Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements?
Abstract
:1. Introduction
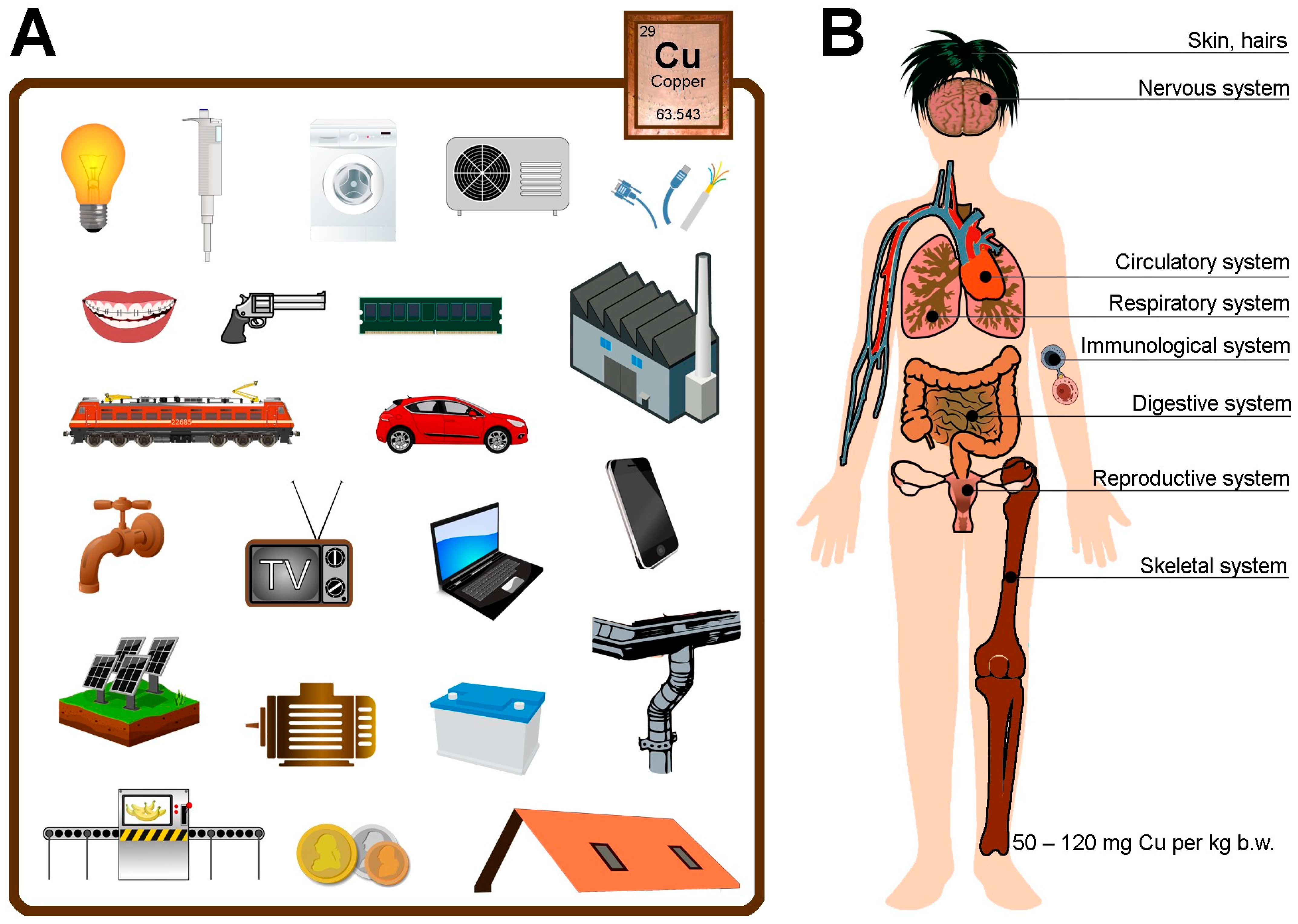
2. Functions of Copper in the Body
2.1. Immunological System
2.2. Skeletal System
2.3. Circulatory System
2.4. Nervous System
2.5. Digestive System
2.6. Respiratory System
2.7. Reproductive System
2.8. Skin
2.9. Cancers
2.10. Copper Transport at the Molecular Level
3. Effects of a Deficiency as Well as an Excess of Copper in the Body
4. Sources of Copper in Food
- -
- Milk and milk products (0.00 mg/kg—milk drink with whey and cream; 0.40 mg/kg—cheese “Oscypek”);
- -
- Eggs (0.03 mg/kg—chicken eggs white; 0.21 mg/kg—chicken eggs whole dried);
- -
- Meat and meat products (0.00 mg/kg—bone broth; 5.50 mg/kg—calf liver);
- -
- Fish and fish products (0.02 mg/kg—sole raw; 0.33 mg/kg—herring smoked “Pikling”);
- -
- Fats and oils (0.00 mg/kg—oil corn; 0.07 mg/kg—oil olive);
- -
- Cereal products (0.01 mg/kg—corn starch; 0.95 mg/kg—wheat bran);
- -
- Vegetables and vegetable products (0.01 mg/kg—aubergine raw; 1.50 mg/kg—soya beans dried);
- -
- Fruits and fruit products (0.00 mg/kg—strawberries sirup; 0.77 mg/kg—apricots dried);
- -
- Nuts (0.28 mg/kg—walnuts; 1.29 mg/kg—hazelnuts);
- -
- Seeds and grains (0.40 mg/kg—linen seeds; 1.87 mg/kg—sunflower seed dried);
