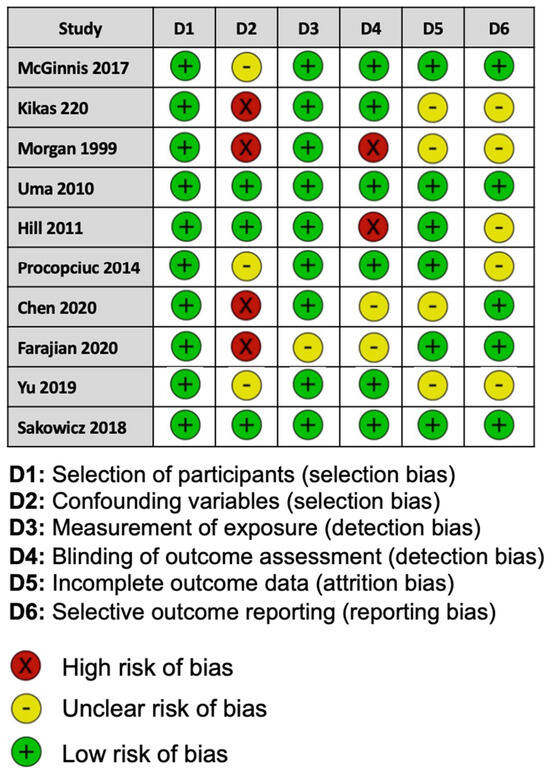
Figure 2.
Assessment of risk of bias for each study in this meta-analysis using the Risk of Bias Assessment tool for Non-randomized Studies.
3.2. Association between Maternal Genetic Variants and Preeclampsia
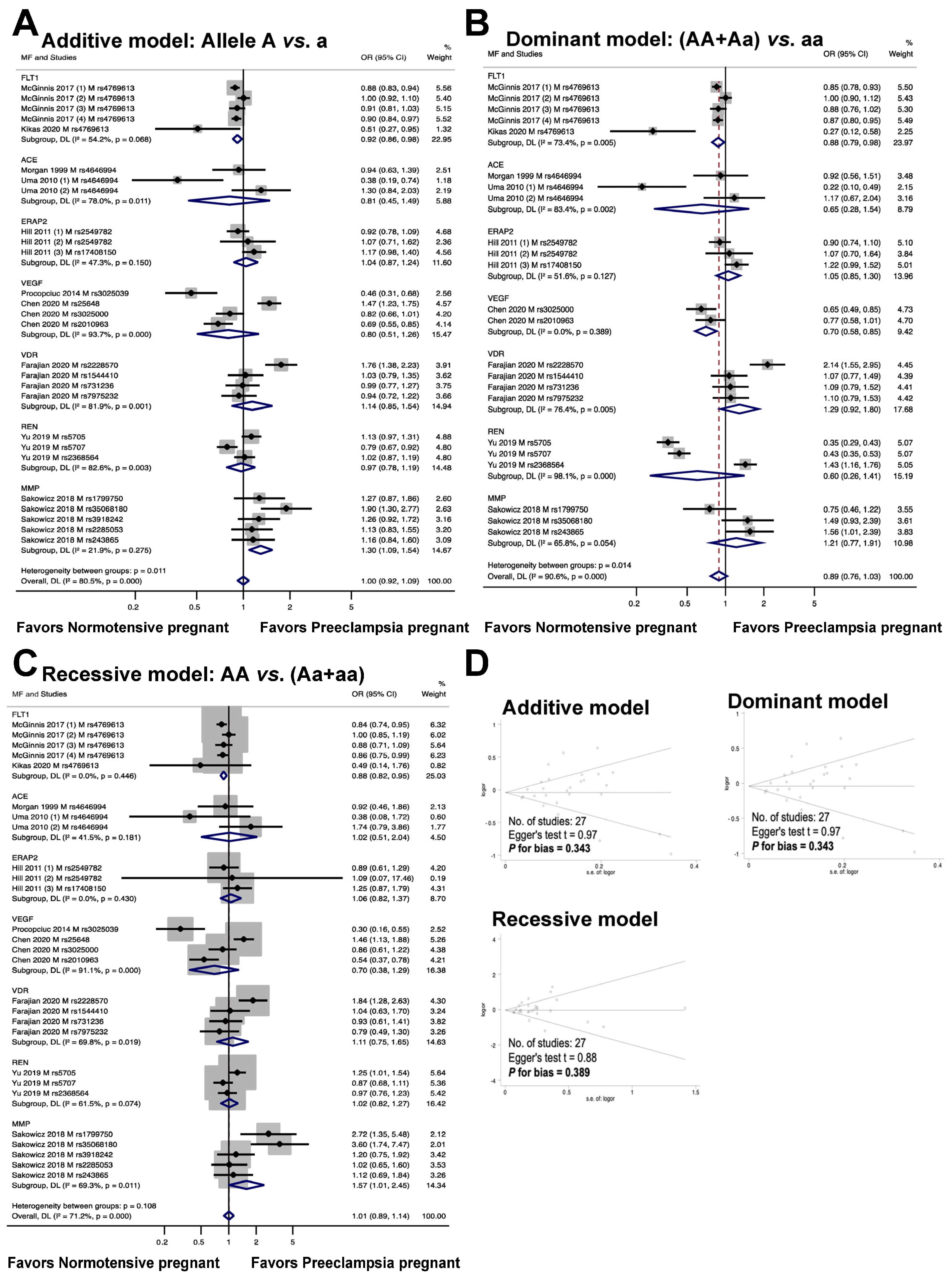
The relationship between maternal genetic variants and risk of developing PE is shown in Figure 3. Seven genes, including FLT1, ACE, ERAP2, VEGF, VDR, REN, and MMP, were analyzed for a total of twenty polymorphisms (FLT1: rs4769613; ACE: rs4646994; ERAP2: rs2549782, rs17408150; VEGF: rs3025039, rs25648, rs3025000, rs2010963; VDR: rs2228570, rs1544410, rs731236, rs7975232; REN: rs5705, rs5707, rs2368564; MMP: rs1799750, rs35068180, rs3918242, rs2285053, rs243865). The meta-analysis results showed that the maternal polymorphism FLT1 rs4769613 was significantly associated with preeclampsia risk in the allelic model (additive model, OR 0.92, 95% CI 0.86 to 0.98, I2 = 54.2%, n = 5). Carriers of the dominant allele (AA and Aa) demonstrated a 12% reduction in preeclampsia susceptibility (dominant model, OR: 0.88, 95% CI 0.79 to 0.98, I2 = 73.4%, n = 5). The recessive model showed a significant association with preeclampsia risk (recessive model, OR 0.88, 95% CI 0.82 to 0.95, I2 = 0.0%, n = 5).
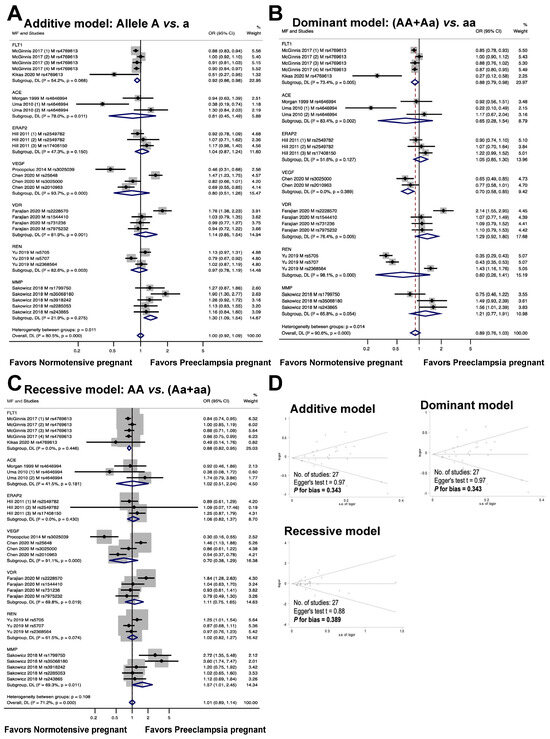
Figure 3.
Association between maternal genetic variants and preeclampsia (A–C). Forest plot of maternal polymorphisms and preeclampsia in an additive model (A), dominant model (B), and recessive model (C). Funnel plot evaluating publication bias among studies included in the meta-analysis (D).
The association between the ACE rs4646994 polymorphism and the risk of preeclampsia is depicted in Figure 3. The results of the analysis of the three genotype models showed no statistically significant association between preeclampsia and maternal ACE rs4646994 polymorphism (additive model, OR 0.81, 95% CI 0.45 to 1.49, I2 = 78%, n = 3; dominant model, OR 0.65, 95% CI 0.28 to 1.54, I2 = 83.4%, n = 3; recessive model, OR 1.02, 95% CI 0.51 to 2.04, I2 = 41.5%, n = 3).
