ELIXCYTE®, an Allogenic Adipose-Derived Stem Cell Product, Mitigates Osteoarthritis by Reducing Inflammation and Preventing Cartilage Degradation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synovial Fluid Collection
2.2. ELIXCYTE® Isolation and Culture
2.3. Chondrocyte Cell Culture
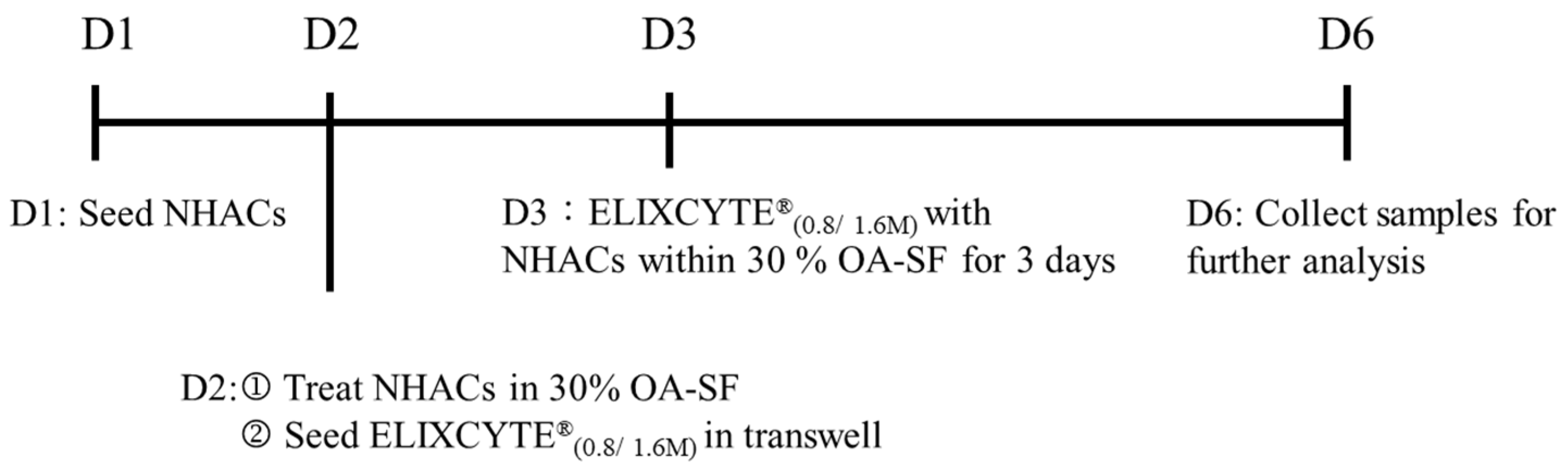
2.4. Coculture of NHACs with ELIXCYTE® in an OA-SF Environment
2.5. Gene Expression Analysis
2.6. Multiplexed Arrays
2.7. IL-11 ELISA
2.8. Data Analysis
3. Results
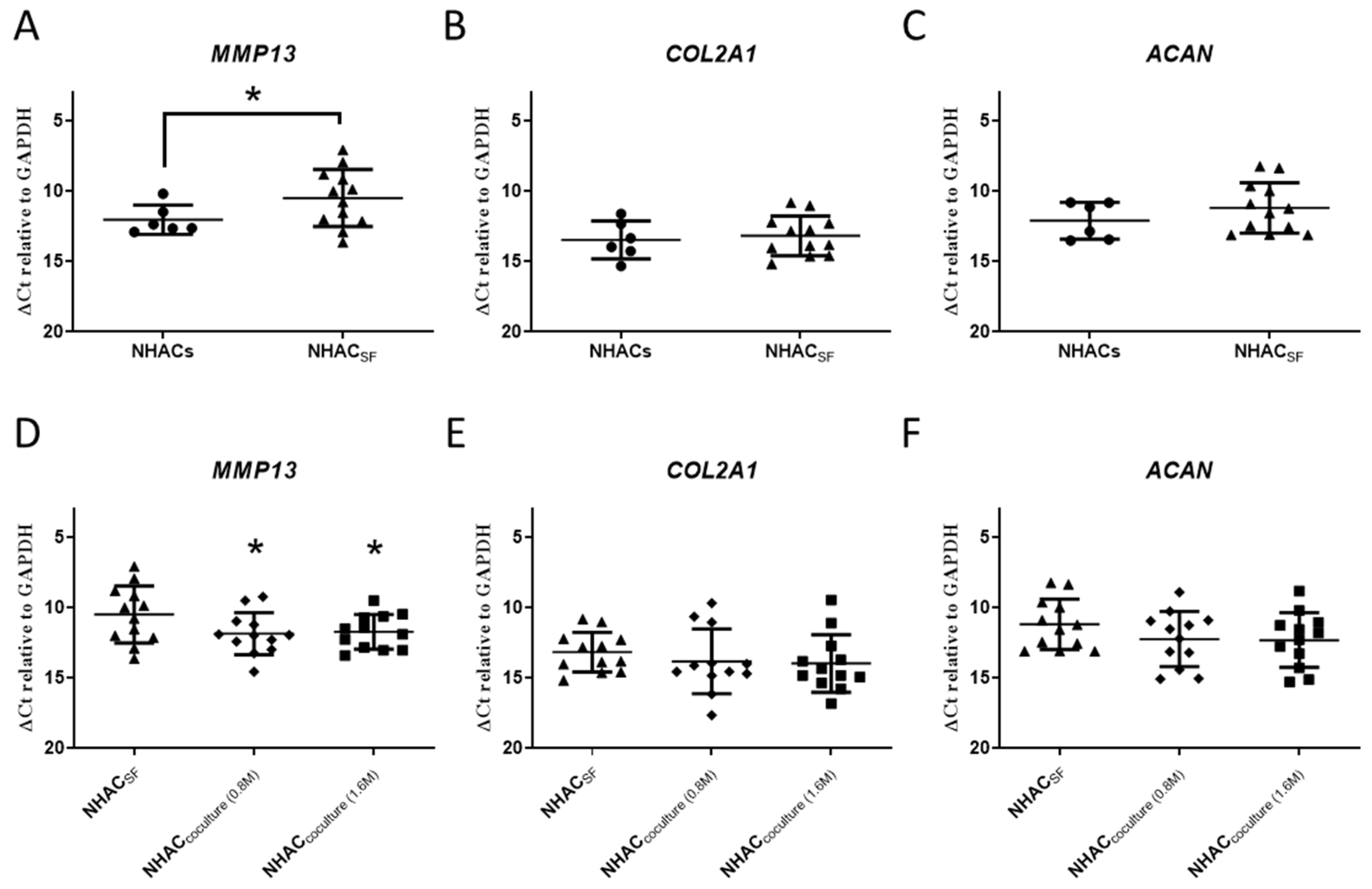
3.1. OA-SF Upregulates MMP13 Gene Expression in Chondrocytes, whereas Coculture with ELIXCYTE® Downregulates MMP13 Expression
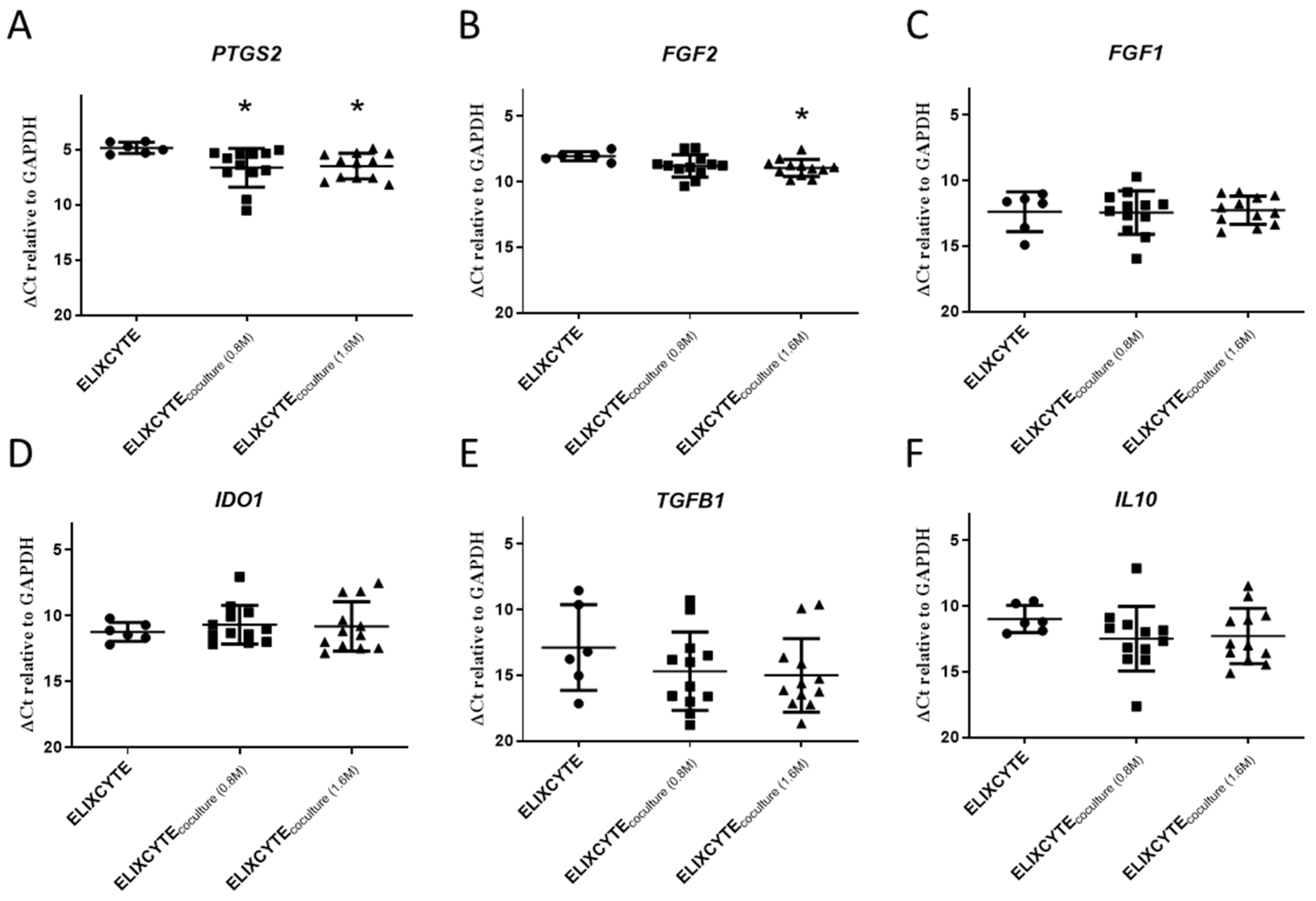
3.2. ELIXCYTE® Protects NHACs against the Effects of OA-SF through Anti-Inflammatory Activity
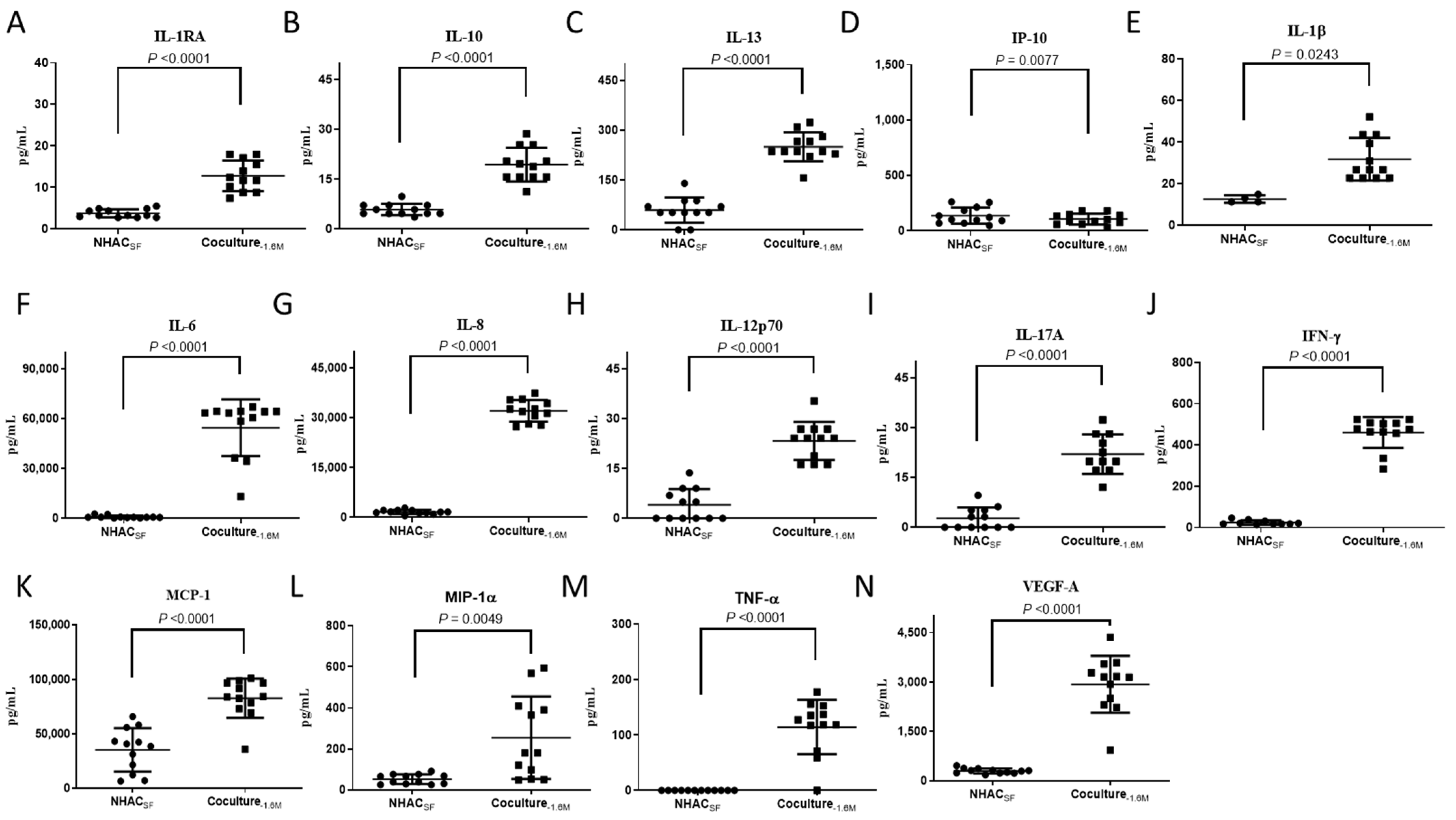
3.3. ELIXCYTE® Triggers Anti-Inflammatory and Pro-Inflammatory Factors in the Coculture System
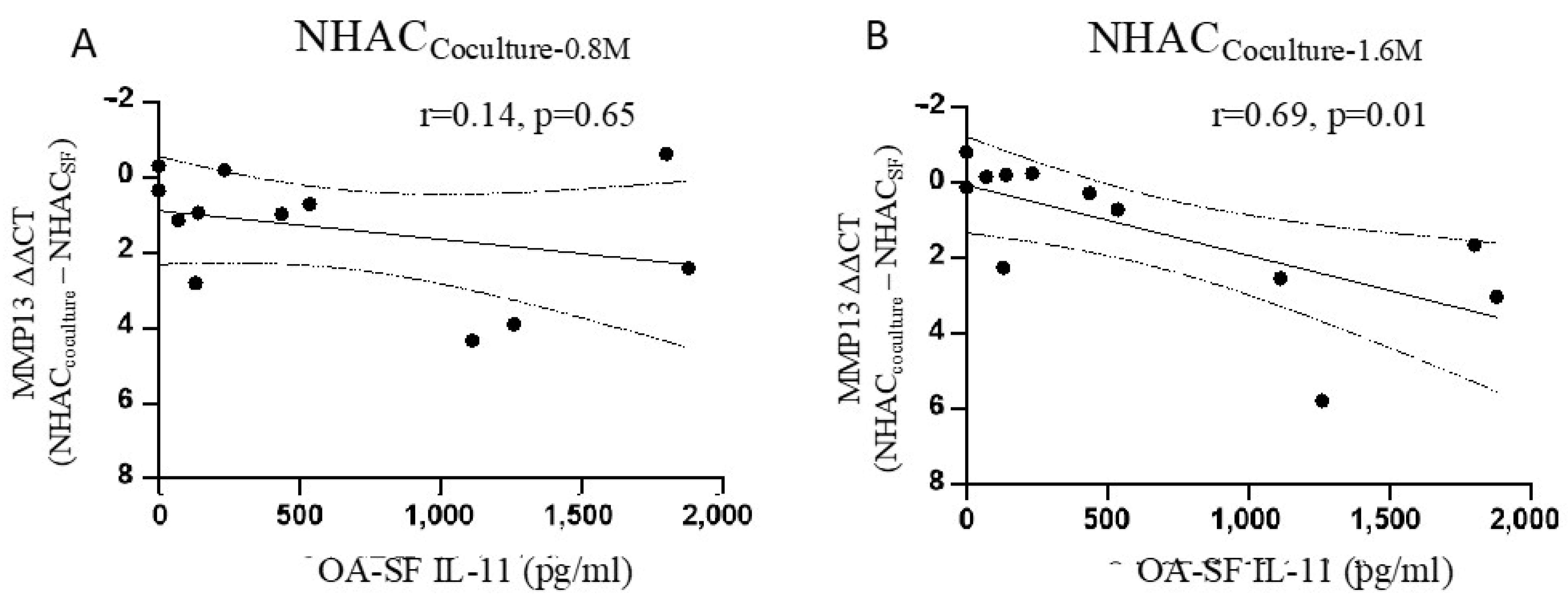
3.4. IL-11 in OA-SF Is Positively Correlated with a Reduction in MMP13 Levels
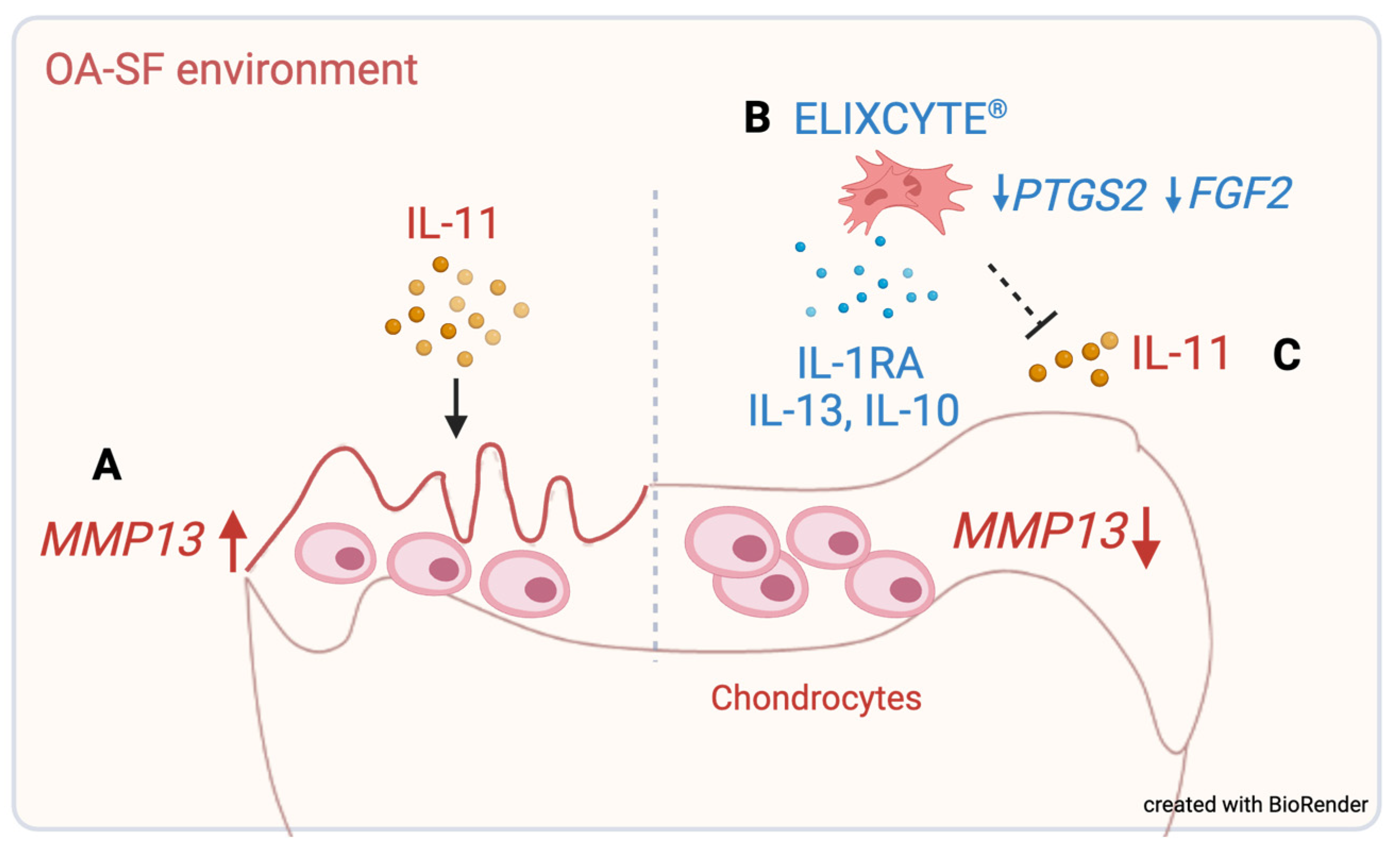
