Therapeutic Application and Structural Features of Adeno-Associated Virus Vector
Abstract
1. Introduction
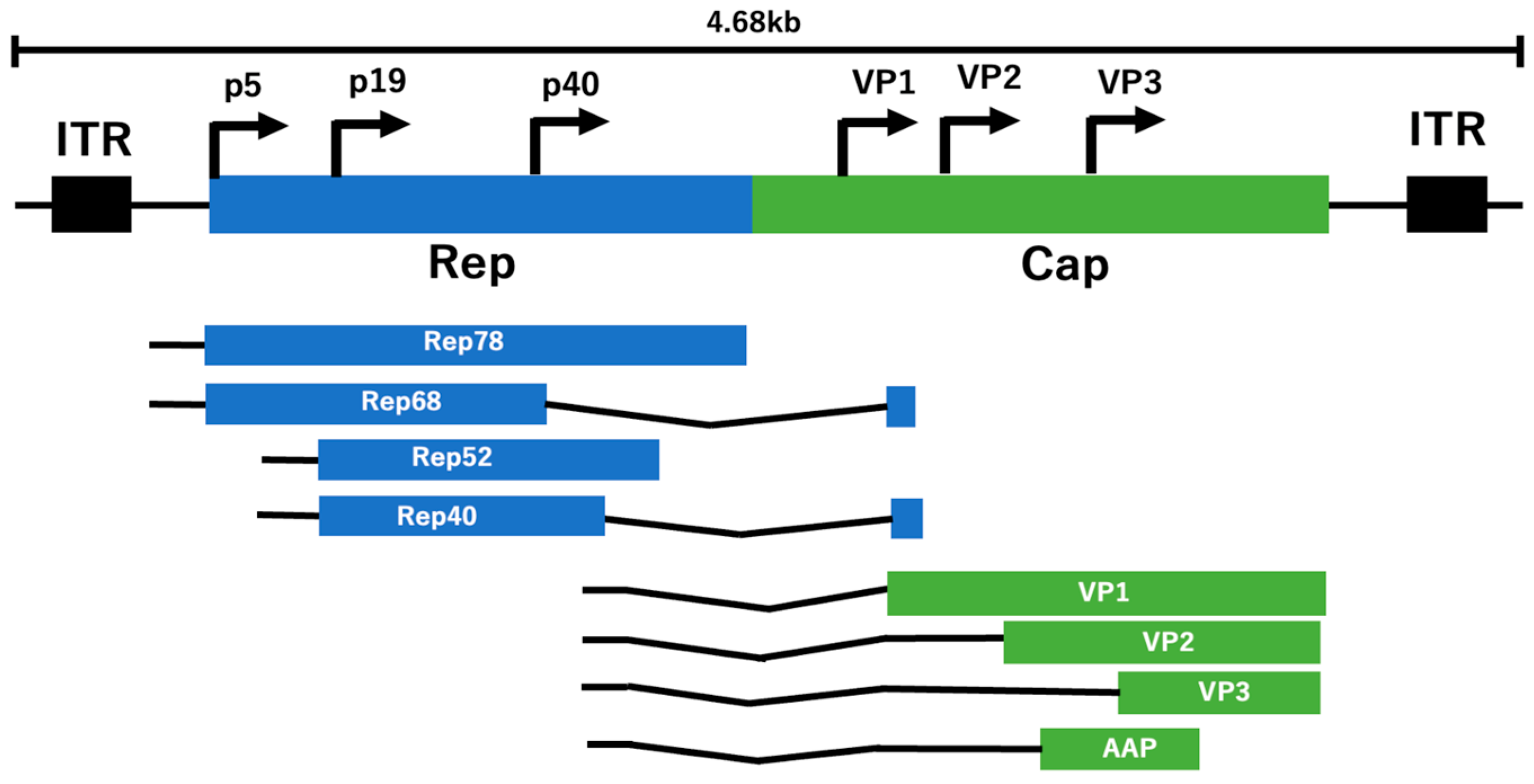
2. Genes and Genomic Structure of AAV
3. Serotype of AAVs
4. Efficacy of Gene Therapy by AAV
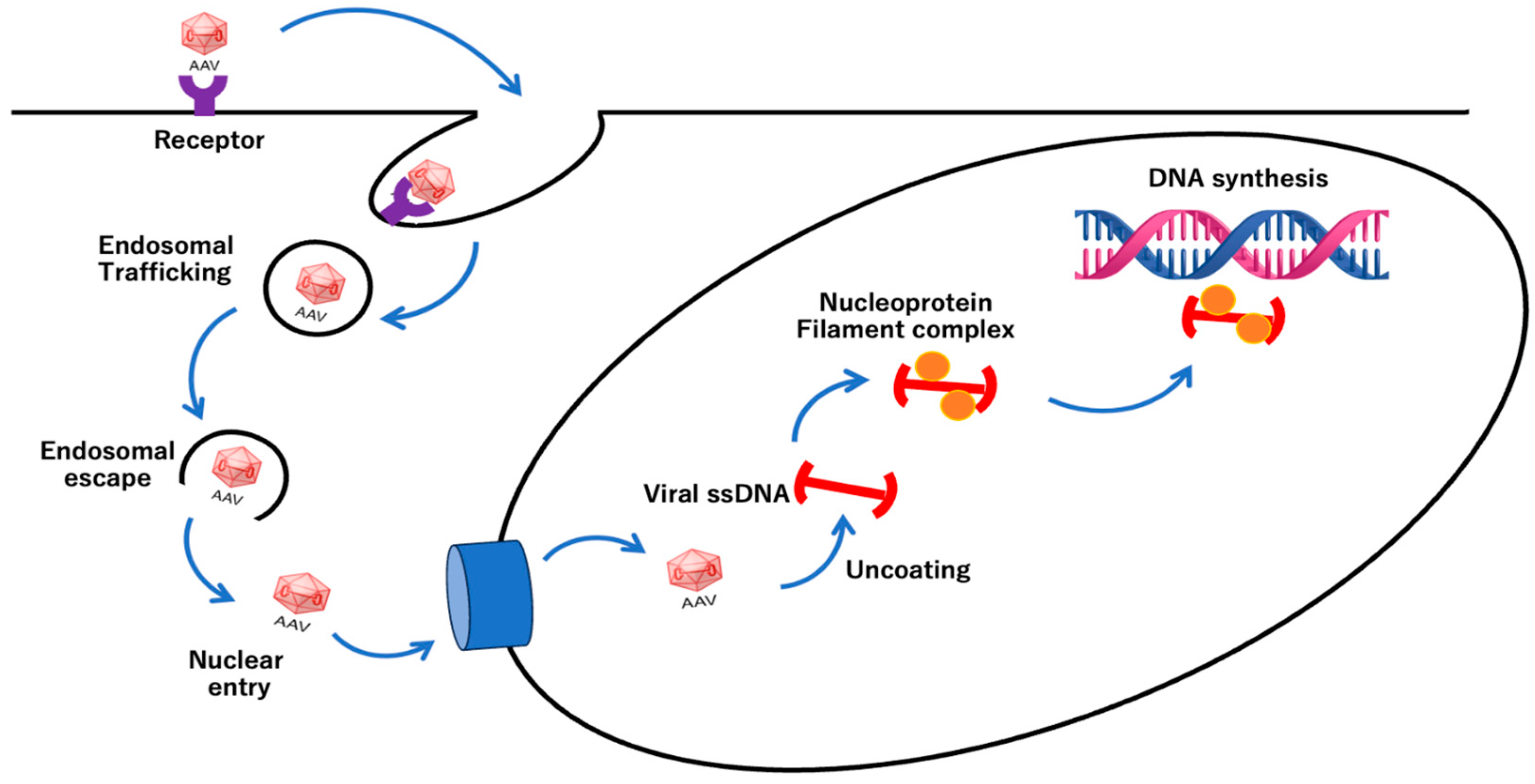
5. AAV Infection Mechanisms
6. Production of Recombinant AAV (rAAV)
7. Safety of AAV Vector
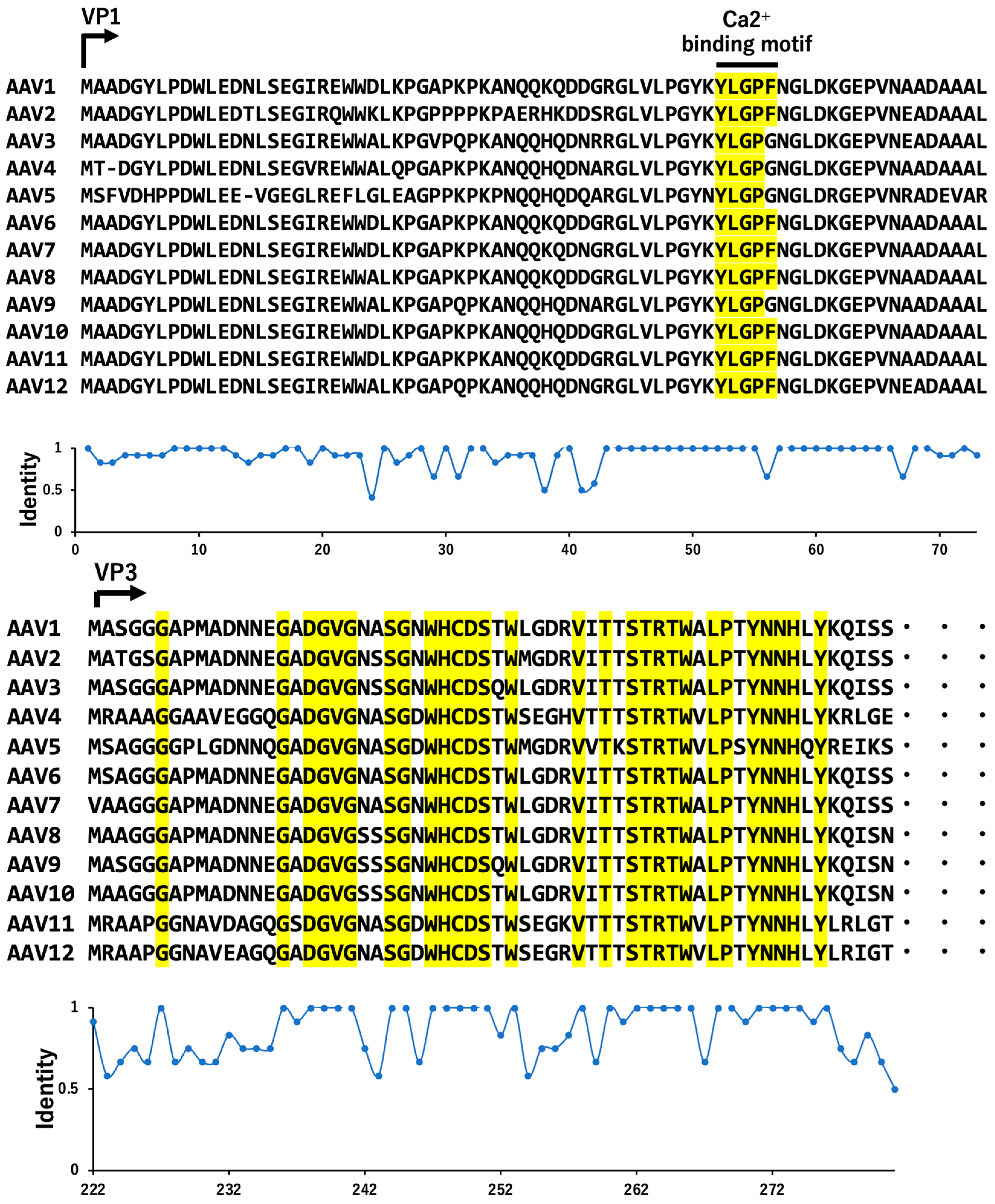
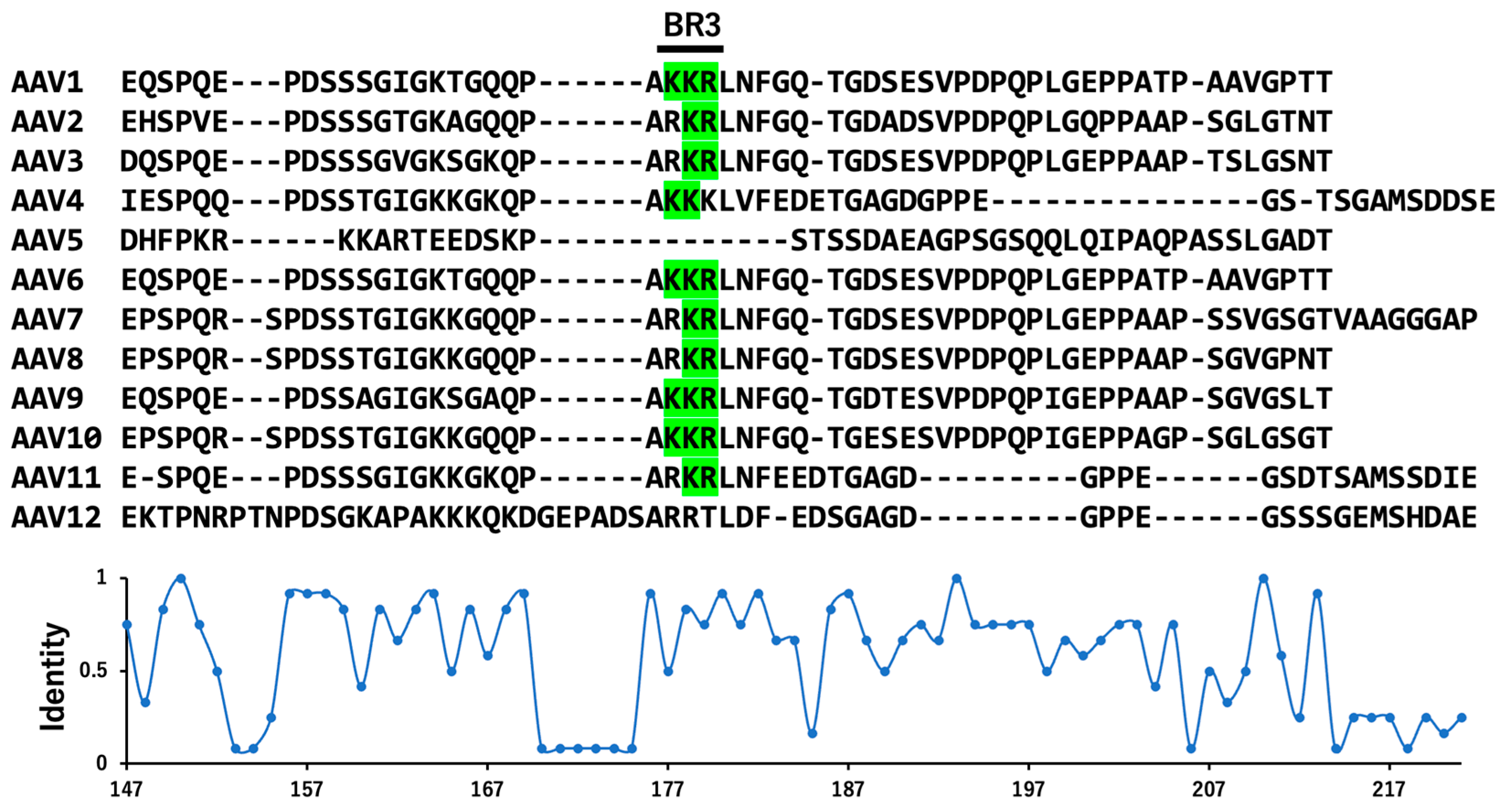
8. Transcription and Serotypes of VP Variants
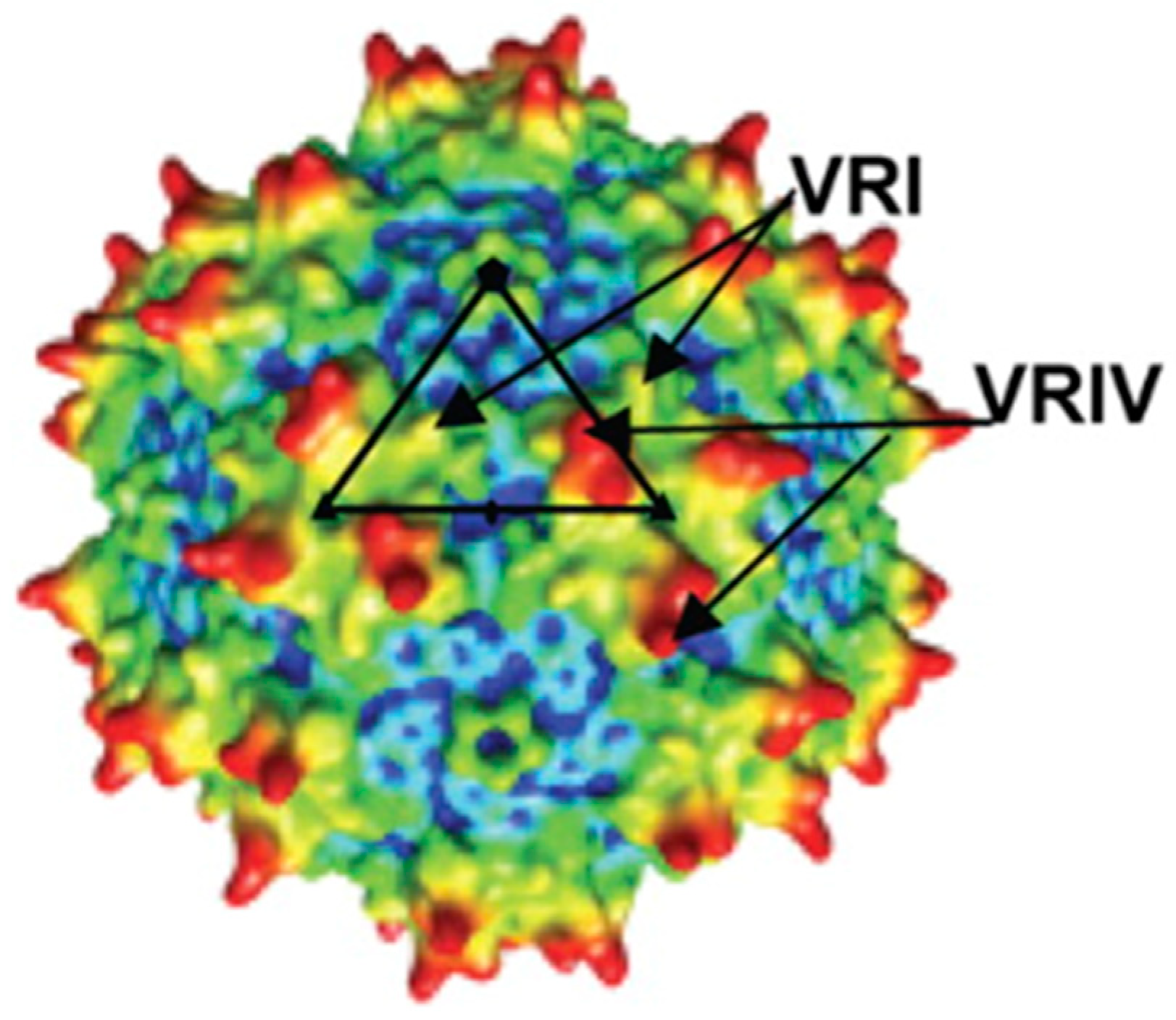
9. Structural Features and Dynamics of AAV Particles
10. Structurally Dynamic Molecules and Hydrogen/Deuterium Exchange–Mass Spectrometry
11. Full and Empty Particles and Secretion of AAV
12. Novel AAV Vectors and Tropisms
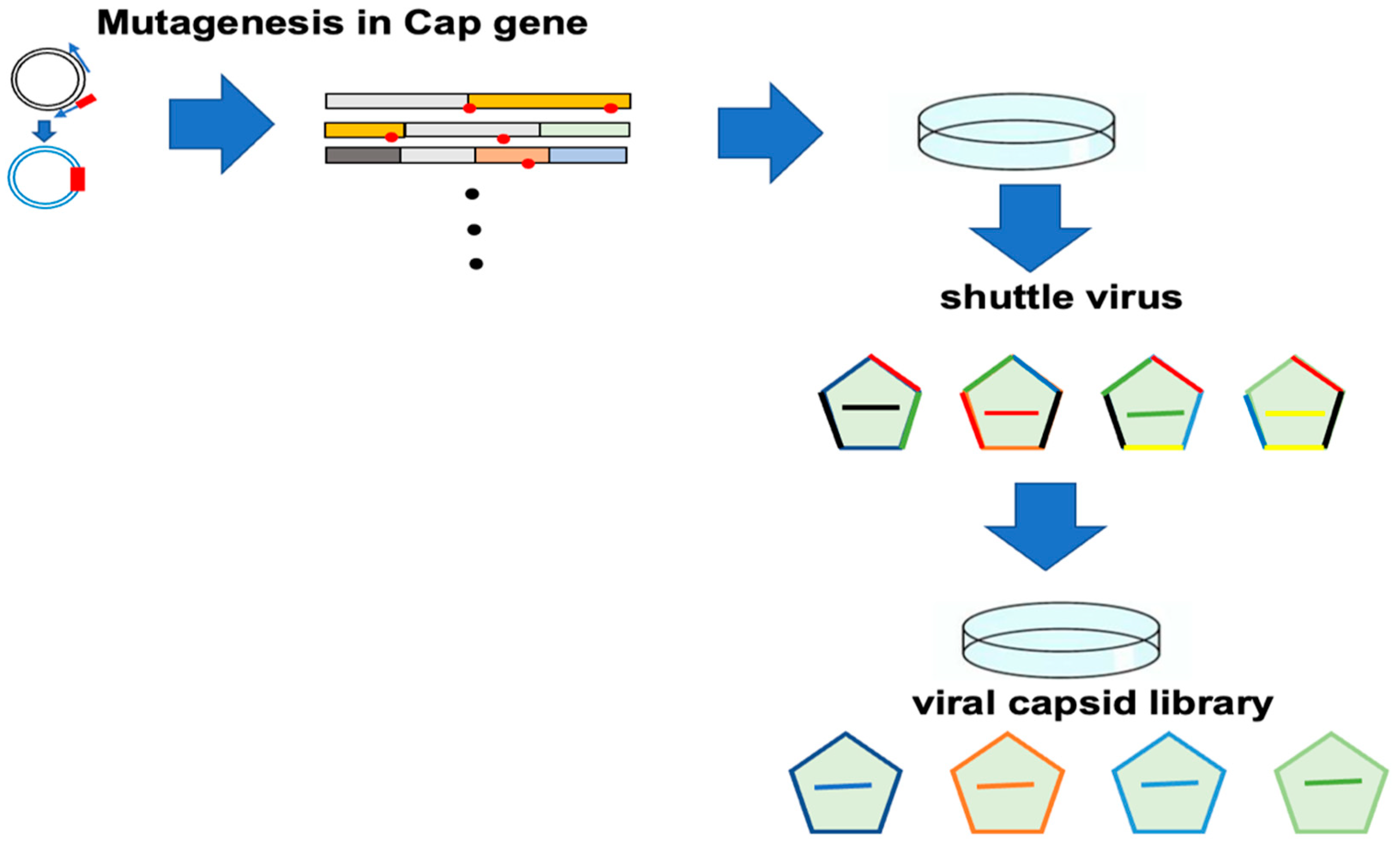
12.1. Screening of Improved AAV Vector
12.2. Viral Packaging of Capsid Library
13. AAV-Based Therapies in Clinical Trials
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Au, H.K.E.; Isalan, M.; Mielcarek, M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2022, 8, 809118. [Google Scholar] [CrossRef] [PubMed]
- Zinn, E.; Vandenberghe, L.H. Adeno-associated virus: Fit to serve. Curr. Opin. Virol. 2014, 8, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Mietzsch, M.; Agbandje-McKenna, M. Understanding capsid assembly and genome packaging for adeno-associated viruses. Future Virol. 2017, 12, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Mao, Q.; Tai, P.W.L.; He, R.; Ai, J.; Su, Q.; Zhu, Y.; Ma, H.; Li, J.; Gong, S.; et al. Short DNA Hairpins Compromise Recombinant Adeno-Associated Virus Genome Homogeneity. Mol. Ther. 2017, 25, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Earley, L.F.; Conatser, L.M.; Lue, V.M.; Dobbins, A.L.; Li, C.; Hirsch, M.L.; Samulski, R.J. Adeno-Associated Virus Serotype-Specific Inverted Terminal Repeat Sequence Role in Vector Transgene Expression. Hum. Gene Ther. 2020, 31, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tian, W.; Liu, C.; Lian, Z.; Dong, X.; Wu, X. Deletion of the B-B’ and C-C’ regions of inverted terminal repeats reduces rAAV productivity but increases transgene expression. Sci. Rep. 2017, 7, 5432. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.S.; Hao, G.G. Biophysical characterization of adeno-associated virus capsid through the viral transduction life cycle. J. Genet. Eng. Biotechnol. 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Ozohanics, O.; Ambrus, A. Hydrogen-Deuterium Exchange Mass Spectrometry: A Novel Structural Biology Approach to Structure, Dynamics and Interactions of Proteins and Their Complexes. Life 2020, 10, 286. [Google Scholar] [CrossRef]
- Engen, J.R. Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Anal. Chem. 2009, 81, 7870–7875. [Google Scholar] [CrossRef]
- Narang, D.; James, D.A.; Balmer, M.T.; Wilson, D.J. Protein Footprinting, Conformational Dynamics, and Core Interface-Adjacent Neutralization “Hotspots” in the SARS-CoV-2 Spike Protein Receptor Binding Domain/Human ACE2 Interaction. J. Am. Soc. Mass. Spectrom. 2021, 32, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Coll De Peña, A.; White, J.D.; Mehta, D.R.; Ben Frej, M.; Tripathi, A. Microfluidic AAV Purity Characterization: New Insights into Serotype and Sample Treatment Variability. ACS Omega 2024, 9, 4027–4036. [Google Scholar] [CrossRef] [PubMed]
- Wörner, T.P.; Bennett, A.; Habka, S.; Snijder, J.; Friese, O.; Powers, T.; Agbandje-McKenna, M.; Heck, A.J.R. Adeno-associated virus capsid assembly is divergent and stochastic. Nat. Commun. 2021, 12, 1642. [Google Scholar] [CrossRef] [PubMed]
- Westhaus, A.; Cabanes-Creus, M.; Jonker, T.; Sallard, E.; Navarro, R.G.; Zhu, E.; Baltazar Torres, G.; Lee, S.; Wilmott, P.; Gonzalez-Cordero, A.; et al. AAV-p40 Bioengineering Platform for Variant Selection Based on Transgene Expression. Hum. Gene Ther. 2022, 33, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Stutika, C.; Gogol-Döring, A.; Botschen, L.; Mietzsch, M.; Weger, S.; Feldkamp, M.; Chen, W.; Heilbronn, R. A Comprehensive RNA Sequencing Analysis of the Adeno-Associated Virus (AAV) Type 2 Transcriptome Reveals Novel AAV Transcripts, Splice Variants, and Derived Proteins. J. Virol. 2015, 90, 1278–1289. [Google Scholar] [CrossRef]
- Cervelli, T.; Backovic, A.; Galli, A. Formation of AAV single stranded DNA genome from a circular plasmid in Saccharomyces cerevisiae. PLoS ONE 2011, 6, e23474. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.; Chen, W.; Muzyczka, N. Complete in vitro reconstitution of adeno-associated virus DNA replication requires the minichromosome maintenance complex proteins. J. Virol. 2008, 82, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Eddington, C.; Jose, A.; His, J.; Chipman, P.; Henley, T.; Choudhry, M.; McKenna, R.; Agbandje-McKenna, M. Improved Genome Packaging Efficiency of Adeno-associated Virus Vectors Using Rep Hybrids. J. Virol. 2021, 95, e0077321. [Google Scholar] [CrossRef]
- Asaad, W.; Volos, P.; Maksimov, D.; Khavina, E.; Deviatkin, A.; Mityaeva, O.; Volchkov, P. AAV genome modification for efficient AAV production. Heliyon 2023, 9, e15071. [Google Scholar] [CrossRef]
- Ohba, K.; Sehara, Y.; Enoki, T.; Mineno, J.; Ozawa, K.; Mizukami, H. Adeno-associated virus vector system controlling capsid expression improves viral quantity and quality. iScience 2023, 26, 106487. [Google Scholar] [CrossRef]
- Cheung, A.K. Specific functions of the Rep and Rep’ proteins of porcine circovirus during copy-release and rolling-circle DNA replication. Virology 2015, 481, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, D.A.; Calabria, A.; Tiyaboonchai, A.; Posey, J.; Naugler, W.E.; Montini, E.; Grompe, M. AAV integration in human hepatocytes. Mol. Ther. 2021, 29, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Maggin, J.E.; James, J.A.; Chappie, J.S.; Dyda, F.; Hickman, A.B. The amino acid linker between the endonuclease and helicase domains of adeno-associated virus type 5 Rep plays a critical role in DNA-dependent oligomerization. J. Virol. 2012, 86, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Shitik, E.M.; Shalik, I.K.; Yudkin, D.V. AAV- based vector improvements unrelated to capsid protein modification. Front. Med. 2023, 10, 1106085. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, K.S.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Volak, A.; Spirig, S.E.; Muller, A.; Sousa, A.A.; Tsai, S.Q.; Bengtsson, N.E.; et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019, 10, 4439. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Ishii, K.; Maruno, T.; Torisu, T.; Uchiyama, S. Characterization of Adeno-Associated Virus Capsid Proteins with Two Types of VP3-Related Components by Capillary Gel Electrophoresis and Mass Spectrometry. Hum. Gene Ther. 2021, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Patel, S.K.; Xing, T.; Yan, Y.; Wang, S.; Li, N. Characterization of Adeno-Associated Virus Capsid Proteins Using Hydrophilic Interaction Chromatography Coupled with Mass Spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113481. [Google Scholar] [CrossRef]