The association between the risk of preeclampsia and the maternal ERAP2 rs2549782 and rs17408150 polymorphisms was assessed through a meta-analysis of three studies. The results are shown in Figure 3. The meta-analysis showed no statistically significant association (additive model, OR 1.04, 95% CI 0.87 to 1.24, I2 = 47.3%, n = 3; dominant model, OR 1.05, 95% CI 0.85 to 1.30, I2 = 51.6%, n = 3; recessive model, OR 1.06, 95% CI 0.82 to 1.37, I2 = 0.0%, n = 3) between the risk of preeclampsia and the maternal ERAP2 rs17408150 polymorphism.
The relationship between maternal VEGF polymorphisms (rs3025039, rs25648, rs3025000, and rs2010963) and preeclampsia risk is shown in Figure 3. In the dominant model, a significant association was observed between maternal VEGF polymorphisms and preeclampsia (OR 0.70, 95% CI, 0.58 to 0.85; I2 = 0.0%; n = 2), but no significant association was detected in the additive model (OR 0.80, 95% CI 0.51 to 1.26; I2 = 93.7%; n = 4) or recessive models (OR 0.70, 95% CI, 0.38 to 1.29; I2 = 91.1%; n = 4).
The meta-analysis depicted in Figure 3 demonstrated that the association between the risk of preeclampsia and maternal VDR polymorphisms (rs2228570, rs1544410, rs731236, and rs7975232) was not statistically significant (additive model, OR 1.14, 95% CI 0.85 to 1.54, I2 = 81.9%; n = 4; dominant model, OR 1.29, 95% CI 0.92 to 1.80, I2 = 76.4%, n = 4; recessive model, OR 1.11, 95% CI 0.75 to 1.65, I2 = 69.8%, n = 4).
The results of the meta-analysis depicted in Figure 3 indicated that there was no statistically significant connection between the risk of preeclampsia and maternal REN polymorphisms (rs5705, rs5707, and rs2368564) under the additive, dominant, or recessive models (OR 0.97, 95% CI 0.78 to 1.19, I2 = 82.6%, n = 3; OR 0.60, 95% CI 0.26 to 1.41, I2 = 98.1%, n = 3; OR 1.02, 95% CI 0.82 to 1.27, I2 = 61.5%, n = 3).
The association between maternal MMP genetic variations (rs1799750, rs35068180, rs3918242, rs2285053, and rs243865) and preeclampsia risk is shown in Figure 3. A meta-analysis revealed that the MMP variant was associated with an increased risk of preeclampsia in an allele-based genetic model (additive model: OR 1.30, 95% CI 1.09 to 1.54, I2 = 21.9%, n = 5). However, no significant association was observed between this genetic variant and the risk of preeclampsia in the dominant (OR 1.21, 95% CI, 0.77 to 1.91; I2 = 65.8%, n = 3) or recessive (OR 1.57, 95% CI 1.01 to 2.45, I2 = 69.3%, n = 5) models.
The graph in Figure 3D illustrates the publication bias in the meta-analysis. There was no evidence of publication bias in the studies, as shown by the symmetric shape of the Begg funnel plot (additive model, Egger’s test t = 0.97, P for bias = 0.343, n = 27; dominant model, Egger’s test t = 0.97, P for bias = 0.343, n = 27; recessive model, Egger’s test t = 0.88, P for bias = 0.389, n = 27).
3.3. Association between Fetal Genetic Variants and Preeclampsia
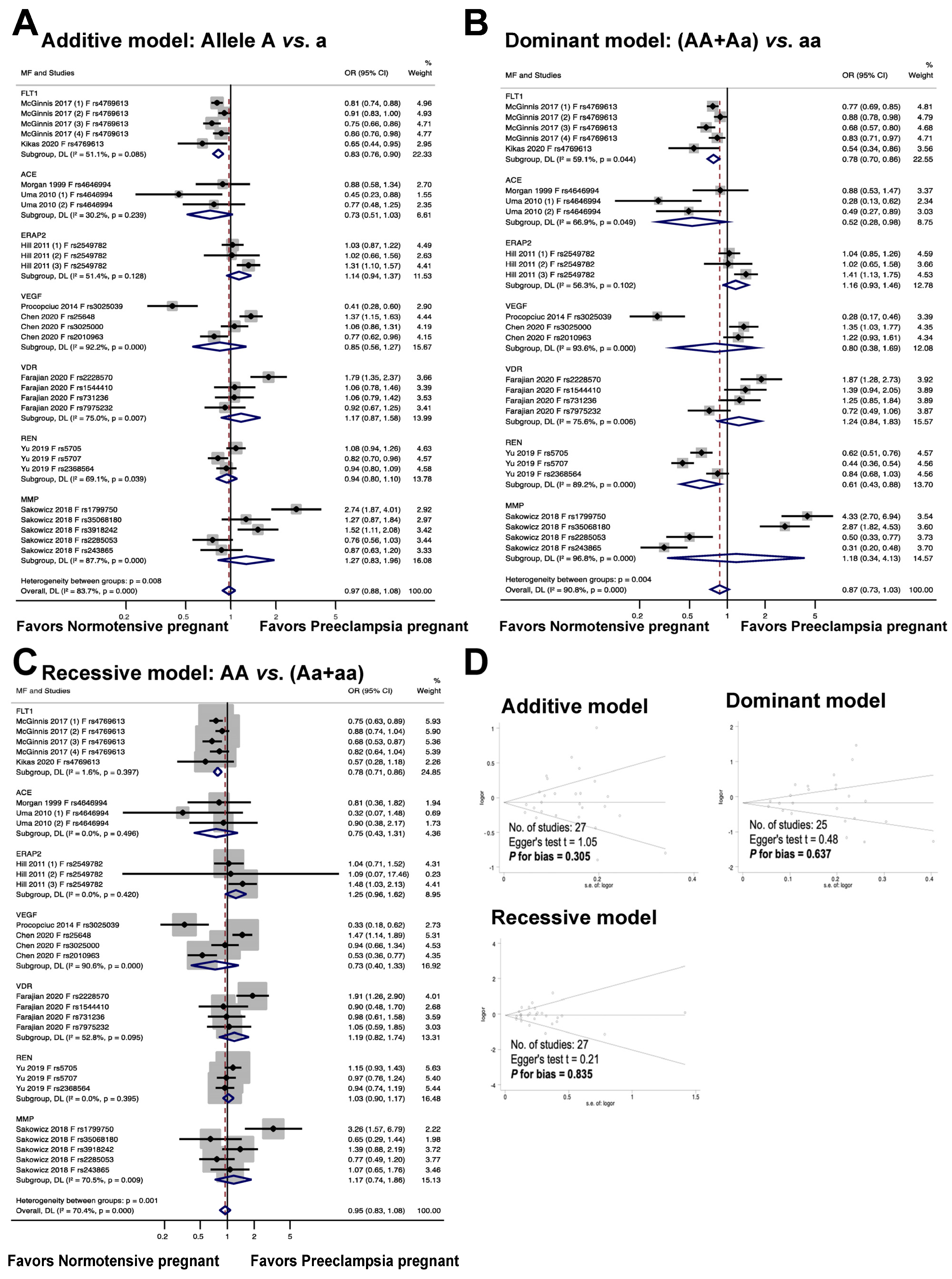
Figure 4 illustrates the association between fetal genetic variants and the risk of preeclampsia. The meta-analysis results reveal that the fetal polymorphism FLT1 rs4769613 is significantly associated with an increased risk of preeclampsia in the allelic model, with an odds ratio of 0.83, a 95% confidence interval of 0.76 to 0.90, and an I2 of 51.1% (n = 5). In the dominant model, individuals with the dominant allele (AA and Aa) had a 22% increased risk of preeclampsia, with an odds ratio of 0.78, 95% confidence interval of 0.70 to 0.86, and I2 of 59.1% (n = 5). The recessive model also showed a significant association with preeclampsia risk, with an odds ratio of 0.78, 95% confidence interval of 0.71 to 0.86, and I2 of 1.6% (n = 5).
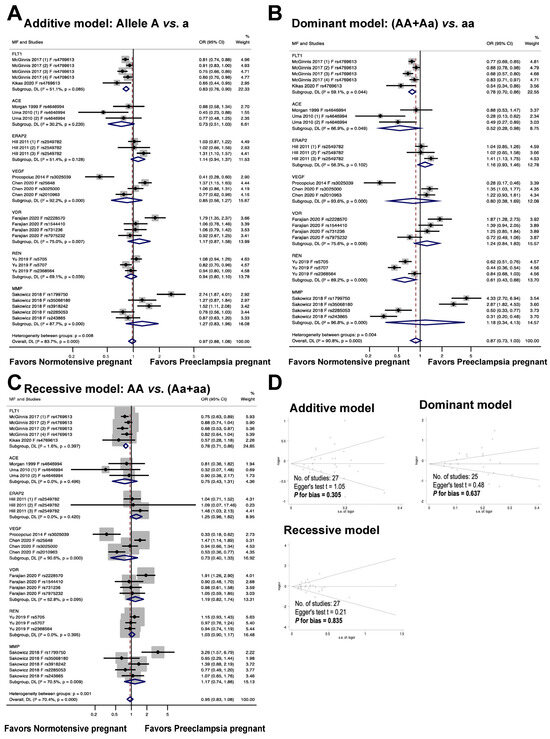
Figure 4.