- -
- Sugar and confectionery (0.01 mg/kg—fruit gums; 3.71 mg/kg—cocoa powder);
- -
- Beverages (0.00 mg/kg—black tea infusion average without sugar; 0.09 mg/kg—grape white juice);
- -
- Yeast (0.09 mg/kg—yeast baker’s compressed);
- -
- Other products (0.00 mg/kg—gelatine; 0.29 mg/kg—potato crisps salted);
- -
- Soups (0.01 mg/kg—soup sauerkraut; 0.11 mg/kg—soup green pea);
- -
- Fish dishes (0.06 mg/kg—cod fillets steamed; 0.11 mg/kg—cod fillets breaded and fried);
- -
- Meat dishes (0.01 mg/kg—chicken fillets steamed; 0.57 mg/kg—pork liver sauté fried);
- -
- Vegetable–meat dishes (0.05 mg/kg—sauerkraut with sausage and meat “Bigos” stewed; 0.10 mg/kg—beans baked with meat in tomato sauce);
- -
- Vegetable dishes (0.03 mg/kg—white cabbage salad; 0.54 mg/kg—mushrooms fried);
- -
- Groat, flour, and potato dishes (0.04 mg/kg—pancakes filled with semi-fat fresh cheese fried; 0.25 mg/kg—milled groats boiled with vegetable fat);
- -
- Eggs dishes (0.04 mg/kg—eggs poached with vegetable fat; 0.11 mg/kg—omelet biscuit baked);
- -
- Desserts (0.02 mg/kg—apple compote with sugar; 0.07 mg/kg—strawberries with whipped cream);
- -
- Other dishes (0.02 mg/kg—cheese Tilsit paste; 0.10 mg/kg—fresh cheese and fish smoked paste) [97].