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef] [PubMed]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Piuzzi, N.S.; Khlopas, A.; Newman, J.M.; Ng, M.; Roche, M.; Husni, M.E.; Spindler, K.P.; Mont, M.A.; Muschler, G. Bone Marrow Cellular Therapies: Novel Therapy for Knee Osteoarthritis. J. Knee Surg. 2018, 31, 22–26. [Google Scholar]
- Loo, S.J.Q.; Wong, N.K. Advantages and challenges of stem cell therapy for osteoarthritis (Review). Biomed. Rep. 2021, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Hu, C.C.; Wu, C.T.; Wu, H.H.; Chang, C.S.; Hung, Y.P.; Tsai, C.C.; Chang, Y. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: A phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res. Ther. 2021, 12, 562. [Google Scholar] [CrossRef]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Buttgereit, F.; Burmester, G.R.; Jakstadt, M.; Gaber, T.; Andreas, K.; Matziolis, G.; Perka, C.; Röhner, E. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int. Orthop. 2013, 37, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Werre, S.R.; Luo, X.; Byron, C.R.; Kalbfleisch, T.S.; MacLeod, J.N.; Dahlgren, L.A. Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB J. 2020, 34, 4430–4444. [Google Scholar] [CrossRef]
- Sayegh, S.; El Atat, O.; Diallo, K.; Rauwel, B.; Degboe, Y.; Cavaignac, E.; Constantin, A.; Cantagrel, A.; Trak-Smayra, V.; Alaaeddine, N.; et al. Rheumatoid Synovial Fluids Regulate the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells Through a TNF/NF-kappaB-Dependent Mechanism. Front. Immunol. 2019, 10, 1482. [Google Scholar]
- Ragni, E.; Colombini, A.; Viganò, M.; Libonati, F.; Perucca Orfei, C.; Zagra, L.; de Girolamo, L. Cartilage Protective and Immunomodulatory Features of Osteoarthritis Synovial Fluid-Treated Adipose-Derived Mesenchymal Stem Cells Secreted Factors and Extracellular Vesicles-Embedded miRNAs. Cells 2021, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Kc, R.; Chen, D.; Xiao, G.; Im, H.J. Bovine lactoferricin-induced anti-inflammation is, in part, via up-regulation of interleukin-11 by secondary activation of STAT3 in human articular cartilage. J. Biol. Chem. 2013, 288, 31655–31669. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernández-Rueda, J.; Zabaleta, L.; Pérez-Ruiz, E.; Sepúlveda, P.; Trigueros, C. Intracellular role of IL-6 in mesenchymal stromal cell immunosuppression and proliferation. Sci. Rep. 2020, 10, 21853. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, M.; Lokau, J.; Garbers, C.; Bertrand, J. Interleukin-11—A New Cytokine in Osteoarthritis? Osteoarthr. Cartil. 2022, 30, S168–S169. [Google Scholar] [CrossRef]
- Fahy, N.; De Vries-Van Melle, M.L.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Haghighitalab, A.; Matin, M.M.; Amin, A.; Minaee, S.; Bidkhori, H.R.; Doeppner, T.R.; Bahrami, A.R. Investigating the effects of IDO1, PTGS2, and TGF-β1 overexpression on immunomodulatory properties of hTERT-MSCs and their extracellular vesicles. Sci. Rep. 2021, 11, 7825. [Google Scholar] [CrossRef]
- Ellman, M.B.; Yan, D.; Ahmadinia, K.; Chen, D.; An, H.S.; Im, H.J. Fibroblast growth factor control of cartilage homeostasis. J. Cell. Biochem. 2013, 114, 735–742. [Google Scholar] [CrossRef] [PubMed]
- El-Seoudi, A.; Abd El Kader, T.; Nishida, T.; Eguchi, T.; Aoyama, E.; Takigawa, M.; Kubota, S. Catabolic effects of FGF-1 on chondrocytes and its possible role in osteoarthritis. J. Cell Commun. Signal. 