- Zoratto, S.; Weiss, V.U.; van der Horst, J.; Commandeur, J.; Buengener, C.; Foettinger-Vacha, A.; Pletzenauer, R.; Graninger, M.; Allmaier, G. Molecular weight determination of adeno-associate virus serotype 8 virus-like particle either carrying or lacking genome via native nES gas-phase electrophoretic molecular mobility analysis and nESI QRTOF mass spectrometry. J. Mass. Spectrom. 2021, 56, e4786. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, N.L.; Berguig, G.Y.; Karim, O.A.; Cortesio, C.L.; De Angelis, R.; Khan, A.A.; Gold, D.; Maga, J.A.; Bhat, V.S. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021, 11, 3012. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Sant’Anna, T.B.; Araujo, N.M. Adeno-associated virus infection and its impact in human health: An overview. Virol. J. 2022, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.L.; Brown, A.; Loveland, A.B.; Lotun, A.; Xu, M.; Luo, L.; Xu, G.; Li, J.; Ren, L.; Su, Q.; et al. Structural characterization of a novel human adeno-associated virus capsid with neurotropic properties. Nat. Commun. 2020, 11, 3279. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.; Gessler, D.J.; Xie, J.; Zhong, L.; Li, J.; Tran, K.; Van Vliet, K.; Ren, L.; Su, Q.; et al. A Rationally Engineered Capsid Variant of AAV9 for Systemic CNS-Directed and Peripheral Tissue-Detargeted Gene Delivery in Neonates. Mol. Ther. Methods Clin. Dev. 2018, 9, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Levy, D.I.; Petropoulos, C.J.; Bashirians, G.; Winburn, I.; Mahn, M.; Somanathan, S.; Cheng, S.H.; Byrne, B.J. Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol. Ther. 2023, 31, 616–630. [Google Scholar] [CrossRef]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef] [PubMed]
- Kuoch, H.; Krotova, K.; Graham, M.L.; Brantly, M.L.; Aslanidi, G. Multiplexing AAV Serotype-Specific Neutralizing Antibodies in Preclinical Animal Models and Humans. Biomedicines 2023, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.J.; Arokiaraj, C.M.; Chuapoco, M.R.; Chen, X.; Goeden, N.; Gradinaru, V.; Fox, A.S. Advances in AAV technology for delivering genetically encoded cargo to the nonhuman primate nervous system. Curr. Res. Neurobiol. 2023, 4, 100086. [Google Scholar] [CrossRef]
- Brown, D.; Altermatt, M.; Dobreva, T.; Chen, S.; Wang, A.; Thomson, M.; Gradinaru, V. Deep Parallel Characterization of AAV Tropism and AAV-Mediated Transcriptional Changes via Single-Cell RNA Sequencing. Front. Immunol. 2021, 12, 730825. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016, 21, 75–80. [Google Scholar] [CrossRef]
- Afione, S.; DiMattia, M.A.; Halder, S.; Di Pasquale, G.; Agbandje-McKenna, M.; Chiorini, J.A. Identification and mutagenesis of the adeno-associated virus 5 sialic acid binding region. J. Virol. 2015, 89, 1660–1672. [Google Scholar] [CrossRef]
- Albright, B.H.; Simon, K.E.; Pillai, M.; Devlin, G.W.; Asokan, A. Modulation of Sialic Acid Dependence Influences the Central Nervous System Transduction Profile of Adeno-associated Viruses. J. Virol. 2019, 93, e00332-19. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.L.; Chapman, M.S. Adeno-associated virus (AAV) cell entry: Structural insights. Trends Microbiol. 2022, 30, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Broecker, F.; Reinhardt, A.; Seeberger, P.H.; Heilbronn, R. Differential adeno-associated virus serotype-specific interaction patterns with synthetic heparins and other glycans. J. Virol. 2014, 88, 2991–3003. [Google Scholar] [CrossRef]
- Rubin, J.D.; Nguyen, T.V.; Allen, K.L.; Ayasoufi, K.; Barry, M.A. Comparison of Gene Delivery to the Kidney by Adenovirus, Adeno-Associated Virus, and Lentiviral Vectors After Intravenous and Direct Kidney Injections. Hum. Gene Ther. 2019, 30, 1559–1571. [Google Scholar] [CrossRef]
- DiMattia, M.A.; Nam, H.J.; Van Vliet, K.; Mitchell, M.; Bennett, A.; Gurda, B.L.; McKenna, R.; Olson, N.H.; Sinkovits, R.S.; Potter, M.; et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J. Virol. 2012, 86, 6947–6958. [Google Scholar] [CrossRef]
- Mingozzi, F.; Meulenberg, J.J.; Hui, D.J.; Basner-Tschakarjan, E.; Hasbrouck, N.C.; Edmonson, S.A.; Hutnick, N.A.; Betts, M.R.; Kastelein, J.J.; Stroes, E.S.; et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 2009, 114, 2077–2086. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, Q.; Huang, B.; Wang, Y.; Wang, X. AAV-mediated gene therapy: Advancing cardiovascular disease treatment. Front. Cardiovasc. Med. 2022, 9, 952755. [Google Scholar] [CrossRef] [PubMed]
- Mollard, A.; Peccate, C.; Forand, A.; Chassagne, J.; Julien, L.; Meunier, P.; Guesmia, Z.; Marais, T.; Bitoun, M.; Piétri-Rouxel, F.; et al. Muscle regeneration affects Adeno Associated Virus 1 mediated transgene transcription. Sci. Rep. 2022, 12, 9674. [Google Scholar] [CrossRef]
- Sands, M.S. AAV-mediated liver-directed gene therapy. Methods Mol. Biol. 2011, 807, 141–157. [Google Scholar] [CrossRef]
- Mücke, M.M.; Fong, S.; Foster, G.R.; Lillicrap, D.; Miesbach, W.; Zeuzem, S. Adeno-associated viruses for gene therapy—Clinical implications and liver-related complications, a guide for hepatologists. J. Hepatol. 2024, 80, 352–361. [Google Scholar] [CrossRef]
- Kang, L.; Jin, S.; Wang, J.; Lv, Z.; Xin, C.; Tan, C.; Zhao, M.; Wang, L.; Liu, J. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J. Control Release 2023, 355, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.F.; Toulmin, S.A.; Brida, K.L.; Eisenlohr, L.C.; Davidson, B.L. Standard screening methods underreport AAV-mediated transduction and gene editing. Nat. Commun. 2019, 10, 3415. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Klose, K.; Neuber, S.; Jiang, M.; Gossen, M.; Stamm, C. Comparative analysis of adeno-associated virus serotypes for gene transfer in organotypic heart slices. J. Transl. Med. 2020, 18, 437. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron. 2019, 101, 839–862. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Kok, C.Y.; Westhaus, A.; Alexander, I.E.; Lisowski, L.; Kizana, E. In Search of Adeno-Associated Virus Vectors with Enhanced Cardiac Tropism for Gene Therapy. Heart Lung Circ. 2023, 32, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Grisch-Chan, H.M.; Schwank, G.; Harding, C.O.; Thöny, B. State-of-the-Art 2019 on Gene Therapy for Phenylketonuria. Hum. Gene Ther. 2019, 30, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Siriwon, N.; Rohrs, J.A.; Wang, P. Generation of Targeted Adeno-Associated Virus (AAV) Vectors for Human Gene Therapy. Curr. Pharm. Des. 2015, 21, 3248–3256. [Google Scholar] [CrossRef]
- Colón-Thillet, R.; Jerome, K.R.; Stone, D. Optimization of AAV vectors to target persistent viral reservoirs. Virol. J. 2021, 18, 85. [Google Scholar] [CrossRef]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef]
- Bates, R.; Huang, W.; Cao, L. Adipose Tissue: An Emerging Target for Adeno-associated Viral Vectors. Mol. Ther. Methods Clin. Dev. 2020, 19, 236–249. [Google Scholar] [CrossRef]
- Kimura, K.; Nagai, Y.; Hatanaka, G.; Fang, Y.; Tanabe, S.; Zheng, A.; Fujiwara, M.; Nakano, M.; Hori, Y.; Takeuchi, R.F.; et al. A mosaic adeno-associated virus vector as a versatile tool that exhibits high levels of transgene expression and neuron specificity in primate brain. Nat. Commun. 2023, 14, 4762. [Google Scholar] [CrossRef]
- Ward, P.; Walsh, C.E. Targeted integration of a rAAV vector into the AAVS1 region. Virology 2012, 433, 356–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meier, A.F.; Fraefel, C.; Seyffert, M. The Interplay between Adeno-Associated Virus and its Helper Viruses. Viruses 2020, 12, 662. [Google Scholar] [CrossRef]
- Dallaire, F.; Schreiner, S.; Blair, G.E.; Dobner, T.; Branton, P.E.; Blanchette, P. The Human Adenovirus Type 5 E4orf6/E1B55K E3 Ubiquitin Ligase Complex Enhances E1A Functional Activity. mSphere 2015, 1, e00015-15. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Muzyczka, N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef]
- Lentz, T.B.; Samulski, R.J. Insight into the mechanism of inhibition of adeno-associated virus by the Mre11/Rad50/Nbs1 complex. J. Virol. 2015, 89, 181–194. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, A.; Wang, M.; Mao, S.; Ou, X.; Yang, Q.; Wu, Y.; Gao, Q.; Liu, M.; Zhang, S.; et al. Duck Hepatitis A Virus Type 1 Induces eIF2α Phosphorylation-Dependent Cellular Translation Shutoff via PERK/GCN2. Front. Microbiol. 2021, 12, 624540. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; Liu, S.; Liu, P.; Chen, X.; Yang, J.; He, L.; Yang, J.; Chen, J. Phosphorylated viral protein evades plant immunity through interfering the function of RNA-binding protein. PLoS Pathog. 2022, 18, e1010412. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Ryu, W.-S. Virus Life Cycle. Mol. Virol. Hum. Pathog. Viruse 2017, 31–45. [Google Scholar] [CrossRef]