5. Copper in the Environment
6. Source of Copper in Different Materials
- -
- roofing, gutters, building fittings, door fittings, locks, architectural decorative elements (also interior design);
- -
- all kinds of installations (electrical, heating, gas, fire-fighting, air-conditioning sprinklers, air conditioners, lighting, heat exchangers, parts of rocket engines, wireless chargers);
- -
- in the hydraulic area, plumbing (cold and hot water pipes, taps);
- -
- in the chemical and food industry apparatus (e.g., coolers, rectification columns, chemical and distillation apparatus, etc.);
- -
- electrical machine components and printed circuits, microelectronic material technology itself as well as optoelectronic materials (solar cells, electro-acoustic relays, batteries);
- -
- in the electrical and energy sector (electrical generators, transformers, wind power stations, types of cabling, wires, sockets);
- -
- in the telecommunications industry (computer connectors, computer chips, graphics cards, hard drives, cables, mobile phones, computers, televisions, household appliances);
- -
- in the automotive industry (trains, aircraft, trucks and cars, including electric vehicles) or mechanical engineering (e.g., sealing of oil injectors);
- -
- in wind and solar energy (supports the proper functioning and efficiency of wind turbines, protects the generator, grounds towers against lightning strikes, conducts electricity);
- -
- in the military sector (weapons, transport);
- -
- in the medical sector (dental products—dental bridges/crowns, intrauterine contraceptive devices—100–150 mg; folk spirit water—13g/L);
- -
- in the mint (Polish one-penny coins are made from manganese brass, containing 59% copper, 40% zinc, 1% manganese);
- -
- in the chemical industry (production of pesticides);
- -
7. Prevention
- -
- governments and major institutions in the country should regularly check Cu levels in food, soil, and air;
- -
- continuously monitor drinkable water systems;
- -
- conduct regular corrosion control, servicing, and regular testing;
- -
- secure drinking sources (especially in schools) with appropriate filtration systems;
- -
- remove old drains, pipes, and sanitation (e.g., taps) from service and replace with new ones;
- -
- use cold water, drained for a minimum of 1 min;
- -
- use appropriate posting if water cannot be consumed in the chosen location (e.g., “Do not use”);
- -
- wash hands regularly;
- -
- replace kitchen appliances with glass/porcelain ones;
- -
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Ancient History of Copper. Available online: https://www.thoughtco.com/copper-history-pt-i-2340112 (accessed on 25 January 2020).
- Copper (Cu) Ore. Available online: https://geologyscience.com/ore-minerals/copper-cu-ore/ (accessed on 23 April 2023).
- Mining 101: Copper—All You ever Wanted to Know about the Red Metal (and Were too Afraid to Ask). Available online: https://www.proactiveinvestors.co.uk/companies/news/1032168/mining-101-copper-all-you-ever-wanted-to-know-about-the-red-metal-and-were-too-afraid-to-ask-1032168.html (accessed on 8 November 2023).
- New Copper Technologies. Available online: https://blog.gorozen.com/blog/new-copper-technologies (accessed on 3 May 2024).
- Barceloux, D.G. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Copper. CAS#: 7440-50-8. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp132-c1-b.pdf (accessed on 26 September 2004).
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Copper. Available online: https://www.hsph.harvard.edu/nutritionsource/copper/ (accessed on 20 March 2023).
- Focarelli, F.; Giachino, A.; Waldron, K.J. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLoS Pathog. 2022, 18, e1010617. [Google Scholar] [CrossRef]
- Copper. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/#en3 (accessed on 18 October 2022).
- Yu, L.; Liou, I.W.; Biggins, S.W.; Yeh, M.; Jalikis, F.; Chan, L.N.; Burkhead, J. Copper Deficiency in Liver Diseases: A Case Series and Pathophysiological Considerations. Hepatol. Commun. 2019, 3, 1159–1165. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.L.; Deniaud, A.; Busser, B. The multifaceted roles of copper in cancer: A trace metal element with dysregulated metabolism, but also a target or a bullet for therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Copper homeostasis: Emerging target for cancer treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef]
- Herman, S.; Lipiński, P.; Ogórek, M.; Starzyński, R.; Grzmil, P.; Bednarz, A.; Lenartowicz, M. Molecular Regulation of Copper Homeostasis in the Male Gonad during the Process of Spermatogenesis. Int. J. Mol. Sci. 2020, 21, 9053. [Google Scholar] [CrossRef]
- Ogórek, M.; Gąsior, Ł.; Pierzchała, O.; Daszkiewicz, R.; Lenartowicz, M. Role of copper in the process of spermatogenesis. Postepy Hig. Med. Dosw. (Online) 2017, 71, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Peng, G.; Lu, Y.; Wang, K.; Ju, Q.; Ju, Y.; Ouyang, M. Relationship between copper and immunity: The potential role of copper in tumor immunity. Front. Oncol. 2022, 12, 1019153. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Copper in Drinking Water. Physiological Role of Copper. In Copper in Drinking Water; National Academies Press (US): Washington, DC, USA, 2000; Chapter 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225407/ (accessed on 11 January 2023).