2017, 11, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Housmans, B.A.C.; Neefjes, M.; Surtel, D.A.M.; Vitik, M.; Cremers, A.; van Rhijn, L.W.; van der Kraan, P.M.; van den Akker, G.G.H.; Welting, T.J.M. Synovial fluid from end-stage osteoarthritis induces proliferation and fibrosis of articular chondrocytes via MAPK and RhoGTPase signaling. Osteoarthr. Cartil. 2022, 30, 862–874. [Google Scholar] [CrossRef]
- Carballo, C.B.; Coelho, T.R.P.; de Holanda Afonso, R.C.; Faria, J.C.O.; Alves, T.; Monte, S.M.; Ventura Matioszek, G.M.; Moura-Neto, V.; Brito, J.M. Osteoarthritic Synovial Fluid and TGF-beta1 Induce Interleukin-18 in Articular Chondrocytes. Cartilage 2020, 11, 385–394. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Colville-Nash, P.R.; Willis, D.; Chivers, J.; Paul-Clark, M.J.; Willoughby, D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Uría, J.A.; Balbín, M.; López, J.M.; Alvarez, J.; Vizoso, F.; Takigawa, M.; López-Otín, C. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am. J. Pathol. 1998, 153, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tardif, G.; Pelletier, J.P.; Dupuis, M.; Geng, C.; Cloutier, J.M.; Martel-Pelletier, J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999, 42, 1147–1158. [Google Scholar] [CrossRef]