- Lim, H.C.; Multhaupt, H.A.; Couchman, J.R. Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Mol. Cancer. 2015, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- García, B.; Merayo-Lloves, J.; Martin, C.; Alcalde, I.; Quirós, L.M.; Vazquez, F. Surface Proteoglycans as Mediators in Bacterial Pathogens Infections. Front. Microbiol. 2016, 7, 220. [Google Scholar] [CrossRef]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, D.D.; McIlwraith, C.W.; Samulski, R.J.; Goodrich, L.R. Adeno-associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured monolayers. Sci. Rep. 2014, 4, 5861. [Google Scholar] [CrossRef] [PubMed]
- Kurian, J.J.; Lakshmanan, R.; Chmely, W.M.; Hull, J.A.; Yu, J.C.; Bennett, A.; McKenna, R.; Agbandje-McKenna, M. Adeno-Associated Virus VP1u Exhibits Protease Activity. Viruses 2019, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, B.P.; Bailey, C.G.; Rasko, J.E.J. Journey to the Center of the Cell: Tracing the Path of AAV Transduction. Trends Mol. Med. 2021, 27, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, Y.J.; Ji, M.; Fang, J.; Siriwon, N.; Zhang, L.I.; Wang, P. Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol. Ther. Methods Clin. Dev. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Bleker, S.; Leuchs, B.; Fischer, R.; Kleinschmidt, J.A. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J. Virol. 2006, 80, 11040–11054. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, B.; Yarbrough, J.; Domsic, J.; Bennett, A.; Bothner, B.; Kozyreva, O.G.; Samulski, R.J.; Muzyczka, N.; McKenna, R.; Agbandje-McKenna, M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 2013, 87, 4974–4984. [Google Scholar] [CrossRef]
- Stahnke, S.; Lux, K.; Uhrig, S.; Kreppel, F.; Hösel, M.; Coutelle, O.; Ogris, M.; Hallek, M.; Büning, H. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology 2011, 409, 77–83. [Google Scholar] [CrossRef]
- Lakshmanan, R.V.; Hull, J.A.; Berry, L.; Burg, M.; Bothner, B.; McKenna, R.; Agbandje-McKenna, M. Structural Dynamics and Activity of B19V VP1u during the pHs of Cell Entry and Endosomal Trafficking. Viruses 2022, 14, 1922. [Google Scholar] [CrossRef] [PubMed]
- Lins-Austin, B.; Patel, S.; Mietzsch, M.; Brooke, D.; Bennett, A.; Venkatakrishnan, B.; Van Vliet, K.; Smith, A.N.; Long, J.R.; McKenna, R.; et al. Adeno-Associated Virus (AAV) Capsid Stability and Liposome Remodeling During Endo/Lysosomal pH Trafficking. Viruses 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Penzes, J.J.; Chipman, P.; Bhattacharya, N.; Zeher, A.; Huang, R.; McKenna, R.; Agbandje-McKenna, M. Adeno-associated Virus 9 Structural Rearrangements Induced by Endosomal Trafficking pH and Glycan Attachment. J. Virol. 2021, 95, e0084321. [Google Scholar] [CrossRef] [PubMed]
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol. Ther. 2020, 207, 107453. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Seyffert, M.; Pereira Bde, A.; Fraefel, C. Viral and Cellular Components of AAV2 Replication Compartments. Open Virol. J. 2013, 7, 98–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, S.; Wang, M.; Cheng, A. The role of nuclear localization signal in parvovirus life cycle. Virol. J. 2017, 14, 80. [Google Scholar] [CrossRef]
- Earley, L.F.; Kawano, Y.; Adachi, K.; Sun, X.X.; Dai, M.S.; Nakai, H. Identification and characterization of nuclear and nucleolar localization signals in the adeno-associated virus serotype 2 assembly-activating protein. J. Virol. 2015, 89, 3038–3048. [Google Scholar] [CrossRef]
- Hoad, M.; Roby, J.A.; Forwood, J.K. Structural characterization of the porcine adeno-associated virus Po1 capsid protein binding to the nuclear trafficking protein importin alpha. FEBS Lett. 2021, 595, 2793–2804. [Google Scholar] [CrossRef]
- Sonntag, F.; Schmidt, K.; Kleinschmidt, J.A. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. USA 2010, 107, 10220–10225. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Sha, S.; Hong, M.S.; Maloney, A.J.; Barone, P.W.; Neufeld, C.; Wolfrum, J.; Springs, S.L.; Sinskey, A.J.; Braatz, R.D. Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells. Mol. Ther. Methods Clin. Dev. 2021, 21, 642–655. [Google Scholar] [CrossRef]
- Penaud-Budloo, M.; François, A.; Clément, N.; Ayuso, E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018, 8, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Bijlani, S.; Pang, K.M.; Sivanandam, V.; Singh, A.; Chatterjee, S. The Role of Recombinant AAV in Precise Genome Editing. Front. Genome Ed. 2022, 3, 799722. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2017, 8, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Oziolor, E.M.; Kumpf, S.W.; Qian, J.; Gosink, M.; Sheehan, M.; Rubitski, D.M.; Newman, L.; Whiteley, L.O.; Lanz, T.A. Comparing molecular and computational approaches for detecting viral integration of AAV gene therapy constructs. Mol. Ther. Methods Clin. Dev. 2023, 29, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D.E.; Bushman, F.D.; Chandler, R.J.; Crystal, R.G.; Davidson, B.L.; Dolmetsch, R.; Eggan, K.C.; Gao, G.; Gil-Farina, I.; Kay, M.A.; et al. Evaluating the state of the science for adeno-associated virus integration: An integrated perspective. Mol. Ther. 2022, 30, 2646–2663. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Turunen, H.T.; Vandenberghe, L.H.; Wolfe, J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol. Biol. 2016, 1382, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, O.; Marsic, D.; Crosson, S.M.; Mendez-Gomez, H.R.; Moskalenko, O.; Mietzsch, M.; Heilbronn, R.; Allison, J.R.; Green, K.B.; Agbandje-McKenna, M.; et al. Direct Head-to-Head Evaluation of Recombinant Adeno-associated Viral Vectors Manufactured in Human versus Insect Cells. Mol. Ther. 2017, 25, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Rumachik, N.G.; Malaker, S.A.; Poweleit, N.; Maynard, L.H.; Adams, C.M.; Leib, R.D.; Cirolia, G.; Thomas, D.; Stamnes, S.; Holt, K.; et al. Methods Matter: Standard Production Platforms for Recombinant AAV Produce Chemically and Functionally Distinct Vectors. Mol. Ther. Methods Clin. Dev. 2020, 18, 98–118. [Google Scholar] [CrossRef]
- Wu, Y.; Mei, T.; Jiang, L.; Han, Z.; Dong, R.; Yang, T.; Xu, F. Development of Versatile and Flexible Sf9 Packaging Cell Line-Dependent OneBac System for Large-Scale Recombinant Adeno-Associated Virus Production. Hum. Gene Ther. Methods. 2019, 30, 172–183. [Google Scholar] [CrossRef]
- Grieger, J.C.; Soltys, S.M.; Samulski, R.J. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector from the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 2016, 24, 287–297. [Google Scholar] [CrossRef]
- Chung, C.H.; Murphy, C.M.; Wingate, V.P.; Pavlicek, J.W.; Nakashima, R.; Wei, W.; McCarty, D.; Rabinowitz, J.; Barton, E. Production of rAAV by plasmid transfection induces antiviral and inflammatory responses in suspension HEK293 cells. Mol. Ther. Methods Clin. Dev. 2023, 28, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Tomono, T.; Hirai, Y.; Okada, H.; Adachi, K.; Ishii, A.; Shimada, T.; Onodera, M.; Tamaoka, A.; Okada, T. Ultracentrifugation-free chromatography-mediated large-scale purification of recombinant adeno-associated virus serotype 1 (rAAV1). Mol. Ther. Methods Clin. Dev. 2016, 3, 15058. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Rombouts, S.; Adriaenssens, E.M. Purification of Bacteriophages Using Anion-Exchange Chromatography. Methods Mol. Biol. 2018, 1681, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Wang, M.; Wu, Y.; Xu, R. Scalable downstream strategies for purification of recombinant adeno- associated virus vectors in light of the properties. Curr. Pharm. Biotechnol. 2015, 16, 684–695. [Google Scholar] [CrossRef]