- Rondanelli, M.; Faliva, M.A.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A.; Peroni, G. Copper as Dietary Supplement for Bone Metabolism: A Review. Nutrients 2021, 13, 2246. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 20, 2197. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Wouters, E.F.; Janssens, W.; Daamen, W.F.; Hagedoorn, P.; de Wit, H.A.; Serré, J.; Gayan-Ramirez, G.; Franssen, F.M.; Reynaert, N.L.; et al. Copper-Heparin Inhalation Therapy to Repair Emphysema: A Scientific Rationale. Int. J. Chron. Obstruct. Pulmon Dis. 2019, 14, 2587–2602. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.; Chow, C. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Qu, X. Serum copper levels are associated with bone mineral density and total fracture. J. Orthop. Transl. 2018, 14, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Shen, J.; Jin, Y.; Chang, C.; Hong, M.; Guo, S.; He, D. Circulating Level of Blood Iron and Copper Associated with Inflammation and Disease Activity of Rheumatoid Arthritis. Biol. Trace Elem. Res. 2023, 201, 90–97. [Google Scholar] [CrossRef]
- Copper Supplement (Oral Route, Parenteral Route). Available online: https://www.mayoclinic.org/drugs-supplements/copper-supplement-oral-route-parenteral-route/proper-use/drg-20070120 (accessed on 1 February 2024).
- Johnson, M.E.; Salvatore, M.F.; Maiolo, S.A.; Bobrovskaya, L. Tyrosine hydroxylase as a sentinel for central and peripheral tissue responses in Parkinson’s progression: Evidence from clinical studies and neurotoxin models. Prog. Neurobiol. 2018, 165–167, 1–25. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Dey, R.S.; Laukkanen, J.A. Circulating serum copper is associated with atherosclerotic cardiovascular disease, but not venous thromboembolism: A prospective cohort study. Pulse 2021, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Margolina, A. Skin Regenerative and Anti-Cancer Actions of Copper Peptides. Cosmetics 2018, 5, 29. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Geels, C.; Sørensen, M.; Ketzel, M.; Khan, J.; Tjønneland, A.; Christensen, J.H.; Brandt, J.; Raaschou-Nielsen, O. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ. Int. 2019, 133 Pt B, 105268. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Oxidation States of Transition Metals. Available online: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/3_d-Block_Elements/1b_Properties_of_Transition_Metals/Oxidation_States_of_Transition_Metals (accessed on 30 July 2023).