Association between fetal genetic variants and preeclampsia (A–C). Forest plot of maternal polymorphisms and preeclampsia in an additive model (A), dominant model (B), and recessive model (C). Funnel plot evaluating publication bias among studies included in the meta-analysis (D).
The findings from the meta-analysis revealed no statistically significant connection between the fetal ACE rs4646994 polymorphism and preeclampsia in the additive and recessive models. In the additive model, the odds ratio was 0.73, with a 95% confidence interval of 0.51 to 1.03 and an I2 of 30.2%. The recessive model showed an odds ratio of 0.75, 95% confidence interval of 0.43 to 1.31, and I2 of 0.0%. In contrast, a significant association was observed between fetal polymorphisms and preeclampsia under a dominant genetic model, with an odds ratio of 0.52, 95% confidence interval of 0.28 to 0.98, and I2 of 66.9%.
The findings from the meta-analysis revealed that there was no statistically significant connection between the risk of preeclampsia and the fetal ERAP2 rs2549782 polymorphism (additive model, OR 1.14, 95% CI 0.94 to 1.37, I2 = 51.4%, n = 3; dominant model, OR 1.16, 95% CI 0.93 to 1.46, I2 = 56.3%, n = 3; recessive model, OR 1.25, 95% CI 0.96 to 1.62, I2 = 0.0%, n = 3).
The association between fetal VEGF polymorphisms (rs3025039, rs25648, rs3025000, and rs2010963) and preeclampsia is illustrated in Figure 4. In the additive, dominant, and recessive models, no significant association was observed between fetal VEGF polymorphisms and preeclampsia (OR 0.85, 95% CI 0.56 to 1.27, I2 = 92.2%, n = 4; OR 0.80, 95% CI 0.38 to 1.69, I2 = 93.6%, n = 3; OR 0.73, 95% CI 0.40 to 1.33, I2 = 90.6%, n = 4).
The association between the risk of preeclampsia and fetal VDR polymorphisms (rs2228570, rs1544410, rs731236, and rs7975232) is illustrated in Figure 4. The meta-analysis showed that the association between the risk of preeclampsia and fetal VDR polymorphisms was not statistically significant (additive model, OR 1.17, 95% CI 0.87 to 1.58; I2 = 75.0%; n = 4. dominant model, OR 1.24, 95% CI 0.84 to1.83; I2 = 75.6%, n = 4. recessive model, OR 1.19, 95% CI 0.82 to 1.74, I2 = 52.8%, n = 4).
The findings show that there was no statistically significant association between the risk of preeclampsia and fetal REN polymorphisms (rs5705, rs5707, and rs2368564) (additive model: OR 0.94, 95% CI 0.80 to 1.10, I2 = 69.1%, n = 3; dominant model: OR 0.61, 95% CI 0.43 to 0.88, I2 = 89.2%, n = 3; recessive model: OR 1.03, 95% CI 0.90 to 1.17, I2 = 0.0%, n = 3).
The results of the meta-analysis indicated that the relationship between fetal MMP gene variations (rs1799750, rs35068180, rs3918242, rs2285053, and rs243865) and the likelihood of developing preeclampsia was not statistically significant across all models tested, including the additive (OR 1.27, 95% CI 0.83 to 1.96, I2 = 87.7%, n = 5), dominant (OR 1.18, 95% CI 0.34 to 4.13, I2 = 96.8%, n = 4), and recessive (OR 1.17, 95% CI 0.74 to 1.86, I2 = 70.5%, n = 5) models.
The meta-analysis illustrates the publication bias among the studies. It is important to note that there was no publication bias detected in the studies (as evidenced by the symmetric plot of the Begg funnel, and the results of the additive model, Egger’s test t = 1.05, P for bias = 0.305, n = 27; the dominant model, Egger’s test t = 0.48, P for bias = 0.637, n = 25; and the recessive model, Egger’s test t = 0.21, P for bias = 0.835, n = 27).
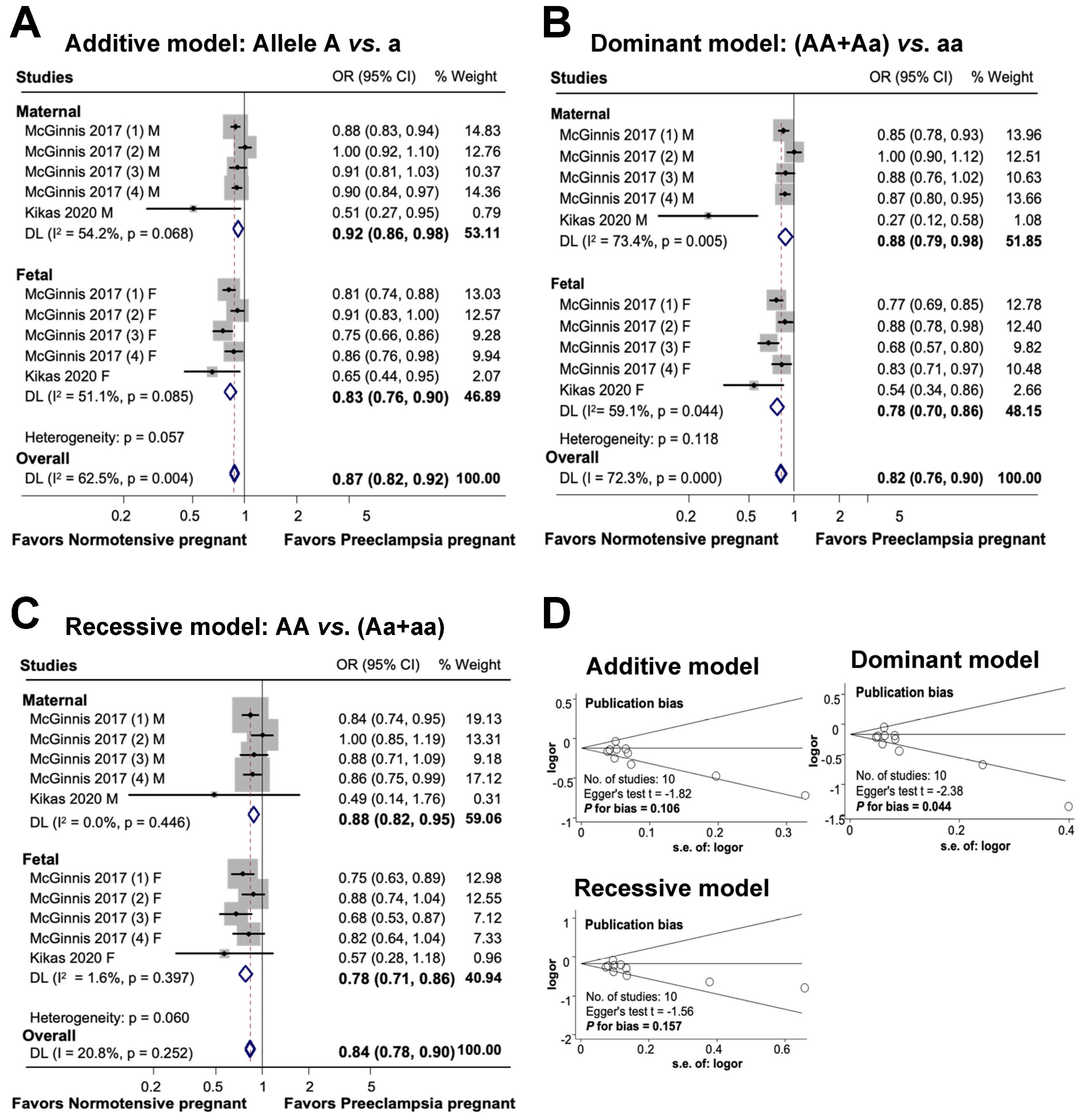
3.4. Association between Maternal and Fetal FLT1 Gene Regulatory Area rs4769613 Polymorphism and Preeclampsia
The findings of this study on the association between maternal and fetal FLT1 rs4769613 polymorphisms and preeclampsia are shown in Figure 5. In an additive model, a substantial association was observed between maternal polymorphisms and preeclampsia risk. Additionally, a significant association was discovered between genetic variants and diseases in the fetal additive model. Overall, the association between maternal and fetal polymorphisms and preeclampsia risk was substantial in the additive model (OR 0.87, 95% CI 0.82 to 0.92, I2 = 62.5%, n = 10). A significant association was observed between FLT1 rs4769613 and preeclampsia in both dominant and recessive models for mothers and fetuses. The overall significant association between maternal and fetal polymorphisms and preeclampsia was determined using a dominant model (OR 0.82, 95% CI 0.76 to 0.90; I2 = 72.3%; n = 10), which demonstrated an 18% reduction in susceptibility to the disease in individuals carrying one dominant allele. Finally, the association between maternal, fetal, and combined polymorphisms and preeclampsia was statistically significant in the recessive model (OR 0.84, 95% CI 0.78 to 0.90; I2 = 20.8%; n = 10).