- Wada, M.; Uchida, N.; Posadas-Herrera, G.; Hayashita-Kinoh, H.; Tsunekawa, Y.; Hirai, Y.; Okada, T. Large-scale purification of functional AAV particles packaging the full genome using short-term ultracentrifugation with a zonal rotor. Gene Ther. 2023, 30, 641–648. [Google Scholar] [CrossRef]
- Lopes, M.M.; Lopes, S.M.; Baganha, R.; Henriques, C.; Silva, A.C.; Lobo, D.D.; Cortes, L.; de Almeida, L.P.; Nobre, R.J. Isolation of Adeno-associated Viral Vectors Through a Single-step and Semi-automated Heparin Affinity Chromatography Protocol. J. Vis. Exp. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Nass, S.A.; Mattingly, M.A.; Woodcock, D.A.; Burnham, B.L.; Ardinger, J.A.; Osmond, S.E.; Frederick, A.M.; Scaria, A.; Cheng, S.H.; O’Riordan, C.R. Universal Method for the Purification of Recombinant AAV Vectors of Differing Serotypes. Mol. Ther. Methods Clin. Dev. 2017, 9, 33–46. [Google Scholar] [CrossRef]
- Miyaoka, R.; Tsunekawa, Y.; Kurosawa, Y.; Sasaki, T.; Onodera, A.; Sakamoto, K.; Kakiuchi, Y.; Wada, M.; Nitahara-Kasahara, Y.; Hayashita-Kinoh, H.; et al. Development of a novel purification method for AAV vectors using tangential flow filtration. Biotechnol. Bioeng. 2023, 120, 3311–3321. [Google Scholar] [CrossRef]
- Kimura, T.; Ferran, B.; Tsukahara, Y.; Shang, Q.; Desai, S.; Fedoce, A.; Pimentel, D.R.; Luptak, I.; Adachi, T.; Ido, Y.; et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci. Rep. 2019, 9, 13601. [Google Scholar] [CrossRef]
- Su, W.; Patrício, M.I.; Duffy, M.R.; Krakowiak, J.M.; Seymour, L.W.; Cawood, R. Self-attenuating adenovirus enables production of recombinant adeno-associated virus for high manufacturing yield without contamination. Nat. Commun. 2022, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Moreno Velasquez, S.D.; Gerstmann, E.; Grimm, D. Goody two plasmids: An optimized transient transfection system for AAV vector production. Mol. Ther. Methods Clin. Dev. 2023, 30, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Zentilin, L.; Giacca, M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ. Res. 2014, 114, 1827–1846. [Google Scholar] [CrossRef]
- Sandro, Q.; Relizani, K.; Benchaouir, R. AAV Production Using Baculovirus Expression Vector System. Methods Mol. Biol. 2019, 1937, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef]
- Jalšić, L.; Lytvyn, V.; Elahi, S.M.; Hrapovic, S.; Nassoury, N.; Chahal, P.S.; Gaillet, B.; Gilbert, R. Inducible HEK293 AAV packaging cell lines expressing Rep proteins. Mol. Ther. Methods Clin. Dev. 2023, 30, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Chahal, P.S.; Schulze, E.; Tran, R.; Montes, J.; Kamen, A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods. 2014, 196, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lee, K.J.; Daris, M.; Lin, Y.; Wolfe, T.; Sheng, J.; Plewa, C.; Wang, S.; Meisen, W.H. Creation of a High-Yield AAV Vector Production Platform in Suspension Cells Using a Design-of-Experiment Approach. Mol. Ther. Methods Clin. Dev. 2020, 18, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Maturana, C.J.; Verpeut, J.L.; Kooshkbaghi, M.; Engel, E.A. Novel tool to quantify with single-cell resolution the number of incoming AAV genomes co-expressed in the mouse nervous system. Gene Ther. 2023, 30, 463–468. [Google Scholar] [CrossRef]
- Mietzsch, M.; Hering, H.; Hammer, E.M.; Agbandje-McKenna, M.; Zolotukhin, S.; Heilbronn, R. OneBac 2.0: Sf9 Cell Lines for Production of AAV1, AAV2, and AAV8 Vectors with Minimal Encapsidation of Foreign DNA. Hum. Gene Ther. Methods 2017, 28, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Lip, F.; Rojas, H.; Anggakusuma. Development of an insect cell-based adeno-associated virus packaging cell line employing advanced Rep gene expression control system. Mol. Ther. Methods Clin. Dev. 2022, 27, 391–403. [Google Scholar] [CrossRef]
- Kurasawa, J.H.; Park, A.; Sowers, C.R.; Halpin, R.A.; Tovchigrechko, A.; Dobson, C.L.; Schmelzer, A.E.; Gao, C.; Wilson, S.D.; Ikeda, Y. Chemically Defined, High-Density Insect Cell-Based Expression System for Scalable AAV Vector Production. Mol. Ther. Methods Clin. Dev. 2020, 19, 330–340. [Google Scholar] [CrossRef]
- Huang, Z.; Li, A.; Pan, M.; Wu, W.; Yuan, M.; Yang, K. Introduction of temperature-sensitive helper and donor plasmids into Bac-to-Bac baculovirus expression systems. Virol. Sin. 2015, 30, 379–385. [Google Scholar] [CrossRef]
- Yu, C.; Trivedi, P.D.; Chaudhuri, P.; Bhake, R.; Johnson, E.J.; Caton, T.; Potter, M.; Byrne, B.J.; Clément, N. NaCl and KCl mediate log increase in AAV vector particles and infectious titers in a specific/timely manner with the HSV platform. Mol. Ther. Methods Clin. Dev. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- La Bella, T.; Imbeaud, S.; Peneau, C.; Mami, I.; Datta, S.; Bayard, Q.; Caruso, S.; Hirsch, T.Z.; Calderaro, J.; Morcrette, G.; et al. Adeno-associated virus in the liver: Natural history and consequences in tumour development. Gut 2020, 69, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Servellita, V.; Sotomayor Gonzalez, A.; Lamson, D.M.; Foresythe, A.; Huh, H.J.; Bazinet, A.L.; Bergman, N.H.; Bull, R.L.; Garcia, K.Y.; Goodrich, J.S.; et al. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature 2023, 617, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Unexplained hepatitis in children may be linked to adeno associated virus 2, studies find. BMJ. 2023, 381, 793. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, D. Potential mechanisms by which adeno-associated virus type 2 causes unexplained hepatitis in children. J. Med. Virol. 2022, 94, 5623–5624. [Google Scholar] [CrossRef]
- Ho, A.; Orton, R.; Tayler, R.; Asamaphan, P.; Herder, V.; Davis, C.; Tong, L.; Smollett, K.; Manali, M.; Allan, J.; et al. Adeno-associated virus 2 infection in children with non-A-E hepatitis. Nature 2023, 617, 555–563. [Google Scholar] [CrossRef]
- Morfopoulou, S.; Buddle, S.; Torres Montaguth, O.E.; Atkinson, L.; Guerra-Assunção, J.A.; Moradi Marjaneh, M.; Zennezini Chiozzi, R.; Storey, N.; Campos, L.; Hutchinson, J.C.; et al. Genomic investigations of unexplained acute hepatitis in children. Nature 2023, 617, 564–573. [Google Scholar] [CrossRef]
- Brüssow, H. Non-A to E hepatitis in children: Detecting a novel viral epidemic during the COVID-19 pandemic. Microb. Biotechnol. 2023, 16, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.M.; Leng, Y.; Boppana, S.; Britt, W.J.; Gutierrez Sanchez, L.H.; Elledge, S.J. Signatures of AAV-2 immunity are enriched in children with severe acute hepatitis of unknown etiology. Sci. Transl. Med. 2023, 15, eadh9917. [Google Scholar] [CrossRef] [PubMed]
- Gates, S.; Andreani, J.; Dewar, R.; Smith, D.B.; Templeton, K.; Child, H.T.; Breuer, J.; Golubchik, T.; Bassano, I.; Wade, M.J.; et al. Postpandemic rebound of adeno-associated virus type 2 (AAV2) infections temporally associated with an outbreak of unexplained severe acute hepatitis in children in the United Kingdom. J. Med. Virol. 2023, 95, e28921. [Google Scholar] [CrossRef]
- Wright, J.F. Quality Control Testing, Characterization and Critical Quality Attributes of Adeno-Associated Virus Vectors Used for Human Gene Therapy. Biotechnol. J. 2021, 16, e2000022. [Google Scholar] [CrossRef]
- Galibert, L.; Hyvönen, A.; Eriksson, R.A.E.; Mattola, S.; Aho, V.; Salminen, S.; Albers, J.D.; Peltola, S.K.; Weman, S.; Nieminen, T.; et al. Functional roles of the membrane-associated AAV protein MAAP. Sci. Rep. 2021, 11, 21698. [Google Scholar] [CrossRef]
- Mary, B.; Maurya, S.; Arumugam, S.; Kumar, V.; Jayandharan, G.R. Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. 2019, 286, 4964–4981. [Google Scholar] [CrossRef]