- Borković-Mitić, S.; Stojsavljević, A.; Vujotić, L.; Matić, S.; Mitić, B.; Manojlović, D. Differences between antioxidant defense parameters and specific trace element concentrations in healthy, benign, and malignant brain tissues. Sci. Rep. 2021, 11, 14766. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and Copper-Based Nanoparticles in Medicine-Perspectives and Challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef] [PubMed]
- Zughaibi, T.A.; Mirza, A.A.; Suhail, M.; Jabir, N.R.; Zaidi, S.K.; Wasi, S.; Zawawi, A.; Tabrez, S. Evaluation of Anticancer Potential of Biogenic Copper Oxide Nanoparticles (CuO NPs) against Breast Cancer. J. Nanomater. 2022, 2022, 5326355. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; Eaton, J.K.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Su, Y.; Cappock, M.; Dobres, S.; Kucine, A.J.; Waltzer, W.C.; Zhu, D. Supplemental mineral ions for bone regeneration and osteoporosis treatment. Eng. Regen. 2023, 4, 170–182. [Google Scholar] [CrossRef]
- Kawada, E.; Moridaira, K.; Itoh, K.; Hoshino, A.; Tamura, J.; Morita, T. In long-term bedridden elderly patients with dietary copper deficiency, biochemical markers of bone resorption are increased with copper supplementation during 12 weeks. Ann. Nutr. Metab. 2006, 50, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Jagielska, A.; Ruszczyńska, A.; Idkowiak, J.; Bobrowska-Korczak, B. Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions. Nutrients 2022, 14, 1285. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Gao, Y.-S.; Wang, Y.; Hu, C.; Sun, Y.; Zhang, C. Dimethyloxaloylglycine increases the bone healing capacity of adipose-derived stem cells by promoting osteogenic differentiation and angiogenic potential. Stem Cells Dev. 2014, 23, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Corredor, O.M.; Guerrero, Y.; de Fernández, M.R.; D’Jesús, I.; Burguera, M.; Burguera, J.L.; Di Bernardo, M.L.; García, M.Y.; Alarcón, A.O. Effect of copper supplementation on lipid profile of Venezuelan hyperlipemic patients. Arch. Latinoam. Nutr. 2004, 54, 413–418. [Google Scholar] [PubMed]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Y.; Liu, H.; Shi, M.; Wang, J.; Wang, Y. The Molecular Mechanisms of Defective Copper Metabolism in Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 5418376. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Elmi, A.; Hallaj-Nezhadi, S. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process Res. 2018, 6, 140. [Google Scholar] [CrossRef]
- Kessler, H.; Bayer, T.A.; Bach, D.; Schneider-Axmann, T.; Supprian, T.; Herrmann, W.; Haber, M.; Multhaup, G.; Falkai, P.; Pajonk, F.-G. Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: A pilot phase 2 clinical trial. J. Neural Transm. 2008, 115, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Simonelli, I.; Pasqualetti, P.; Mariani, S.; Caprara, D.; Bucossi, S.; Ventriglia, M.; Molinario, R.; Antenucci, M.; Rongioletti, M.; et al. Association between Serum Ceruloplasmin Specific Activity and Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 50, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Lanza, V.; Milardi, D.; Di Natale, G.; Pappalardo, G. Repurposing of Copper(II)-chelating Drugs for the Treatment of Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Rembach, A.; Doecke, J.D.; Roberts, B.R.; Watt, A.D.; Faux, N.G.; Volitakis, I.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; et al. Longitudinal analysis of serum copper and ceruloplasmin in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Aschner, M. Commonalities between Copper Neurotoxicity and Alzheimer’s Disease. Toxics 2021, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Dobson, R.J.B.; Newhouse, S.J. A Meta-Analysis of Alzheimer’s Disease Brain Transcriptomic Data. J. Alzheimer’s Dis. 2019, 68, 1635–1656. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Ayton, S.; Agrawal, S.; Dhana, K.; Bennett, D.A.; Barnes, L.L.; Leurgans, S.E.; Bush, A.I.; Schneider, J.A. Brain copper may protect from cognitive decline and Alzheimer’s disease pathology: A community-based study. Mol. Psychiatry 2022, 27, 4307–4313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ji, X.; Cui, N.; Cao, S.; Liu, C.; Liu, J. Association between Serum Copper Status and Working Memory in Schoolchildren. Nutrients 2015, 7, 7185–7196. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Lek. Listy 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Dzieżyc, K.; Litwin, T.; Sobańska, A.; Członkowska, A. Symptomatic copper deficiency in three Wilson’s disease patients treated with zinc sulphate. Neurol. Neurochir. Pol. 2014, 48, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Mohr, I.; Weiss, K.H. Biochemical Markers for the Diagnosis and Monitoring of Wilson Disease. Clin. Biochem. Rev. 2019, 40, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yue, Y.; Zhang, Q.; Wang, X. Copper homeostasis dysregulation in respiratory diseases: A review of current knowledge. Front. Physiol. 2024, 15, 1243629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; van Donkelaar, A.; Bai, L.; Martin, R.V.; Copes, R.; et al. A Population-Based Cohort Study of Respiratory Disease and Long-Term Exposure to Iron and Copper in Fine Particulate Air Pollution and Their Combined Impact on Reactive Oxygen Species Generation in Human Lungs. Environ. Sci. Technol. 2021, 55, 3807–3818. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Fischer, P.H.; Janssen, N.A.; Kos, G.P.; Weijers, E.P.; Cassee, F.R.; van der Zee, S.C.; de Hartog, J.J.; Meliefste, K.; Wang, M.; et al. Respiratory effects of a reduction in outdoor air pollution concentrations. Epidemiology 2013, 24, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Unar, A.; Afridi, H.I.; Ali, A.; Ali, N.; Qureshi, T. Determination of Electrolytes and Trace Elements in Biological Samples from Patients with Altered Semen Parameters: A Correlational Analysis. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef] [PubMed]
- Omeljaniuk, W.J.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E.; Laudański, T.; Kulikowski, M.; Kobylec, E. Antioxidant status in women who have had a miscarriage. Adv. Med. Sci. 2015, 60, 329–334. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Orywal, K.; Czygier, M.; Szmitkowski, M.; Mroczko, B.; Maślach, D.; Szpak, A. Concentration of Selected Elements and Antioxidative Potential in a Group of Males Working in the Metal Industry: Elements And Antioxidative Potential In Men. Am. J. Men’s Health 2019, 13, 1557988319851954. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Jamiołkowski, J.; Pędziński, B.; Krzyżak, M.; Maślach, D.; Szpak, A.; Omeljaniuk, W.J. Changes in Dietary Patterns and the Nutritional Status in Men in the Metallurgical Industry in Poland over A 21-Year Period. Ann. Nutr. Metab. 2018, 72, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, A.; Shokri, S.; Pirhadi, M.; Karimi, M.; Abbaszadeh, S.; Mirzaei, G.; Bahmani, M. The Effects of Toxic Heavy Metals Lead, Cadmium and Copper on the Epidemiology of Male and Female Infertility. JBRA Assist. Reprod. 2022, 26, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Roshankhah, S.; Barani, N.; Salahshoor, M.R.; Rezakhani, L.; Khazaei, M. The Relationship between Body Mass Index, Metal Elements, and Antioxidant Capacity of Semen on the Human Sperm Chromatin. Int. J. Fertil. Steril. 2023, 17, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Turgut, A.; Ozler, A.; Goruk, N.Y.; Tunc, S.Y.; Evliyaoglu, O.; Gul, T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1472–1478. [Google Scholar]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Peacey, L.; Peacey, C.; Gutzinger, A.; Jones, C.E. Copper(II) Binding by the Earliest Vertebrate Gonadotropin-Releasing Hormone, the Type II Isoform, Suggests an Ancient Role for the Metal. Int. J. Mol. Sci. 2020, 21, 7900, Erratum in Int. J. Mol. Sci. 2021, 22, 3431. [Google Scholar] [CrossRef]
- Gupta, P.K. Fundamentals of Toxicology. Essential Concepts and Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128054260. [Google Scholar]