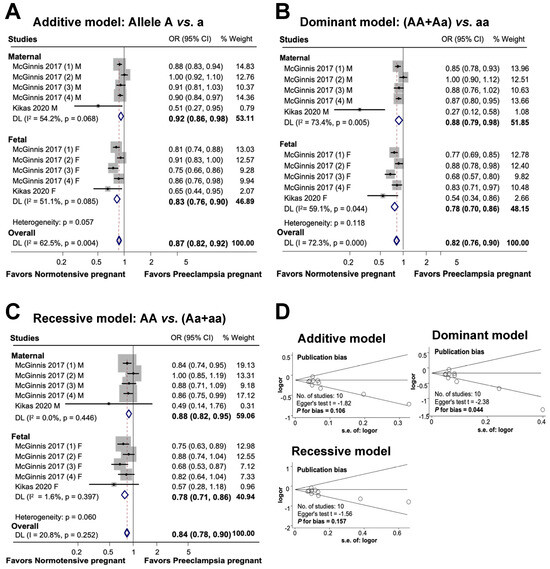
Figure 5.
Association between maternal and fetal FLT1 rs4769613 polymorphism and preeclampsia (A–C). Forest plot of maternal and fetal FLT1 rs4769613 polymorphism and preeclampsia in additive (A), dominant (B), and recessive (C) models. Funnel plot evaluating publication bias among studies included in the meta-analysis (D).
The results of the additive and recessive models indicated no publication bias, as evidenced by the symmetric Begg funnel plot. Egger’s test for the additive model produced a value of t = −1.82, with a P for bias of 0.106 and n = 10. Similarly, Egger’s test for the recessive model yielded a value of t = −1.56, P for bias of 0.157, and n = 10. However, the dominant model showed evidence of publication bias, as indicated by Egger’s test, which produced a value of t = −2.38, P for bias of 0.044, and n = 10.
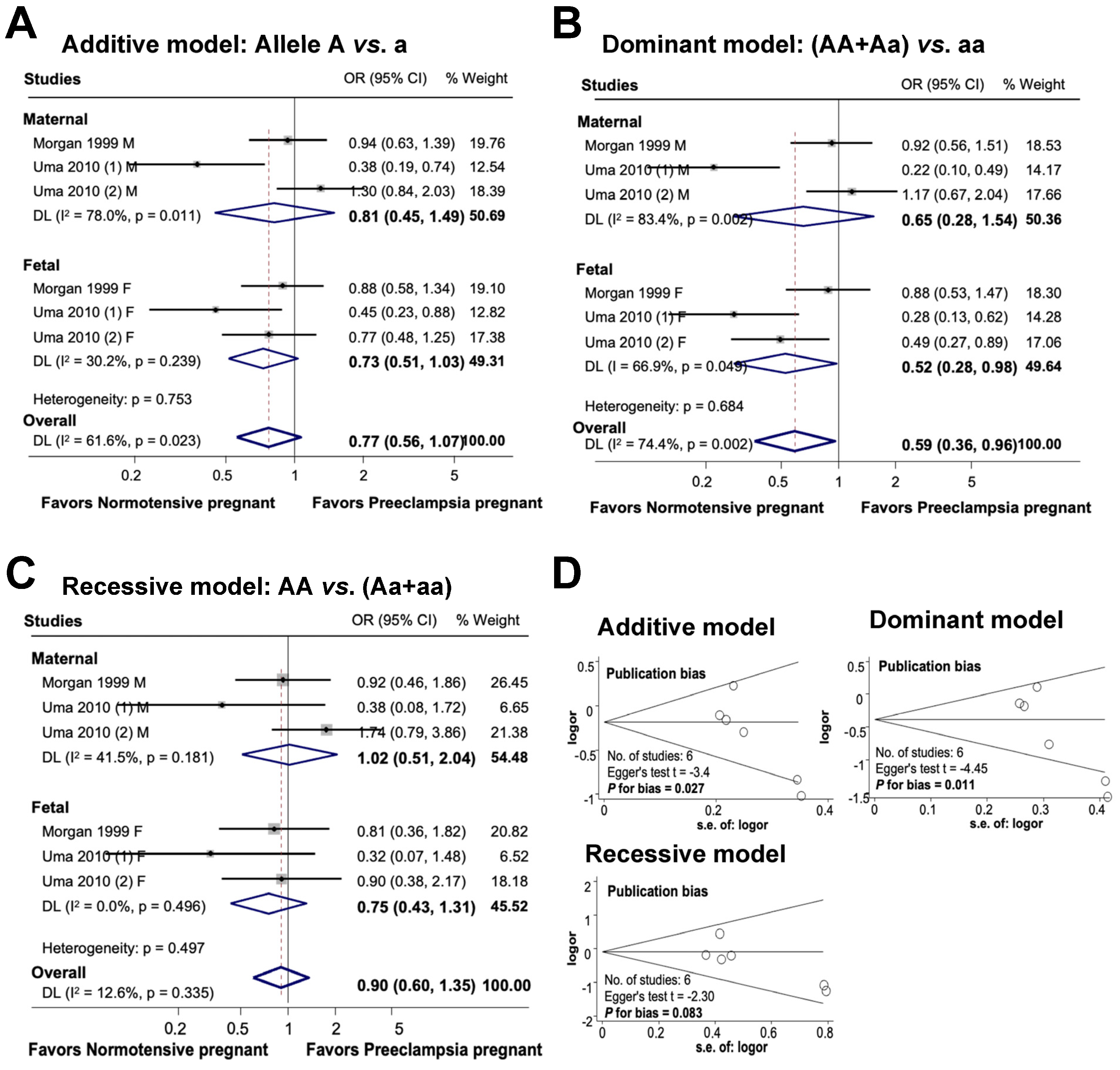
3.5. Association between Maternal and Fetal ACE rs4646994 Polymorphism and Preeclampsia
Figure 6 provides an overview of the association between the maternal and fetal ACE rs4646994 polymorphism and the risk of preeclampsia. In the additive model, there was no significant association between maternal genetic variations and the risk of preeclampsia or between fetal polymorphisms and preeclampsia. The combined influence of maternal and fetal polymorphisms on the risk of preeclampsia appeared to be significant under the additive model, with an odds ratio of 0.77 (95% CI 0.56 to 1.07; I2 = 61.6%; n = 6). In the dominant model, while there was no significant association between maternal polymorphisms and preeclampsia, the fetal ACE rs4646994 polymorphism was significantly associated with preeclampsia risk. Overall, the subjects carrying the dominant genotype had a 41% reduced risk of preeclampsia (OR 0.59, 95% CI, 0.36 to 0.96; I2 = 74.4%; n = 6). In the recessive model, no significant association was detected between maternal polymorphisms, fetal polymorphisms, and the risk of preeclampsia. The overall association between maternal and fetal polymorphisms and preeclampsia was not significant (OR 0.90, 95% CI 0.60 to 1.35; I2 = 12.6%; n = 6).
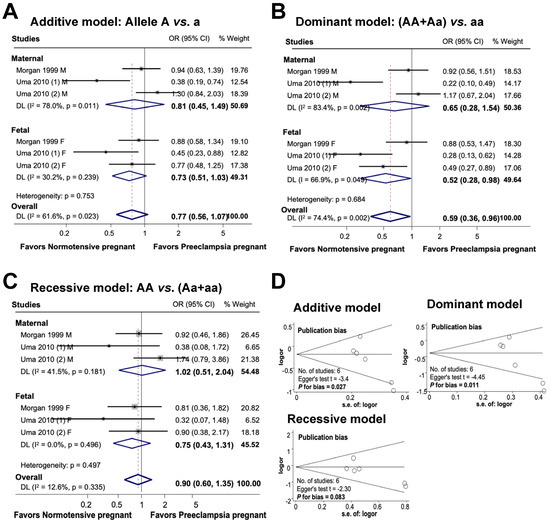
Figure 6.
Association between maternal and fetal ACE rs4646994 polymorphisms and preeclampsia (A–C). Forest plot of maternal and fetal ACE rs4646994 polymorphisms and preeclampsia in an additive (A), dominant (B), and recessive (C) models. Funnel plot evaluating publication bias among studies included in the meta-analysis (D).