- Ebberink, E.H.T.M.; Ruisinger, A.; Nuebel, M.; Thomann, M.; Heck, A.J.R. Assessing production variability in empty and filled adeno-associated viruses by single molecule mass analyses. Mol. Ther. Methods Clin. Dev. 2022, 27, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Korneyenkov, M.A.; Zamyatnin, A.A., Jr. Next Step in Gene Delivery: Modern Approaches and Further Perspectives of AAV Tropism Modification. Pharmaceutics 2021, 13, 750. [Google Scholar] [CrossRef]
- Martinez-Fernandez de la Camara, C.; McClements, M.E.; MacLaren, R.E. Accurate Quantification of AAV Vector Genomes by Quantitative PCR. Genes 2021, 12, 601. [Google Scholar] [CrossRef]
- Erles, K.; Rohde, V.; Thaele, M.; Roth, S.; Edler, L.; Schlehofer, J.R. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum. Reprod. 2001, 16, 2333–2337. [Google Scholar] [CrossRef]
- Rohde, V.; Erles, K.; Sattler, H.P.; Derouet, H.; Wullich, B.; Schlehofer, J.R. Detection of adeno-associated virus in human semen: Does viral infection play a role in the pathogenesis of male infertility? Fertil. Steril. 1999, 72, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Jiang, Z.; Stawiski, E.W.; Gnad, F.; Liu, J.; Mayba, O.; Du, P.; Diao, J.; Johnson, S.; Wong, K.F.; et al. Diverse modes of genomic alteration in hepatocellular carcinoma. Genome Biol. 2014, 15, 436. [Google Scholar] [CrossRef]
- Kan, Z.; Zheng, H.; Liu, X.; Li, S.; Barber, T.D.; Gong, Z.; Gao, H.; Hao, K.; Willard, M.D.; Xu, J.; et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013, 23, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Long, X.; Tsang, S.Y.; Hu, T.; Yang, J.F.; Mat, W.K.; Wang, H.; Xue, H. Genomic subtyping of liver cancers with prognostic application. BMC Cancer 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Lee, J.; Park, C.K.; Mao, M.; Shi, Y.; Gong, Z.; Zheng, H.; Li, Y.; Zhao, Y.; Wang, G.; et al. Whole-genome sequencing of matched primary and metastatic hepatocellular carcinomas. BMC Med. Genom. 2014, 7, 2. [Google Scholar] [CrossRef]
- Berns, K.I.; Byrne, B.J.; Flotte, T.R.; Gao, G.; Hauswirth, W.W.; Herzog, R.W.; Muzyczka, N.; VandenDriessche, T.; Xiao, X.; Zolotukhin, S.; et al. Adeno-Associated Virus Type 2 and Hepatocellular Carcinoma? Hum. Gene Ther. 2015, 26, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Meumann, N.; Schmithals, C.; Elenschneider, L.; Hansen, T.; Balakrishnan, A.; Hu, Q.; Hook, S.; Schmitz, J.; Bräsen, J.H.; Franke, A.C.; et al. Hepatocellular Carcinoma Is a Natural Target for Adeno-Associated Virus (AAV) 2 Vectors. Cancers 2022, 14, 427. [Google Scholar] [CrossRef]
- Hadi, M.; Qutaiba BAllela, O.; Jabari, M.; Jasoor, A.M.; Naderloo, O.; Yasamineh, S.; Gholizadeh, O.; Kalantari, L. Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma. Virol. J. 2024, 21, 17. [Google Scholar] [CrossRef]
- Maurya, S.; Sarangi, P.; Jayandharan, G.R. Safety of Adeno-associated virus-based vector-mediated gene therapy-impact of vector dose. Cancer Gene Ther. 2022, 29, 1305–1306. [Google Scholar] [CrossRef]
- Suoranta, T.; Laham-Karam, N.; Ylä-Herttuala, S. Strategies to improve safety profile of AAV vectors. Int. J. Mol. Med. 2022, 2, 1054069. [Google Scholar] [CrossRef]
- Sobh, M.; Lagali, P.S.; Ghiasi, M.; Montroy, J.; Dollin, M.; Hurley, B.; Leonard, B.C.; Dimopoulos, I.; Lafreniere, M.; Fergusson, D.A.; et al. Safety and Efficacy of Adeno-Associated Viral Gene Therapy in Patients with Retinal Degeneration: A Systematic Review and Meta-Analysis. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. Rationale and strategies for the development of safe and effective optimized AAV vectors for human gene therapy. Mol. Ther. Nucleic Acids 2023, 2, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Radukic, M.T.; Müller, K.M. Adeno-associated virus capsid protein expression in Escherichia coli and chemically defined capsid assembly. Sci. Rep. 2019, 9, 18631. [Google Scholar] [CrossRef]
- Jaafar, Z.A.; Kieft, J.S. Viral RNA structure-based strategies to manipulate translation. Nat. Rev. Microbiol. 2019, 17, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.Y.; Zhu, Z.; Marazzi, I. Unconventional viral gene expression mechanisms as therapeutic targets. Nature. 2021, 593, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Nonaka, M.; Maruno, T.; Yamaguchi, Y.; Fukuhara, M.; Torisu, T.; Maeda, M.; Abbatiello, S.; Haris, A.; Richardson, K.; et al. Enhancement of recombinant adeno-associated virus activity by improved stoichiometry and homogeneity of capsid protein assembly. Mol. Ther. Methods Clin. Dev. 2023, 31, 101142. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Liu, W.; Ma, K.; Bennett, A.; Nelson, A.R.; Gliwa, K.; Chipman, P.; Fu, X.; Bechler, S.; McKenna, R.; et al. Production and characterization of an AAV1-VP3-only capsid: An analytical benchmark standard. Mol. Ther. Methods Clin. Dev. 2023, 29, 460–472. [Google Scholar] [CrossRef]
- Bosma, B.; du Plessis, F.; Ehlert, E.; Nijmeijer, B.; de Haan, M.; Petry, H.; Lubelski, J. Optimization of viral protein ratios for production of rAAV serotype 5 in the baculovirus system. Gene Ther. 2018, 25, 415–424. [Google Scholar] [CrossRef]
- Viney, L.; Bürckstümmer, T.; Eddington, C.; Mietzsch, M.; Choudhry, M.; Henley, T.; Agbandje-McKenna, M. Adeno-associated Virus (AAV) Capsid Chimeras with Enhanced Infectivity Reveal a Core Element in the AAV Genome Critical for both Cell Transduction and Capsid Assembly. J. Virol. 2021, 95, e02023-20. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Hu, H.; Chen, C.; Yan, M.; Ling, F.; Wang, K.C.; Wang, X.; Deng, Z.; Zhou, X.; et al. Directed evolution of adeno-associated virus 5 capsid enables specific liver tropism. Mol. Ther. Nucleic Acids. 2022, 28, 293–306. [Google Scholar] [CrossRef]
- Jain, N.K.; Ogden, P.J.; Church, G.M. Comprehensive mutagenesis maps the effect of all single codon mutations in the AAV2 rep gene on AAV production. bioRxiv 2023. [Google Scholar] [CrossRef]
- Khanal, O.; Kumar, V.; Jin, M. Adeno-associated viral capsid stability on anion exchange chromatography column and its impact on empty and full capsid separation. Mol. Ther. Methods Clin. Dev. 2023, 31, 101112. [Google Scholar] [CrossRef] [PubMed]
- Jarand, C.W.; Baker, K.; Petroff, M.; Jin, M.; Reed, W.F. DNA Released by Adeno-Associated Virus Strongly Alters Capsid Aggregation Kinetics in a Physiological Solution. Biomacromolecules 2024, 25, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, V.; Kruse, S.; Kant, R.; Venkatakrishnan, B.; Movahed, N.; Brooke, D.; Lins, B.; Bennett, A.; Potter, T.; McKenna, R.; et al. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013, 87, 13150–13160. [Google Scholar] [CrossRef] [PubMed]
- Earley, L.F.; Powers, J.M.; Adachi, K.; Baumgart, J.T.; Meyer, N.L.; Xie, Q.; Chapman, M.S.; Nakai, H. Adeno-associated Virus (AAV) Assembly-Activating Protein Is Not an Essential Requirement for Capsid Assembly of AAV Serotypes 4, 5, and 11. J. Virol. 2017, 91, e01980-16. [Google Scholar] [CrossRef] [PubMed]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef]
- Brimble, M.A.; Cheng, P.H.; Winston, S.M.; Reeves, I.L.; Souquette, A.; Spence, Y.; Zhou, J.; Wang, Y.D.; Morton, C.L.; Valentine, M.; et al. Preventing packaging of translatable P5-associated DNA contaminants in recombinant AAV vector preps. Mol. Ther. Methods Clin. Dev. 2022, 24, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Barnes, C.; Hull, J.A.; Chipman, P.; Xie, J.; Bhattacharya, N.; Sousa, D.; McKenna, R.; Gao, G.; Agbandje-McKenna, M. Comparative Analysis of the Capsid Structures of AAVrh.10, AAVrh.39, and AAV8. J. Virol. 2020, 94, e01769-19. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Jose, A.; Chipman, P.; Bhattacharya, N.; Daneshparvar, N.; McKenna, R.; Agbandje-McKenna, M. Completion of the AAV Structural Atlas: Serotype Capsid Structures Reveals Clade-Specific Features. Viruses 2021, 13, 101. [Google Scholar] [CrossRef]
- Mietzsch, M.; Pénzes, J.J.; Agbandje-McKenna, M. Twenty-Five Years of Structural Parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef]