- Geng, X.; Liu, K.; Wang, J.; Su, X.; Shi, Y.; Zhao, L. Preparation of Ultra-Small Copper Nanoparticles-Loaded Self-Healing Hydrogels with Antibacterial, Inflammation-Suppressing and Angiogenesis-Enhancing Properties for Promoting Diabetic Wound Healing. Int. J. Nanomed. 2023, 18, 3339–3358. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.S.; Fitch, C.A.; Levenson, C.W. Tumor Suppressor Protein p53 mRNA and Subcellular Localization Are Altered by Changes in Cellular Copper in Human Hep G2 Cells. J. Nutr. 2001, 131, 1427–1432. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Tang, X.; Yan, Z.; Miao, Y.; Ha, W.; Li, Z.; Yang, L.; Mi, D. Copper in cancer: From limiting nutrient to therapeutic target. Front. Oncol. 2023, 13, 1209156. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.B.; Bhat, V.R.; Upadhyay, D.; Udupa, S.L. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J. Physiol. Pharm. 2003, 47, 108–110. [Google Scholar]
- Wazir, S.M.; Ghobrial, I. Copper deficiency, a new triad: Anemia, leucopenia, and myeloneuropathy. J. Community Hosp. Internal Med. Perspect. 2017, 7, 265–268. [Google Scholar] [CrossRef]
- Voli, F.; Valli, E.; Lerra, L.; Kimpton, K.; Saletta, F.; Giorgi, F.M.; Mercatelli, D.; Rouaen, J.R.C.; Shen, S.; Murray, J.E.; et al. Intratumoral Copper Modulates PD-L1 Expression and Influences Tumor Immune Evasion. Cancer Res. 2020, 80, 4129–4144. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.A.; Nayef, U.M.; AlMalki, F.A.; et al. Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 1212, 16165. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakm, L.O.; Kandil, E.I.; Mansour, S.Z.; El-Sonbaty, S.M. Chrysin Encapsulated Copper Nanoparticles with Low Dose of Gamma Radiation Elicit Tumor Cell Death through p38 MAPK/NF-κB Pathways. Biol. Element Res. 2023, 201, 5278–5297. [Google Scholar] [CrossRef] [PubMed]
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 99, 12613. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 5561–5573. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Mikrobiol. Biotechnol 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Gupta, A.; Lutsenko, S. Human copper transporters: Mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 2009, 1, 1125–1142. [Google Scholar] [CrossRef]
- Miyajima, H. Genetic disorders affecting proteins of iron and copper metabolism: Clinical implications. Intern. Med. 2002, 41, 762–769. [Google Scholar] [CrossRef]
- Liu, J.; Luan, J.; Zhou, X.; Cui, Y.; Han, J. Epidemiology, diagnosis, and treatment of Wilson’s disease. Intractable Rare Dis. Res. 2017, 6, 249–255. [Google Scholar] [CrossRef]
- Szeligowska, J.; Ilczuk, T.; Nehring, P.; Górnicka, B.; Litwin, T.; Członkowska, A.; Przybyłkowski, A. Liver injury in Wilson’s disease: An immunohistochemical study. Adv. Med. Sci. 2022, 67, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Panel on Micronutrients, and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Dietary Reference Intakes; National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Nutrition Standards for the Population of Poland and Their Application. Available online: https://ncez.pzh.gov.pl/wp-content/uploads/2021/03/normy_zywienia_2020web.pdf (accessed on 1 February 2020).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Food Composition Tables, 2nd ed.; PZWL Medical Publishers: Warsaw, Poland, 2017. [Google Scholar]
- Lead and Copper in School Drinking Water Sampling Results. Summary and Detailed Lead and Copper in Drinking Water Data in School Drinking Water. Available online: https://www.mass.gov/info-details/lead-and-copper-in-school-drinking-water-sampling-results (accessed on 26 April 2016).
- Ishizuka, O.; Yuasa, M.; Uto, K. Evidence of porphyry copper-type hydrothermal activity from a submerged remnant back-arc volcano of the Izu-Bonin arc implications for the volcanotectonic history of back-arc seamounts. Earth Planet. Sci. Lett. 2002, 198, 381–399. [Google Scholar] [CrossRef]
- Basic Information about Copper (Podstawowe Informacje o Miedzi). Available online: https://www.pgi.gov.pl/psg-1/psg-2/informacja-i-szkolenia/wiadomosci-surowcowe/9795-miedz-i-srebro.html (accessed on 25 January 2019).