Publication bias was detected in both the additive and dominant models (additive model, Egger’s test t = −3.4, P for bias = 0.027, n = 6; dominant model, Egger’s test t = −4.45, P for bias = 0.011, n = 6). However, no publication bias was observed in the recessive model (Egger’s test t = −2.30, P for bias = 0.083, n = 6).
4. Discussion
The results of this study demonstrate that the FLT1 rs4769513 polymorphism in both the mother and fetus affects the likelihood of preeclampsia. In contrast, the ACE rs4646994 polymorphism in the fetus is associated with the risk of preeclampsia, whereas the ACE rs4646994 polymorphism in the mother is not. However, other polymorphisms did not display any substantial association with preeclampsia risk.
This meta-analysis integrated studies that concurrently examined both maternal and fetal genotypes and their association with preeclampsia. However, previous meta-analyses have only focused on studies that explore the association between maternal and fetal genotypes. Studies on individual gene polymorphisms are limited. Therefore, we advocate additional individual studies exploring the link between maternal and fetal gene polymorphisms and preeclampsia to complement the findings of this meta-analysis
A strong relationship is present between the mother and fetus throughout pregnancy and the placenta. The embryo obtains nourishment from its mother’s blood, and when the blood flows back, it contains various macromolecules such as proteins and miRNAs [46]. The process of the mother’s physiological response to this interaction is still being studied. However, the genetics of the fetus can also affect the health of the mother. A study of haplotype genetic scores in 10,734 mother–infant pairs of European ancestry revealed that alleles that raise fetal birth weight can also affect gestation duration and maternal blood pressure [47]. Additionally, research conducted by Rizzo et al. on a Hispanic population showed that the expression of fetal genes, regulated by DNA methylation, is associated with the risk of obesity and diabetes in the mother [48].
Preeclampsia is the most widely studied pregnancy-related disorder, and the advent of modern methods for obtaining fetal samples, such as amniocentesis and chorionic villus sampling, has provided scientists with greater opportunities to investigate fetal genetic material [49]. As discovered by Konecna et al., fetal RNA fragments in maternal blood can trigger an autoimmune response associated with preeclampsia [50]. Banadakoppa et al. found that certain haplotypes of fetal and maternal genetic variants can increase the risk of developing preeclampsia [51]. Steinthorsdottir et al. determined that preeclampsia results from a combination of maternal and fetal genetic materials and that late-onset preeclampsia tends to be linked to fetal genes [52].
The relationships between maternal and fetal variants on the development of preeclampsia (PE) is undeniable because FLT1 plays a crucial role in placentation progression. Protein malfunction can result in vasoconstriction and PE [38,52,53,54]. GCM1 and TGF-β3 can affect maternal risk only if the mother carries the risk allele via fetal carriage [55,56]. However, some studies have revealed that fetal single-nucleotide polymorphisms (SNPs) may not significantly predispose mothers to PE [33,34,35]. The effects of maternal and fetal genes were investigated, including the interaction between fetal HLA-C2 and maternal KIR2DL1 on the KIRAA genotype [57] and the association of maternal and placental TABf haplotypes of the VDR Taq1, Apa1, Bsm1, and Fok1 polymorphisms with a lower PE risk [43]. Additionally, paternal influence on PE risk was indicated through the SOD2 Ala16Val SNP inherited from the father by the fetus [58].
An imbalance between pro- and anti-angiogenic factors is crucial for preeclampsia development. Elevated levels of sFlt-1, a splice variant of the VEGF endothelial receptor, have been observed in pregnant women with preeclampsia [59,60,61,62,63]. Many studies have shown that excessive circulating sFlt1 secreted by the placenta precedes the onset of preeclampsia and is correlated with disease severity [61,64,65,66,67]. sFlt-1 has a greater affinity for VEGF than cell-associated VEGFRboactivities and the balance of VEGF signaling in vascular development. sFlt1 inhibits the function of PIGF by inhibiting its interaction with intrinsic receptors, thereby diminishing the activities of VEGF and PIGF. Nonetheless, in cases of preeclampsia, the surplus release of this substance by the placenta disrupts the equilibrium between pro-angiogenic and anti-angiogenic elements, resulting in endothelial impairment, elevated blood pressure, and protein in the urine. The levels of sFlt-1 and the sFlt-1/PIGF ratio were found to be higher in preeclamptic patients than in the normal group, and can be used as markers for the differential diagnosis of preeclampsia [68]. The sFlt-1/PIGF ratio has been reported to have higher accuracy in differentiating preeclampsia patients from those without preeclampsia [69].
In FLT1, variant rs4769613, positioned on chromosome 13 (GRCh38.p14), has been linked to an increased risk of preeclampsia (PE) in the placenta. This variant, known as a single-nucleotide variant (SNV), modulates gene expression in the placenta and is referred to as the expression quantitative trait locus (eQTL). The FLT1 rs4769613 variant (C > T) functions as a conditional eQTL, with the C allele thought to alter the regulation of the FLT1 gene and enhance its expression. Studies have shown that placentas from women with the CC genotype have higher FLT1 expression levels than placentas with the CT/TT genotype [70]. Previous studies have also identified the C/T variant rs4769613, located near FLT1, as a strong risk factor for preeclampsia [38,39,71]. McGinnis et al. (2017) were the first to provide evidence that alterations in the FLT1 locus in the human fetal genome are associated with an increased risk of preeclampsia. This finding was replicated in multiple European cohorts and led to the identification of the lead risk SNP rs4769613 as a significant factor in preeclampsia. The association between rs4769613 genotype and late-onset preeclampsia has also been investigated [38]. Furthermore, the risk of PE related to FLT1 variants has been suggested to be influenced by fetal gene expression, regardless of maternal or paternal origin [53].
During pregnancy, the renin–angiotensin system (RAS) plays a pivotal role in the regulation of the blood volume and blood pressure balance [72]. Angiotensin-converting enzyme 2 (ACE2) is a key component of this system as it transforms angiotensin (ANG) II to Ang-(1–7) and is associated with preeclampsia and pregnancy outcomes [73,74]. ACE2 exhibits protective effects in the heart, lungs, and kidneys, which are crucial for maintaining blood pressure homeostasis [75,76].
ACE2 is involved in balancing the activities of the heart, lungs, and kidneys, and its levels are important for maintaining blood pressure homeostasis. It cleaves Angiotensin I, forming Angiotensin II, which increases the level of plasminogen activator inhibitor-1 (PAI-1), a regulator of the fibrinolytic system, and normal development of pregnancy [77]. However, in preeclamptic women, ACE concentration is higher than that in normal pregnancies, leading to an imbalance in the Angiotensin II and Ang-(1–7) pathways, which is consistent with the development of hypertension [78]. Increased ACE activity causes abnormal placental circulation and angiogenesis, resulting in an increased risk of the disease. Variants of ACE have been reported to be associated with preeclampsia risk [74,79,80,81,82].
ACE is situated at locus 17q23.3, encompasses 26 exons and 25 introns, and encodes an angiotensin-converting enzyme. One of the most widely investigated and prevalent single-nucleotide polymorphisms (SNPs) discovered in ACE is the insertion/deletion (I/D) variation (rs4646994). The presence of the insertion (I) allele or deletion (D) allele of an Alu repeat sequence in intron 16 results in three genotypes: DD, II, and ID. ACE I/D gene polymorphism is correlated with plasma ACE activity, and the DD genotype of the I/D polymorphism in ACE is the most susceptible to the disease [83]. In contrast, heterozygote ID individuals have demonstrated intermediate concentrations of ACE in the plasma and tissues [84], and those with both the I allele (II) are associated with the lowest ACE levels, potentially reducing the risk of disease [85]. D-allele carriers have been reported to exhibit increased ACE concentrations, which significantly affect hypertension [84,86], and high ACE activity in the DD genotype of the ACE I/D polymorphism is associated with preeclampsia [87]. Furthermore, body mass index and oxidative damage have been suggested to contribute to the development of preeclampsia, alongside the polymorphism rs4646994 (González-Garrido et al. 2017). Additionally, ACE I/D polymorphism has been linked to severe proteinuria and renal dysfunction in preeclamptic patients [88].
Meta-analyses conducted across various studies have investigated the association between genetic polymorphisms and the risk of preeclampsia (PE), yielding mixed results. Although some polymorphisms have been linked to an increased risk of PE, others have shown no significant association. For instance, IL-10 polymorphism [89], RAAS [90], FOXP3 [91], thrombophilia [92], and NOS3 [93] were found to be associated with an increased risk of PE. Conversely, the MMP9-1562C > T polymorphism [94], TLR4 polymorphisms [29], and GST deletions [95] were not significantly associated with PE risk. Weicheng Duan and colleagues conducted a meta-analysis for two SNPs (rs3025039 and rs2010963) in the vascular endothelial growth factor. The findings revealed that the two VEGF gene polymorphisms were connected to an increased risk of PE. Nevertheless, insufficient studies for rs3025039 and rs2010963 SNP in fetal genetics prevented the current meta-analysis from drawing definitive conclusions about each SNP [18].