- Varghese, S.; Rydziel, S.; Canalis, E. Basic fibroblast growth factor stimulates collagenase-3 promoter activity in osteoblasts through an activator protein-1-binding site. Endocrinology 2000, 141, 2185–2191. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Orito, K.; Koshino, T.; Saito, T. Fibroblast growth factor 2 in synovial fluid from an osteoarthritic knee with cartilage regeneration. J. Orthop. Sci. 2003, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Z.; Guo, H.; Wang, Z.; Sun, K.; Yang, X.; Zhao, X.; Ma, L.; Wang, J.; Meng, Z.; et al. Extensive cytokine analysis in synovial fluid of osteoarthritis patients. Cytokine 2021, 143, 155546. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, Y.; Li, C.; Zhang, T.; Meng, F.; Zhang, J.; Li, J.; Chen, S.; Wang, Q.; Wang, Y.; et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef] [PubMed]
- Putoczki, T.; Ernst, M. More than a sidekick: The IL-6 family cytokine IL-11 links inflammation to cancer. J. Leukoc. Biol. 2010, 88, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Seyedsadr, M.; Wang, Y.; Elzoheiry, M.; Shree Gopal, S.; Jang, S.; Duran, G.; Chervoneva, I.; Kasimoglou, E.; Wrobel, J.A.; Hwang, D.; et al. IL-11 induces NLRP3 inflammasome activation in monocytes and inflammatory cell migration to the central nervous system. Proc. Natl. Acad. Sci. USA 2023, 120, e2221007120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Croker, B.A.; Campbell, I.K.; Gauci, S.J.; Alexander, W.S.; Tonkin, B.A.; Walsh, N.C.; Linossi, E.M.; Nicholson, S.E.; Lawlor, K.E.; et al. Key Role of Suppressor of Cytokine Signaling 3 in Regulating gp130 Cytokine–Induced Signaling and Limiting Chondrocyte Responses During Murine Inflammatory Arthritis. Arthritis Rheumatol. 2014, 66, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Wu, C.C.; Song, I.W.; Chuang, H.P.; Lu, L.S.; Chang, J.H.; Kuo, S.Y.; Lee, C.H.; Wu, J.Y.; Chen, Y.T.; et al. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Res. Ther. 2013, 15, R190. [Google Scholar] [CrossRef]