- COPPER—From the 1st Metal Used by Humans to the Essential Element in Technology Development_PART II—How Is Copper Capable to Harness the Technology Development in Digital Age? Available online: https://americanbobcat.blog/2021/05/17/copper-from-the-1st-metal-used-by-humans-to-the-essential-element-in-technology-development_part-ii-how-is-copper-capable-to-harness-the-technology-development-in-digital-age/ (accessed on 17 May 2017).
- Copper, Silver. Available online: https://www.pgi.gov.pl/images/muzeum/kopalnia_wiedzy/surowce/foldery/miedz_i_srebro.pdf (accessed on 31 July 2020).
- Ivanova, I.A.; Daskalova, D.S.; Yordanova, L.P.; Pavlova, E.L. Copper and Copper Nanoparticles Applications and Their Role against Infections: A Minireview. Processes 2024, 12, 352. [Google Scholar] [CrossRef]
- Montagnino, E.; Lytle, D.A.; Rose, J.; Cwiertny, D.; Whelton, A.J. School and childcare center drinking water: Copper chemistry, health effects, occurrence, and remediation. AWWA Water. Sci. 2022, 4, e1270. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]
- Cradock, A.L.; Everett Jones, S.; Merlo, C. Examining differences in the implementation of school water-quality practices and water-access policies by school demographic characteristics. Prev. Med. Rep. 2019, 14, 100823. [Google Scholar] [CrossRef]
| Human Body | Effects of Exposure to Copper |
|---|---|
| Immunological system |
|
| Skeletal system |
|
| Circulatory system |
|
| Nervous system |
|
| Digestive system |
|
| Respiratory system |
|
| Reproductive system |
|
| Skin |
|
| Cancers |
|
| Copper Deficiency | Copper Excess |
|---|---|
|
|
| Food Product | Threshold Content (mg/kg Fresh Matter) |
|---|---|
| Milk and milk products | 0.00–0.40 |
| Eggs | 0.03–0.21 |
| Meat and meat products | 0.00–5.50 |
| Fish and fish products | 0.02–0.33 |
| Fats and oils | 0.00–0.07 |
| Cereal products | 0.01–0.95 |
| Vegetables and vegetable products | 0.01–1.50 |
| Fruits and fruit products | 0.00–0.77 |
| Nuts | 0.28–1.29 |
| Seeds and grains | 0.40–1.87 |
| Sugar and confectionery | 0.01–3.71 |
| Beverages | 0.00–0.09 |
| Yeast | 0.09 |
| Other products | 0.00–0.29 |
| Soups | 0.01–0.11 |
| Fish dishes | 0.06-.011 |
| Meat dishes | 0.01–0.57 |
| Vegetable–meat dishes | 0.05–0.10 |
| Vegetable dishes | 0.03–0.54 |
| Groat, flour, and potato dishes | 0.04–0.25 |
| Eggs dishes | 0.04–0.11 |
| Desserts | 0.02–0.07 |
| Other dishes | 0.02–0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkiewicz, A.E. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Curr. Issues Mol. Biol. 2024, 46, 8441-8463. https://doi.org/10.3390/cimb46080498
Charkiewicz AE. Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements? Current Issues in Molecular Biology. 2024; 46(8):8441-8463. https://doi.org/10.3390/cimb46080498
Chicago/Turabian StyleCharkiewicz, Angelika Edyta. 2024. "Is Copper Still Safe for Us? What Do We Know and What Are the Latest Literature Statements?" Current Issues in Molecular Biology 46, no. 8: 8441-8463. https://doi.org/10.3390/cimb46080498