Shaik et al. conducted a meta-analysis of ACE gene polymorphisms and their correlation with preeclampsia risk. The results revealed no significant association between ACE polymorphisms and preeclampsia risk [96]. Similarly, the current meta-analysis found no significant association between the maternal ACE rs4646994 genetic variation and preeclampsia risk. However, the fetal ACE rs4646994 polymorphism was significantly associated with preeclampsia risk in a dominant model.
The heterogeneity index of several analyses in this study was acceptable. However, some analyses have revealed a relatively high heterogeneity index. The high heterogeneity between the studies is a limitation of the present study. Nonetheless, the results of the subgroup analysis, stratified by gene and maternal or fetal factors, showed that the heterogeneity index was not high for each subgroup analysis.
The current meta-analysis evaluated articles that examined the genotypes of both fetal and maternal genetic variations and preeclampsia. There are limited studies on fetal gene polymorphisms, which restrict the ability to categorize the analysis based on study design, publication year, etc. Although maternal and fetal genotypes may be related, the included studies did not consider this correlation, which could have influenced the outcomes of the meta-analysis.
5. Conclusions
In conclusion, a meta-analysis of 10 studies that assessed the association between 20 polymorphisms in 7 genes and the risk of preeclampsia in 16,025 maternal and fetal cases and 2,994,233 maternal and fetal controls found that maternal and fetal polymorphisms play a significant role in increasing the risk of preeclampsia, which could be used as a predictive factor. Future research should be conducted to understand the underlying mechanisms linking maternal and fetal polymorphisms to preeclampsia and provide guidance on screening for these polymorphisms in pregnant women.
Author Contributions
Project administration: T.N.-T.; experimental design: T.N.-T. and T.-M.-T.H.; experimental performance: P.-T.N.-V., Q.-A.L.-T. and T.-N.P.-T.; data analysis: T.N.-T.; manuscript draft: T.N.-T., P.-T.N.-V., Q.-A.L.-T., T.-N.P.-T. and T.-M.-T.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Research Projects in Science and Technology of the Vietnamese Ministry of Education and Training (B2021-DHH-19). The Group on Regenerative Medicine (NCTB.DHH.2024.02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This study was partially supported by the Research Projects in Science and Technology of the Vietnamese Ministry of Education and Training (B2021-DHH-19). The authors acknowledge the partial support of Hue University under the Core Research Program (Research Group on Regenerative Medicine, NCTB.DHH.2024.02).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, D.D.; Chandresh, N.R.; Javed, A.; Girgis, P.; Zeeshan, M.; Fatima, S.S.; Arab, T.T.; Gopidasan, S.; Daddala, V.C.; Vaghasiya, K.V.; et al. The Management of Preeclampsia: A Comprehensive Review of Current Practices and Future Directions. Cureus 2024, 16, e51512. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef]
- Gyselaers, W. Preeclampsia Is a Syndrome with a Cascade of Pathophysiologic Events. J. Clin. Med. 2020, 9, 2245. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Seow, K.M.; Chen, K.H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef]
- Parada-Niño, L.; Castillo-León, L.F.; Morel, A. Preeclampsia, Natural History, Genes, and miRNAs Associated with the Syndrome. J. Pregnancy 2022, 2022, 3851225. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.D.; Sun, G.X.; Xia, S.S.; Yang, Z.W. Gene Expression Network Analysis Identifies Potential Targets for Prevention of Preeclampsia. Int. J. Gen. Med. 2022, 15, 1023–1032. [Google Scholar] [CrossRef]
- Tabacco, S.; Ambrosii, S.; Polsinelli, V.; Fantasia, I.; D’Alfonso, A.; Ludovisi, M.; Cecconi, S.; Guido, M. Pre-Eclampsia: From Etiology and Molecular Mechanisms to Clinical Tools—A Review of the Literature. Curr. Issues Mol. Biol. 2023, 45, 6202–6215. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Meng, T.; Zhao, G. Identification of Differentially Expressed Genes and Signaling Pathways in Placenta Tissue of Early-Onset and Late-Onset Pre-Eclamptic Pregnancies by Integrated Bioinformatics Analysis. Med. Sci. Monit. 2020, 26, e921997. [Google Scholar] [CrossRef]
- Mohamad, M.A.; Mohd Manzor, N.F.; Zulkifli, N.F.; Zainal, N.; Hayati, A.R.; Ahmad Asnawi, A.W. A Review of Candidate Genes and Pathways in Preeclampsia-An Integrated Bioinformatical Analysis. Biology 2020, 9, 62. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, C.; Liu, Y.; Wang, N.; Gao, S.; Qiu, S.; Wang, Z.; Ding, J.; Zhang, L.; Wang, H.; et al. Identifying key genes and drug screening for preeclampsia based on gene expression profiles. Oncol. Lett. 2020, 20, 1585–1596. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, Y.; Gao, Y.; Liang, G.; Chen, Q.; Jiang, S.; Yang, X.; Fan, C.; Wang, H.; Wang, J.; et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 2021, 11, 5028–5044. [Google Scholar] [CrossRef]
- He, L.; Wu, X.; Zhan, F.; Li, X.; Wu, J. Protective role of metformin in preeclampsia via the regulation of NF-kappaB/sFlt-1 and Nrf2/HO-1 signaling pathways by activating AMPK. Placenta 2023, 143, 91–99. [Google Scholar] [CrossRef]
- Michita, R.T.; Kaminski, V.L.; Chies, J.A.B. Genetic Variants in Preeclampsia: Lessons from Studies in Latin-American Populations. Front. Physiol. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Bokuda, K. Solving preeclampsia from SNP in IGFBP-1 gene. Hypertens. Res. 2023, 46, 2430–2432. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Xia, C.; Wang, K.; Duan, Y.; Cheng, P.; Xiong, B. A meta-analysis of the vascular endothelial growth factor polymorphisms associated with the risk of pre-eclampsia. Biosci. Rep. 2020, 40, BSR20190209. [Google Scholar] [CrossRef]
- Pacheco-Romero, J.; Acosta, O.; Huerta, D.; Cabrera, S.; Vargas, M.; Mascaro, P.; Huamán, M.; Sandoval, J.; López, R.; Mateus, J.; et al. Genetic markers for preeclampsia in Peruvian women. Colomb. Med. 2021, 52, e2014437. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Shakiba, M.; Rezavand, N.; Rahimi, Z.; Vaisi-Raygani, A.; Rahimi, Z.; Shakiba, E. Gene variants and haplotypes of Vitamin D biosynthesis, transport, and function in preeclampsia. Hypertens. Pregnancy 2021, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Magiełda-Stola, J.; Kurzawińska, G.; Ożarowski, M.; Karpiński, T.M.; Drews, K.; Seremak-Mrozikiewicz, A. The Significance of VDR Genetic Polymorphisms in the Etiology of Preeclampsia in Pregnant Polish Women. Diagnostics 2021, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Gomes, C.E.M.; Duggal, P.; De Paula Holanda, I.; de Lima, A.S.; do Nascimento, P.R.P.; Jeronimo, S.M.B. Genetic association of ERAP1 and ERAP2 with eclampsia and preeclampsia in northeastern Brazilian women. Sci. Rep. 2021, 11, 6764. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, G.; Zhao, G.; Meng, T. Gene polymorphism associated with TGF-β1 and susceptibility to preeclampsia: A meta-analysis and trial sequential analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.; Hamdan, H.Z.; Kamis, A.H.; Adam, I. The association of the prothrombin G20210A single-nucleotide polymorphism and the risk of preeclampsia: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 162–169. [Google Scholar] [CrossRef]