- Large, E.E.; Silveria, M.A.; Zane, G.M.; Weerakoon, O.; Chapman, M.S. Adeno-Associated Virus (AAV) Gene Delivery: Dissecting Molecular Interactions upon Cell Entry. Viruses 2021, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Prantner, A.; Maar, D. Genome concentration, characterization, and integrity analysis of recombinant adeno-associated viral vectors using droplet digital PCR. PLoS ONE 2023, 18, e0280242. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, M.; Negrini, M.; Hauser, S.; Svanbergsson, A.; Lockowandt, M.; Tomasello, G.; Manfredsson, F.P.; Heuer, A. A comparison of AAV-vector production methods for gene therapy and preclinical assessment. Sci. Rep. 2020, 10, 21532. [Google Scholar] [CrossRef]
- Damry, A.M.; Mayer, M.M.; Broom, A.; Goto, N.K.; Chica, R.A. Origin of conformational dynamics in a globular protein. Commun. Biol. 2019, 2, 433. [Google Scholar] [CrossRef] [PubMed]
- Audagnotto, M.; Czechtizky, W.; De Maria, L.; Käck, H.; Papoian, G.; Tornberg, L.; Tyrchan, C.; Ulander, J. Machine learning/molecular dynamic protein structure prediction approach to investigate the protein conformational ensemble. Sci. Rep. 2022, 12, 10018. [Google Scholar] [CrossRef]
- Feng, J.; Shukla, D. Characterizing Conformational Dynamics of Proteins Using Evolutionary Couplings. J. Phys. Chem. B 2018, 122, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, C.; Bernard, D.N.; Bafna, K.; Gagné, D.; Agarwal, P.K.; Doucet, N. Ligand-Induced Variations in Structural and Dynamical Properties Within an Enzyme Superfamily. Front. Mol. Biosci. 2018, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Mamounis, K.J.; Yukl, E.T.; Davidson, V.L. Roles of active-site residues in catalysis, substrate binding, cooperativity, and the reaction mechanism of the quinoprotein glycine oxidase. J. Biol. Chem. 2020, 295, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- Maria-Solano, M.A.; Serrano-Hervás, E.; Romero-Rivera, A.; Iglesias-Fernández, J.; Osuna, S. Role of conformational dynamics in the evolution of novel enzyme function. Chem. Commun. 2018, 54, 6622–6634. [Google Scholar] [CrossRef]
- Masson, G.R.; Burke, J.E.; Ahn, N.G.; Anand, G.S.; Borchers, C.; Brier, S.; Bou-Assaf, G.M.; Engen, J.R.; Englander, S.W.; Faber, J.; et al. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods 2019, 16, 595–602. [Google Scholar] [CrossRef]
- James, E.I.; Murphree, T.A.; Vorauer, C.; Engen, J.R.; Guttman, M. Advances in Hydrogen/Deuterium Exchange Mass Spectrometry and the Pursuit of Challenging Biological Systems. Chem. Rev. 2022, 122, 7562–7623. [Google Scholar] [CrossRef]
- Jia, R.; Martens, C.; Shekhar, M.; Pant, S.; Pellowe, G.A.; Lau, A.M.; Findlay, H.E.; Harris, N.J.; Tajkhorshid, E.; Booth, P.J.; et al. Hydrogen-deuterium exchange mass spectrometry captures distinct dynamics upon substrate and inhibitor binding to a transporter. Nat. Commun. 2020, 11, 6162. [Google Scholar] [CrossRef]
- So, P.K. Hydrogen-Deuterium Exchange Mass Spectrometry for Probing Changes in Conformation and Dynamics of Proteins. Methods Mol. Biol. 2021, 2199, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Seetaloo, N.; Kish, M.; Phillips, J.J. HDfleX: Software for Flexible High Structural Resolution of Hydrogen/Deuterium-Exchange Mass Spectrometry Data. Anal. Chem. 2022, 94, 4557–4564. [Google Scholar] [CrossRef]
- Martens, C.; Shekhar, M.; Lau, A.M.; Tajkhorshid, E.; Politis, A. Integrating hydrogen-deuterium exchange mass spectrometry with molecular dynamics simulations to probe lipid-modulated conformational changes in membrane proteins. Nat. Protoc. 2019, 14, 3183–3204. [Google Scholar] [CrossRef]
- Nguyen, D.; Mayne, L.; Phillips, M.C.; Walter Englander, S. Reference Parameters for Protein Hydrogen Exchange Rates. J. Am. Soc. Mass. Spectrom. 2018, 29, 1936–1939. [Google Scholar] [CrossRef]
- Gessner, C.; Steinchen, W.; Bédard, S.J.; Skinner, J.; Woods, V.L.; Walsh, T.J.; Bange, G.; Pantazatos, D.P. Computational method allowing Hydrogen-Deuterium Exchange Mass Spectrometry at single amide Resolution. Sci. Rep. 2017, 7, 3789. [Google Scholar] [CrossRef] [PubMed]
- Zöller, J.; Hong, S.; Eisinger, M.L.; Anderson, M.; Radloff, M.; Desch, K.; Gennis, R.; Langer, J.D. Ligand binding and conformational dynamics of the E. coli nicotinamide nucleotide transhydrogenase revealed by hydrogen/deuterium exchange mass spectrometry. Comput. Struct. Biotechnol. J. 2022, 20, 5430–5439. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Khananshvili, D. Hydrogen-Deuterium Exchange Mass-Spectrometry of Secondary Active Transporters: From Structural Dynamics to Molecular Mechanisms. Front. Pharmacol. 2020, 11, 70. [Google Scholar] [CrossRef]
- Kammari, R.; Topp, E.M. Effects of Secondary Structure on Solid-State Hydrogen-Deuterium Exchange in Model α-Helix and β-Sheet Peptides. Mol. Pharm. 2020, 17, 3501–3512. [Google Scholar] [CrossRef]
- Habibi, Y.; Thibodeaux, C.J. A Hydrogen-Deuterium Exchange Mass Spectrometry (HDX-MS) Platform for Investigating Peptide Biosynthetic Enzymes. J. Vis. Exp. 2020, 4, 159. [Google Scholar] [CrossRef]
- Jia, R.; Bradshaw, R.T.; Calvaresi, V.; Politis, A. Integrating Hydrogen Deuterium Exchange-Mass Spectrometry with Molecular Simulations Enables Quantification of the Conformational Populations of the Sugar Transporter XylE. J. Am. Chem. Soc. 2023, 145, 7768–7779. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Thompson, E.J.; Barrow, S.L.; Zhang, W.; Iavarone, A.T.; Klinman, J.P. Hydrogen-Deuterium Exchange within Adenosine Deaminase, a TIM Barrel Hydrolase, Identifies Networks for Thermal Activation of Catalysis. J. Am. Chem. Soc. 2020, 142, 19936–19949. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Smith, J.K.; Yu, J.C.; Banala, V.; Emmanuel, S.N.; Jose, A.; Chipman, P.; Bhattacharya, N.; McKenna, R.; Agbandje-McKenna, M. Characterization of AAV-Specific Affinity Ligands: Consequences for Vector Purification and Development Strategies. Mol. Ther. Methods Clin. Dev. 2020, 19, 362–373. [Google Scholar] [CrossRef]
- Havlik, L.P.; Simon, K.E.; Smith, J.K.; Klinc, K.A.; Tse, L.V.; Oh, D.K.; Fanous, M.M.; Meganck, R.M.; Mietzsch, M.; Kleinschmidt, J.; et al. Coevolution of Adeno-associated Virus Capsid Antigenicity and Tropism through a Structure-Guided Approach. J. Virol. 2020, 94, e00976-20. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.D.; Zdechlik, A.C.; He, Y.; Nedrud, D.; Aslanidi, G.; Gordon, W.; Schmidt, D. Multiparametric domain insertional profiling of Adeno-Associated Virus VP1. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mietzsch, M.; McKenna, R.; Väisänen, E.; Yu, J.C.; Ilyas, M.; Hull, J.A.; Kurian, J.; Smith, J.K.; Chipman, P.; Lasanajak, Y.; et al. Structural Characterization of Cuta- and Tusavirus: Insight into Protoparvoviruses Capsid Morphology. Viruses 2020, 12, 653. [Google Scholar] [CrossRef]
- Large, E.E.; Chapman, M.S. Adeno-associated virus receptor complexes and implications for adeno-associated virus immune neutralization. Front. Microbiol. 2023, 14, 1116896. [Google Scholar] [CrossRef] [PubMed]
- Schramm, H.M.; Tamadate, T.; Hogan, C.J.; Clowers, B.H. Ion-neutral clustering alters gas-phase hydrogen-deuterium exchange rates. Phys. Chem. Chem. Phys. 2023, 25, 4959–4968. [Google Scholar] [CrossRef]
- Seyffert, M.; Glauser, D.L.; Schraner, E.M.; de Oliveira, A.P.; Mansilla-Soto, J.; Vogt, B.; Büning, H.; Linden, R.M.; Ackermann, M.; Fraefel, C. Novel Mutant AAV2 Rep Proteins Support AAV2 Replication without Blocking HSV-1 Helpervirus Replication. PLoS ONE 2017, 12, e0170908. [Google Scholar] [CrossRef]
- Bennett, A.; Hull, J.; Jolinon, N.; Tordo, J.; Moss, K.; Binns, E.; Mietzsch, M.; Hagemann, C.; Linden, R.; Serio, A.; et al. Comparative structural, biophysical, and receptor binding study of true type and wild type AAV2. J. Struct. Biol. 2021, 213, 107795. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Lee, K.K. Isotope Labeling of Biomolecules: Structural Analysis of Viruses by HDX-MS. Methods Enzymol. 2016, 566, 405–426. [Google Scholar] [CrossRef]