- Kuo, C.L.; Liu, S.T.; Chang, Y.L.; Wu, C.C.; Huang, S.M. Zac1 regulates IL-11 expression in osteoarthritis. Oncotarget 2018, 9, 32478–32495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Hung, Y.-P.; Chen, C.-Y.; Chen, Y.-T.; Tsai, T.-C.; Yang, J.-J.; Wu, C.-C. ELIXCYTE®, an Allogenic Adipose-Derived Stem Cell Product, Mitigates Osteoarthritis by Reducing Inflammation and Preventing Cartilage Degradation In Vitro. Curr. Issues Mol. Biol. 2024, 46, 8395-8406. https://doi.org/10.3390/cimb46080495
Chen Y-H, Hung Y-P, Chen C-Y, Chen Y-T, Tsai T-C, Yang J-J, Wu C-C. ELIXCYTE®, an Allogenic Adipose-Derived Stem Cell Product, Mitigates Osteoarthritis by Reducing Inflammation and Preventing Cartilage Degradation In Vitro. Current Issues in Molecular Biology. 2024; 46(8):8395-8406. https://doi.org/10.3390/cimb46080495
Chicago/Turabian StyleChen, Yu-Hsiu, Yi-Pei Hung, Chih-Ying Chen, Yi-Ting Chen, Tai-Chen Tsai, Jui-Jung Yang, and Chia-Chun Wu. 2024. "ELIXCYTE®, an Allogenic Adipose-Derived Stem Cell Product, Mitigates Osteoarthritis by Reducing Inflammation and Preventing Cartilage Degradation In Vitro" Current Issues in Molecular Biology 46, no. 8: 8395-8406. https://doi.org/10.3390/cimb46080495