- Hu, K.; Liu, X.; Bai, H.; Zhou, M.; Jiang, C.; Fan, P. Association of CYBA C242T and superoxide dismutase 2 A16V genetic variants with preeclampsia. Int. J. Gynecol. Obstet. 2022, 158, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, G.; Jahan, S.; Bibi, N.; Ullah, A.; Faryal, R.; Almajwal, A.; Afsar, T.; Al-Disi, D.; Abulmeaty, M.; Al Khuraif, A.A.; et al. Association of endothelial nitric oxide synthase gene variants with preeclampsia. Reprod. Health 2021, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guan, C.; Sui, J.; Zang, Y.; Wu, Y.; Zhang, R.; Qi, X.; Piao, S. Association between polymorphisms rs2228001 and rs2228000 in XPC and genetic susceptibility to preeclampsia: A case control study. BMC Pregnancy Childbirth 2021, 21, 787. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rouault, C.; Clément, K.; Zhu, W.; Degrelle, S.A.; Charles, M.A.; Heude, B.; Fournier, T. C1431T Variant of PPARγ Is Associated with Preeclampsia in Pregnant Women. Life 2021, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiang, H.; Meng, T.; Liu, P.; Chen, H. Association Between TLR4 Gene Polymorphisms and Risk of Preeclampsia: Systematic Review and Meta-Analysis. Med. Sci. Monit. 2021, 27, e930438. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Yang, M.; Liu, M.; Tian, S.; Yu, M.; Li, W.P.; Zhang, C. The Impact of PTPRK and ROS1 Polymorphisms on the Preeclampsia Risk in Han Chinese Women. Int. J. Hypertens. 2021, 27, 3275081. [Google Scholar] [CrossRef]
- Morgan, L.; Foster, F.; Hayman, R.; Crawshaw, S.; Baker, P.N.; Broughton Pipkin, F.; Kalsheker, N. Angiotensin-converting enzyme insertion-deletion polymorphism in normotensive and pre-eclamptic pregnancies. J. Hypertens. 1999, 17, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.; Crawshaw, S.; Baker, P.N.; Broughton Pipkin, F.; Kalsheker, N. Maternal and fetal angiotensinogen gene allele sharing in pre-eclampsia. Br. J. Obstet. Gynaecol. 1999, 106, 244–251. [Google Scholar] [CrossRef]
- Livingston, J.C.; Barton, J.R.; Park, V.; Haddad, B.; Phillips, O.; Sibai, B.M. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am. J. Obstet. Gynecol. 2001, 185, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rokni, M.; Salimi, S.; Sohrabi, T.; Asghari, S.; Teimoori, B.; Saravani, M. Association between miRNA-152 polymorphism and risk of preeclampsia susceptibility. Arch. Gynecol. Obstet. 2019, 299, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Pappa, K.I.; Roubelakis, M.; Vlachos, G.; Marinopoulos, S.; Zissou, A.; Anagnou, N.P.; Antsaklis, A. Variable effects of maternal and paternal-fetal contribution to the risk for preeclampsia combining GSTP1, eNOS, and LPL gene polymorphisms. J. Matern. Fetal Neonatal Med. 2011, 24, 628–635. [Google Scholar] [CrossRef]
- Procopciuc, L.M.; Caracostea, G.; Zaharie, G.; Stamatian, F. Maternal/newborn VEGF-C936T interaction and its influence on the risk, severity and prognosis of preeclampsia, as well as on the maternal angiogenic profile. J. Matern. Fetal Neonatal Med. 2014, 27, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Yu, S.J.; Wei, M.H.; Li, C.Y.; Yan, W.R. Effects of maternal and fetal vascular endothelial growth factor a single nucleotide polymorphisms on pre-eclampsia: A hybrid design study. Cytokine 2020, 127, 154995. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, R.; Steinthorsdottir, V.; Williams, N.O.; Thorleifsson, G.; Shooter, S.; Hjartardottir, S.; Bumpstead, S.; Stefansdottir, L.; Hildyard, L.; Sigurdsson, J.K.; et al. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat. Genet. 2017, 49, 1255–1260. [Google Scholar] [CrossRef]
- Kikas, T.; Inno, R.; Ratnik, K.; Rull, K.; Laan, M. C-allele of rs4769613 Near FLT1 Represents a High-Confidence Placental Risk Factor for Preeclampsia. Hypertension 2020, 76, 884–891. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Uma, R.; Forsyth, S.J.; Struthers, A.D.; Fraser, C.G.; Godfrey, V.; Murphy, D.J. Polymorphisms of the angiotensin converting enzyme gene in early-onset and late-onset pre-eclampsia. J. Matern. Fetal Neonatal Med. 2010, 23, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.D.; Hilliard, D.D.; York, T.P.; Srinivas, S.; Kusanovic, J.P.; Gomez, R.; Elovitz, M.A.; Romero, R.; Strauss, J.F., 3rd. Fetal ERAP2 variation is associated with preeclampsia in African Americans in a case-control study. BMC Med. Genet. 2011, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Farajian-Mashhadi, F.; Eskandari, F.; Rezaei, M.; Eskandari, F.; Najafi, D.; Teimoori, B.; Moradi-Sharbabak, M.; Salimi, S. The possible role of maternal and placental vitamin D receptor polymorphisms and haplotypes in pathogenesis of preeclampsia. Clin. Exp. Hypertens. 2020, 42, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Peng, W.; Zhang, H.; Li, C.; Chen, X.; Wei, M.; Yan, W. The association between maternal and foetal REN gene polymorphisms and preeclampsia/eclampsia: A hybrid design study. Pregnancy Hypertens. 2019, 18, 150–155. [Google Scholar] [CrossRef]
- Sakowicz, A.; Lisowska, M.; Biesiada, L.; Rybak-Krzyszkowska, M.; Gach, A.; Sakowicz, B.; Grzesiak, M.; Huras, H.; Pietrucha, T. Association of Maternal and Fetal Single-Nucleotide Polymorphisms in Metalloproteinase (MMP1, MMP2, MMP3, and MMP9) Genes with Preeclampsia. Dis. Markers 2018, 2018, 1371425. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, A.; Vilella, F. Mother and Embryo Cross-Communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef]
- Chen, J.; Bacelis, J.; Sole-Navais, P.; Srivastava, A.; Juodakis, J.; Rouse, A.; Hallman, M.; Teramo, K.; Melbye, M.; Feenstra, B.; et al. Dissecting maternal and fetal genetic effects underlying the associations between maternal phenotypes, birth outcomes, and adult phenotypes: A mendelian-randomization and haplotype-based genetic score analysis in 10,734 mother-infant pairs. PLoS Med. 2020, 17, e1003305. [Google Scholar] [CrossRef]
- Rizzo, H.E.; Escaname, E.N.; Alana, N.B.; Lavender, E.; Gelfond, J.; Fernandez, R.; Hibbs, M.A.; King, J.M.; Carr, N.R.; Blanco, C.L. Maternal diabetes and obesity influence the fetal epigenome in a largely Hispanic population. Clin. Epigenet. 2020, 12, 34. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, H.; Wang, Z.; Li, J. Novel insights into the complex interplay of immune dysregulation and inflammatory biomarkers in preeclampsia and fetal growth restriction: A two-step Mendelian randomization analysis. J. Transl. Autoimmun. 2024, 8, 100226. [Google Scholar] [CrossRef]
- Konečná, B.; Vlková, B.; Celec, P. Role of fetal DNA in preeclampsia (review). Int. J. Mol. Med. 2015, 35, 299–304. [Google Scholar] [CrossRef]
- Banadakoppa, M.; Balakrishnan, M.; Yallampalli, C. Common variants of fetal and maternal complement genes in preeclampsia: Pregnancy specific complotype. Sci. Rep. 2020, 10, 4811. [Google Scholar] [CrossRef] [PubMed]
- Steinthorsdottir, V.; McGinnis, R.; Williams, N.O.; Stefansdottir, L.; Thorleifsson, G.; Shooter, S.; Fadista, J.; Sigurdsson, J.K.; Auro, K.M.; Berezina, G.; et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat. Commun. 2020, 11, 5976. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Saxena, R.; Karumanchi, S.A. Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. Am. J. Obstet. Gynecol. 2018, 218, 211–218. [Google Scholar] [CrossRef]
- Macias-Salas, A.; Sosa-Macias, M.; Barragan-Zuniga, L.J.; Blanco-Castaneda, R.; Damiano, A.; Garcia-Robles, R.; Ayala-Ramirez, P.; Bueno-Sanchez, J.; Giachini, F.R.; Escudero, C.; et al. Preeclampsia association of placental nucleotide variations in eNOS, VEGFA, and FLT-1 genes in Latin American pregnant women. Placenta 2023, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Desmond, D.H.; Goodwin, T.M.; Miller, D.A.; Ingles, S.A. Maternal and fetal variants in the TGF-beta3 gene and risk of pregnancy-induced hypertension in a predominantly Latino population. Am. J. Obstet. Gynecol. 2009, 201, 295.e1–295.e5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, M.L.; Brueggmann, D.; Desmond, D.H.; Mandeville, J.E.; Goodwin, T.M.; Ingles, S.A. A fetal variant in the GCM1 gene is associated with pregnancy induced hypertension in a predominantly hispanic population. Int. J. Mol. Epidemiol. Genet. 2011, 2, 196–206. [Google Scholar] [PubMed]
- Kelemu, T.; Erlandsson, L.; Seifu, D.; Hansson, E.; Abebe, M.; Teklu, S.; Girma, S.; Traherne, J.A.; Moffett, A.; Hansson, S.R. Polymorphism in killer cell immunoglobulin-like receptors and human leukocyte antigen-c and predisposition to preeclampsia in Ethiopian pregnant women population. J. Reprod. Immunol. 2020, 141, 103169. [Google Scholar] [CrossRef]
- Luo, Z.C.; Julien, P.; Wei, S.Q.; Audibert, F.; Fraser, W.D. Association of pre-eclampsia with SOD2 Ala16Val polymorphism among mother-father-infant triads. Int. J. Gynaecol. Obstet. 2018, 142, 221–227. [Google Scholar] [CrossRef] [PubMed]