- Redhair, M.; Clouser, A.F.; Atkins, W.M. Hydrogen-deuterium exchange mass spectrometry of membrane proteins in lipid nanodiscs. Chem. Phys. Lipids 2019, 220, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Balsbaugh, J.L.; Gao, S.; Ahn, N.G.; Klinman, J.P. Hydrogen deuterium exchange defines catalytically linked regions of protein flexibility in the catechol O-methyltransferase reaction. Proc. Natl. Acad. Sci. USA 2020, 117, 10797–10805. [Google Scholar] [CrossRef]
- Ahmadi, E.; Ravanshad, M.; Xie, J.; Panigrahi, R.; Jubbal, S.S.; Guru, S.K.; Guangping, G.; Ziyaeyan, M.; Fingeroth, J. Serotype-dependent recombinant adeno-associated vector (AAV) infection of Epstein-Barr virus-positive B-cells, towards recombinant AAV-based therapy of focal EBV + lymphoproliferative disorders. Virol. J. 2021, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Flores, C.; López, T.; Zamudio, F.; Sandoval-Jaime, C.; Pérez, E.I.; López, S.; DuBois, R.; Arias, C.F. The Capsid Precursor Protein of Astrovirus VA1 Is Proteolytically Processed Intracellularly. J. Virol. 2022, 96, e0066522. [Google Scholar] [CrossRef]
- Elmore, Z.C.; Patrick Havlik, L.; Oh, D.K.; Anderson, L.; Daaboul, G.; Devlin, G.W.; Vincent, H.A.; Asokan, A. The membrane associated accessory protein is an adeno-associated viral egress factor. Nat. Commun. 2021, 12, 6239. [Google Scholar] [CrossRef]
- Büning, H.; Srivastava, A. Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors. Mol. Ther. Methods Clin. Dev. 2019, 12, 248–265. [Google Scholar] [CrossRef]
- Hull, J.A.; Mietzsch, M.; Chipman, P.; Strugatsky, D.; McKenna, R. Structural characterization of an envelope-associated adeno-associated virus type 2 capsid. Virology 2022, 565, 22–28. [Google Scholar] [CrossRef]
- Dudek, A.M.; Porteus, M.H. Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing. Front. Immunol. 2021, 12, 660302. [Google Scholar] [CrossRef]
- Bee, J.S.; O’Berry, K.; Zhang, Y.Z.; Phillippi, M.K.; Kaushal, A.; DePaz, R.A.; Marshall, T. Quantitation of Trace Levels of DNA Released from Disrupted Adeno-Associated Virus Gene Therapy Vectors. J. Pharm. Sci. 2021, 110, 3183–3187. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.F.; Draper, B.E.; Jarrold, M.F. Analysis of thermally driven structural changes, genome release, disassembly, and aggregation of recombinant AAV by CDMS. Mol. Ther. Methods Clin. Dev. 2022, 27, 327–336. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, P.; Zhang, J.; Chrzanowski, M.; Chew, H.; Firrman, J.A.; Sang, N.; Diao, Y.; Xiao, W. Effects of Thermally Induced Configuration Changes on rAAV Genome’s Enzymatic Accessibility. Mol. Ther. Methods Clin. Dev. 2020, 18, 328–334. [Google Scholar] [CrossRef]
- Bernaud, J.; Rossi, A.; Fis, A.; Gardette, L.; Aillot, L.; Büning, H.; Castelnovo, M.; Salvetti, A.; Faivre-Moskalenko, C. Characterization of AAV vector particle stability at the single-capsid level. J. Biol. Phys. 2018, 44, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Dalby, P.A. Challenges in scaling up AAV-based gene therapy manufacturing. Trends Biotechnol. 2023, 41, 1268–1281. [Google Scholar] [CrossRef]
- Kostelic, M.M.; Ryan, J.P.; Brown, L.S.; Jackson, T.W.; Hsieh, C.C.; Zak, C.K.; Sanders, H.M.; Liu, Y.; Chen, V.S.; Byrne, M.; et al. Stability and Dissociation of Adeno-Associated Viral Capsids by Variable Temperature-Charge Detection-Mass Spectrometry. Anal. Chem. 2022, 94, 11723–11727. [Google Scholar] [CrossRef]
- Strasser, L.; Morgan, T.E.; Guapo, F.; Füssl, F.; Forsey, D.; Anderson, I.; Bones, J. A Native Mass Spectrometry-Based Assay for Rapid Assessment of the Empty:Full Capsid Ratio in Adeno-Associated Virus Gene Therapy Products. Anal. Chem. 2021, 93, 12817–12821. [Google Scholar] [CrossRef]
- Sun, C.P.; Chiu, C.W.; Wu, P.Y.; Tsung, S.I.; Lee, I.J.; Hu, C.W.; Hsu, M.F.; Kuo, T.J.; Lan, Y.H.; Chen, L.Y.; et al. Development of AAV-delivered broadly neutralizing anti-human ACE2 antibodies against SARS-CoV-2 variants. Mol. Ther. 2023, 31, 3322–3336. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rodnin, M.V.; Ladokhin, A.S.; Gross, M.L. Hydrogen-deuterium exchange and mass spectrometry reveal the pH-dependent conformational changes of diphtheria toxin T domain. Biochemistry 2014, 53, 6849–8656. [Google Scholar] [CrossRef]
- Heldt, C.L.; Areo, O.; Joshi, P.U.; Mi, X.; Ivanova, Y.; Berrill, A. Empty and Full AAV Capsid Charge and Hydrophobicity Differences Measured with Single-Particle AFM. Langmuir 2023, 39, 5641–5648. [Google Scholar] [CrossRef]
- Werle, A.K.; Powers, T.W.; Zobel, J.F.; Wappelhorst, C.N.; Jarrold, M.F.; Lyktey, N.A.; Sloan, C.D.K.; Wolf, A.J.; Adams-Hall, S.; Baldus, P.; et al. Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.E.; Keifer, D.Z.; Asokan, A.; Jarrold, M.F. Resolving Adeno-Associated Viral Particle Diversity with Charge Detection Mass Spectrometry. Anal. Chem. 2016, 88, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Z.; Zheng, M.; Shi, L.; Gu, M.; Liu, G.; Miao, F.; Chang, Y.; Huang, F.; Tang, N. Recombinant adeno-associated virus 8 vector in gene therapy: Opportunities and challenges. Genes Dis. 2023, 11, 283–293. [Google Scholar] [CrossRef]
- Xie, Q.; Yoshioka, C.K.; Chapman, M.S. Adeno-Associated Virus (AAV-DJ)-Cryo-EM Structure at 1.56 Å Resolution. Viruses 2020, 12, 1194. [Google Scholar] [CrossRef]
- van de Waterbeemd, M.; Llauró, A.; Snijder, J.; Valbuena, A.; Rodríguez-Huete, A.; Fuertes, M.A.; de Pablo, P.J.; Mateu, M.G.; Heck, A.J.R. Structural Analysis of a Temperature-Induced Transition in a Viral Capsid Probed by HDX-MS. Biophys. J. 2017, 112, 1157–1165. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Sandoval, A.; Pekrun, K.; Tsuji, S.; Zhang, F.; Hung, K.L.; Chang, H.Y.; Kay, M.A. The AAV capsid can influence the epigenetic marking of rAAV delivered episomal genomes in a species dependent manner. Nat. Commun. 2023, 14, 2448. [Google Scholar] [CrossRef]
- Cole, L.; Fernandes, D.; Hussain, M.T.; Kaszuba, M.; Stenson, J.; Markova, N. Characterization of Recombinant Adeno-Associated Viruses (rAAVs) for Gene Therapy Using Orthogonal Techniques. Pharmaceutics 2021, 13, 586. [Google Scholar] [CrossRef]
- Srivastava, A.; Mallela, K.M.G.; Deorkar, N.; Brophy, G. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 2021, 110, 2609–2624. [Google Scholar] [CrossRef]
- Meyer, N.L.; Hu, G.; Davulcu, O.; Xie, Q.; Noble, A.J.; Yoshioka, C.; Gingerich, D.S.; Trzynka, A.; David, L.; Stagg, S.M.; et al. Structure of the gene therapy vector, adeno-associated virus with its cell receptor, AAVR. eLife 2019, 8, e44707. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Wec, A.Z.; Lin, K.S.; Kwasnieski, J.C.; Sinai, S.; Gerold, J.; Kelsic, E.D. Overcoming Immunological Challenges Limiting Capsid-Mediated Gene Therapy With Machine Learning. Front. Immunol. 2021, 2, 674021. [Google Scholar] [CrossRef]
- Becker, J.; Fakhiri, J.; Grimm, D. Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning. Pathogens 2022, 11, 756. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; van Bakel, H.; Hajjar, R.J.; Weber, T. High capsid-genome correlation facilitates creation of AAV libraries for directed evolution. Mol. Ther. 2015, 23, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, J.; Weis, S.; Sippel, J.; Tulalamba, W.; Remes, A.; El Andari, J.; Herrmann, A.K.; Pham, Q.H.; Borowski, C.; Hille, S.; et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 2020, 11, 5432. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.J.; Simon, K.E.; Blondel, L.O.; Fanous, M.M.; Roger, A.L.; Maysonet, M.S.; Devlin, G.W.; Smith, T.J.; Oh, D.K.; Havlik, L.P.; et al. Cross-species evolution of a highly potent AAV variant for therapeutic gene transfer and genome editing. Nat. Commun. 2022, 13, 5947. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, K.S.; Meltzer, J.C.; Buzhdygan, T.; Cheng, M.J.; Sena-Esteves, M.; Bennett, R.E.; Sullivan, T.P.; Razmpour, R.; Gong, Y.; Ng, C.; et al. Selection of an Efficient AAV Vector for Robust CNS Transgene Expression. Mol. Ther. Methods Clin. Dev. 2019, 15, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.D.; Kummer, M.; Kondratov, O.; Banerjee, A.; Moskalenko, O.; Zolotukhin, S. Applying machine learning to predict viral assembly for adeno-associated virus capsid libraries. Mol. Ther. Methods Clin. Dev. 2020, 20, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Domenger, C.; Grimm, D. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum. Mol. Genet. 2019, 28, R3–R14. [Google Scholar] [CrossRef]