- de Jesús, G.R.; Lacerda, M.I. Soluble Flt-1, Placental Growth Factor, and Vascular Endothelial Growth Factor Serum Levels to Differentiate between Active Lupus Nephritis during Pregnancy and Preeclampsia. Arthritis Care Res. 2021, 73, 717–721. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Carmeliet, P. Soluble VEGF receptor Flt1: The elusive preeclampsia factor discovered? J. Clin. Investig. 2003, 111, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Su, M. HMOX1 Participates in Pre-Eclampsia by Regulating the Proliferation, Apoptosis, and Angiogenesis Modulation Potential of Mesenchymal Stem Cells via VEGF. Biochem. Genet. 2024, 62, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Wathén, K.A.; Tuutti, E.; Stenman, U.H.; Alfthan, H.; Halmesmäki, E.; Finne, P.; Ylikorkala, O.; Vuorela, P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J. Clin. Endocrinol. Metab. 2006, 91, 180–184. [Google Scholar] [CrossRef][Green Version]
- McKeeman, G.C.; Ardill, J.E.S.; Caldwell, C.M.; Hunter, A.J.; McClure, N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am. J. Obstet. Gynecol. 2004, 191, 1240–1246. [Google Scholar] [CrossRef]
- Hertig, A.; Berkane, N.; Lefevre, G.; Toumi, K.; Marti, H.-P.; Capeau, J.; Uzan, S.; Rondeau, E. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin. Chem. 2004, 50, 1702–1703. [Google Scholar] [CrossRef]
- De Vivo, A.; Baviera, G.; Giordano, D.; Todarello, G.; Corrado, F.; D’Anna, R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet. Gynecol. Scand. 2008, 87, 837–842. [Google Scholar] [CrossRef]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80. [Google Scholar] [CrossRef]
- Kikas, T.; Laan, M.; Kasak, L. Current knowledge on genetic variants shaping placental transcriptome and their link to gestational and postnatal health. Placenta 2021, 116, 2–11. [Google Scholar] [CrossRef]
- Ohwaki, A.; Nishizawa, H.; Kato, A.; Kato, T.; Miyazaki, J.; Yoshizawa, H.; Noda, Y.; Sakabe, Y.; Ichikawa, R.; Sekiya, T.; et al. Placental Genetic Variants in the Upstream Region of the FLT1 Gene in Pre-eclampsia. J. Reprod. Infertil. 2020, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Drozdz, D.; Tomasik, P.J. Classical and Alternative Pathways of the Renin-Angiotensin-Aldosterone System in Regulating Blood Pressure in Hypertension and Obese Adolescents. Biomedicines 2024, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.; Konoshita, T.; Moodley, J.; Gathiram, P. Association of gene polymorphisms of four components of renin-angiotensin-aldosterone system and preeclampsia in South African black women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.B.; Rahman, M.M.; Howlader, M.Z.H.; Kabir, Y. Assessment of angiotensin converting enzyme gene polymorphism in preeclampsia mothers of Bangladesh. J. Obstet. Gynaecol. 2021, 41, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, S.; Lumbers, E.R.; Morosin, S.K.; Delforce, S.J.; Pringle, K.G. ACE2: A key modulator of the renin-angiotensin system and pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R833–R843. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, S.; Clifton, V.L.; Rae, K.; van Helden, D.F.; Lumbers, E.R.; Pringle, K.G. Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front. Physiol. 2020, 11, 590787. [Google Scholar] [CrossRef] [PubMed]
- Gintoni, I.; Adamopoulou, M.; Yapijakis, C. The Angiotensin-converting Enzyme Insertion/Deletion Polymorphism as a Common Risk Factor for Major Pregnancy Complications. In Vivo 2021, 35, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.C.; Karoly, M.; Chen, K.; Ferrario, C.M.; Brosnihan, K.B. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine 2002, 18, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, X.; Liu, H.; Huang, S. Three polymorphisms of renin-angiotensin system and preeclampsia risk. J. Assist. Reprod. Genet. 2020, 37, 3121–3142. [Google Scholar] [CrossRef]
- Dmitrenko, O.P.; Karpova, N.S.; Nurbekov, M.K.; Papysheva, O.V. I/D Polymorphism Gene ACE and Risk of Preeclampsia in Women with Gestational Diabetes Mellitus. Dis. Markers 2020, 2020, 8875230. [Google Scholar] [CrossRef]
- Shaheen, G.; Sajid, S.; Razak, S.; Mazhar, S.B.; Afsar, T.; Almajwal, A.; Alam, I.; Jahan, S. Role of ACE I/D polymorphism in pathological assessment of preeclampsia in Pakistan. Mol. Genet. Genom. Med. 2019, 7, e00799. [Google Scholar] [CrossRef]
- Lee, N.R.; Hwang, I.W.; Kim, H.J.; Kang, Y.D.; Park, J.W.; Jin, H.J. Genetic Association of Angiotensin-Converting Enzyme (ACE) Gene I/D Polymorphism with Preterm Birth in Korean Women: Case-Control Study and Meta-Analysis. Medicina 2019, 55, 264. [Google Scholar] [CrossRef]
- Agerholm-Larsen, B.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. ACE gene polymorphism in cardiovascular disease: Meta-analyses of small and large studies in whites. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, S.; Ahmadalipour, A.; Salehi, M. Evaluation of ACE gene I/D polymorphism in Iranian elite athletes. Adv. Biomed. Res. 2014, 3, 207. [Google Scholar] [CrossRef]
- Yapijakis, C.; Koronellos, N.; Spyridonidou, S.; Vylliotis, A.; Avgoustidis, D.; Goutas, N.; Vlachodimitropoulos, D.; Vairaktaris, E. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with decreased risk for basal cell carcinoma. Arch. Dermatol. Res. 2013, 305, 333–339. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Fan, C.; Yu, M.; Wallar, G.; Zhang, Z.F.; Wang, L.; Zhang, X.; Hu, R. Associations of ACE gene insertion/deletion polymorphism, ACE activity, and ACE mRNA expression with hypertension in a Chinese population. PLoS ONE 2013, 8, e75870. [Google Scholar] [CrossRef]
- González-Garrido, J.A.; García-Sánchez, J.R.; Tovar-Rodríguez, J.M.; Olivares-Corichi, I.M. Preeclampsia is associated with ACE I/D polymorphism, obesity and oxidative damage in Mexican women. Pregnancy Hypertens. 2017, 10, 22–27. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Fu, Q.; Wang, L. Angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensin II type 1 receptor (AT1R) gene polymorphism and its association with preeclampsia in Chinese women. Hypertens. Pregnancy 2007, 26, 293–301. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Z.; Wang, J.; Ye, W.; Ding, Y. Evaluation of association of maternalIL-10 polymorphisms with risk of preeclampsia by A meta-analysis. J. Cell. Mol. Med. 2014, 18, 2466–2477. [Google Scholar] [CrossRef]
- Wang, X.; Kong, Y.; Chen, X.; Weng, Z.; Li, B. Pertinence between risk of preeclampsia and the renin-angiotensin-aldosterone system (RAAS) gene polymorphisms: An updated meta-analysis based on 73 studies. J. Obstet. Gynaecol. 2023, 43, 2171782. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.; Zidanloo, S.G.; Jahantigh, D. Influence of FOXP3 gene polymorphisms on the risk of preeclampsia: A meta-analysis and a bioinformatic approach. Clin. Exp. Hypertens. 2022, 44, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, T.; Liu, S.; Pan, H.; Wang, B. Association between Thrombophilia Gene Polymorphisms and Preeclampsia: A Meta-Analysis. PLoS ONE 2014, 9, e100789. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zhu, S.; Wong, M.C.-S.; Yang, Z.; Tang, J.; Li, K.; Su, X. Associations between nitric oxide synthase 3 gene polymorphisms and preeclampsia risk: A meta-analysis. Sci. Rep. 2016, 6, 23407. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.-L.; Liu, H.; Liu, L.-H. Lack of association between matrix metalloproteinase-9 gene-1562C/T polymorphism and preeclampsia: A meta-analysis. Hypertens. Pregnancy 2014, 33, 389–394. [Google Scholar] [CrossRef]
- Jakovljevic, T.S.; Jacimovic, J.; Nikolic, N.; Milasin, J. Lack of association between glutathione S-transferase M1 and T1 gene polymorphisms and susceptibility to preeclampsia: An updated systematic review and meta-analysis. Am. J. Reprod. Immunol. 2020, 84, e13303. [Google Scholar] [CrossRef]
- Shaik, A.P.; Sultana, A.; Bammidi, V.K.; Sampathirao, K.; Jamil, K. A meta-analysis of eNOS and ACE gene polymorphisms and risk of pre-eclampsia in women. J. Obstet. Gynaecol. 2011, 31, 603–607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).