- Nance, M.E.; Duan, D. Perspective on Adeno-Associated Virus Capsid Modification for Duchenne Muscular Dystrophy Gene Therapy. Hum. Gene Ther. 2015, 26, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Xiao, B.; Li, J.; Xiao, X. Directed Evolution of AAV Serotype 5 for Increased Hepatocyte Transduction and Retained Low Humoral Seroreactivity. Mol. Ther. Methods Clin. Dev. 2020, 20, 122–132. [Google Scholar] [CrossRef]
- Lee, S.O.; Fried, S.D. An error prone PCR method for small amplicons. Anal. Biochem. 2021, 628, 114266. [Google Scholar] [CrossRef]
- Börner, K.; Kienle, E.; Huang, L.Y.; Weinmann, J.; Sacher, A.; Bayer, P.; Stüllein, C.; Fakhiri, J.; Zimmermann, L.; Westhaus, A.; et al. Pre-arrayed Pan-AAV Peptide Display Libraries for Rapid Single-Round Screening. Mol. Ther. 2020, 28, 1016–1032. [Google Scholar] [CrossRef]
- Zhang, X.; Chai, Z.; Samulski, R.J.; Li, C. Bound Protein- and Peptide-Based Strategies for Adeno-Associated Virus Vector-Mediated Gene Therapy: Where Do We Stand Now? Hum. Gene Ther. 2020, 31, 1146–1154. [Google Scholar] [CrossRef]
- Hu, G.; Silveria, M.A.; Zane, G.M.; Chapman, M.S.; Stagg, S.M. Adeno-Associated Virus Receptor-Binding: Flexible Domains and Alternative Conformations through Cryo-Electron Tomography of Adeno-Associated Virus 2 (AAV2) and AAV5 Complexes. J. Virol. 2022, 96, e0010622. [Google Scholar] [CrossRef]
- Cabanes-Creus, M.; Ginn, S.L.; Amaya, A.K.; Liao, S.H.Y.; Westhaus, A.; Hallwirth, C.V.; Wilmott, P.; Ward, J.; Dilworth, K.L.; Santilli, G.; et al. Codon-Optimization of Wild-Type Adeno-Associated Virus Capsid Sequences Enhances DNA Family Shuffling while Conserving Functionality. Mol. Ther. Methods Clin. Dev. 2018, 12, 71–84. [Google Scholar] [CrossRef]
- Herrmann, A.K.; Bender, C.; Kienle, E.; Grosse, S.; El Andari, J.; Botta, J.; Schürmann, N.; Wiedtke, E.; Niopek, D.; Grimm, D. A Robust and All-Inclusive Pipeline for Shuffling of Adeno-Associated Viruses. ACS Synth. Biol. 2019, 8, 194–206. [Google Scholar] [CrossRef]
- Kremer, L.P.M.; Cerrizuela, S.; Dehler, S.; Stiehl, T.; Weinmann, J.; Abendroth, H.; Kleber, S.; Laure, A.; El Andari, J.; Anders, S.; et al. High throughput screening of novel AAV capsids identifies variants for transduction of adult NSCs within the subventricular zone. Mol. Ther. Methods Clin. Dev. 2021, 23, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Rode, L.; Bär, C.; Groß, S.; Rossi, A.; Meumann, N.; Viereck, J.; Abbas, N.; Xiao, K.; Riedel, I.; Gietz, A.; et al. AAV capsid engineering identified two novel variants with improved in vivo tropism for cardiomyocytes. Mol. Ther. 2022, 30, 3601–3618. [Google Scholar] [CrossRef] [PubMed]
- Tabebordbar, M.; Lagerborg, K.A.; Stanton, A.; King, E.M.; Ye, S.; Tellez, L.; Krunnfusz, A.; Tavakoli, S.; Widrick, J.J.; Messemer, K.A.; et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 2021, 184, 4919–4938.e22. [Google Scholar] [CrossRef]
- Zinn, E.; Pacouret, S.; Khaychuk, V.; Turunen, H.T.; Carvalho, L.S.; Andres-Mateos, E.; Shah, S.; Shelke, R.; Maurer, A.C.; Plovie, E.; et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep. 2015, 12, 1056–1068. [Google Scholar] [CrossRef]
- Metsky, H.C.; Welch, N.L.; Pillai, P.P.; Haradhvala, N.J.; Rumker, L.; Mantena, S.; Zhang, Y.B.; Yang, D.K.; Ackerman, C.M.; Weller, J.; et al. Designing sensitive viral diagnostics with machine learning. Nat. Biotechnol. 2022, 40, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Gligorijević, V.; Renfrew, P.D.; Kosciolek, T.; Leman, J.K.; Berenberg, D.; Vatanen, T.; Chandler, C.; Taylor, B.C.; Fisk, I.M.; Vlamakis, H.; et al. Structure-based protein function prediction using graph convolutional networks. Nat. Commun. 2021, 12, 3168. [Google Scholar] [CrossRef]
- AlQuraishi, M. Machine learning in protein structure prediction. Curr. Opin. Chem. Biol. 2021, 65, 1–8. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Schmit, P.F.; Pacouret, S.; Zinn, E.; Telford, E.; Nicolaou, F.; Broucque, F.; Andres-Mateos, E.; Xiao, R.; Penaud-Budloo, M.; Bouzelha, M.; et al. Cross-Packaging and Capsid Mosaic Formation in Multiplexed AAV Libraries. Mol. Ther. Methods Clin. Dev. 2019, 17, 107–121. [Google Scholar] [CrossRef]
- Tang, Q.; Keeler, A.M.; Zhang, S.; Su, Q.; Lyu, Z.; Cheng, Y.; Gao, G.; Flotte, T.R. Two-Plasmid Packaging System for Recombinant Adeno-Associated Virus. BioRes. Open Access 2020, 9, 219–228. [Google Scholar] [CrossRef]
- Han, Z.; Luo, N.; Wang, F.; Cai, Y.; Yang, X.; Feng, W.; Zhu, Z.; Wang, J.; Wu, Y.; Ye, C.; et al. Computer-Aided Directed Evolution Generates Novel AAV Variants with High Transduction Efficiency. Viruses 2023, 15, 848. [Google Scholar] [CrossRef] [PubMed]
- Pekrun, K.; De Alencastro, G.; Luo, Q.J.; Liu, J.; Kim, Y.; Nygaard, S.; Galivo, F.; Zhang, F.; Song, R.; Tiffany, M.R.; et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight 2019, 4, e131610. [Google Scholar] [CrossRef]
- Xie, Y.; Butler, M. N-glycomic profiling of capsid proteins from adeno-associated virus serotypes. Glycobiology. 2023, 29, cwad074. [Google Scholar] [CrossRef]
- Crudele, J.M.; Chamberlain, J.S. AAV-based gene therapies for the muscular dystrophies. Hum. Mol. Genet. 2019, 28, R102–R107. [Google Scholar] [CrossRef]
- Nyberg, W.A.; Ark, J.; To, A.; Clouden, S.; Reeder, G.; Muldoon, J.J.; Chung, J.Y.; Xie, W.H.; Allain, V.; Steinhart, Z.; et al. An evolved AAV variant enables efficient genetic engineering of murine T cells. Cell 2023, 186, 446–460.e19. [Google Scholar] [CrossRef]
- Biswas, M.; Marsic, D.; Li, N.; Zou, C.; Gonzalez-Aseguinolaza, G.; Zolotukhin, I.; Kumar, S.R.P.; Rana, J.; Butterfield, J.S.S.; Kondratov, O.; et al. Engineering and In Vitro Selection of a Novel AAV3B Variant with High Hepatocyte Tropism and Reduced Seroreactivity. Mol. Ther. Methods Clin. Dev. 2020, 19, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Gross, D.A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.A.; Wright, J.F. Challenges Posed by Immune Responses to AAV Vectors: Addressing Root Causes. Front. Immunol. 2021, 12, 675897. [Google Scholar] [CrossRef] [PubMed]
- Arjomandnejad, M.; Dasgupta, I.; Flotte, T.R.; Keeler, A.M. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs 2023, 37, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, K.S.; Cheng, M.; De La Cruz, D.; Patel, N.; Santoscoy, M.C.; Gong, Y.; Ng, C.; Nguyen, D.M.; Nammour, J.; Clark, S.W.; et al. In vivo selection in non-human primates identifies superior AAV capsids for on-target CSF delivery to spinal cord. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mendell, J.R.; Connolly, A.M.; Lehman, K.J.; Griffin, D.A.; Khan, S.Z.; Dharia, S.D.; Quintana-Gallardo, L.; Rodino-Klapac, L.R. Testing preexisting antibodies prior to AAV gene transfer therapy: Rationale, lessons and future considerations. Mol. Ther. Methods Clin. Dev. 2022, 25, 74–83. [Google Scholar] [CrossRef]
- Chandra, S.; Long, B.R.; Fonck, C.; Melton, A.C.; Arens, J.; Woloszynek, J.; O’Neill, C.A. Safety Findings of Dosing Gene Therapy Vectors in NHP With Pre-existing or Treatment-Emergent Anti-capsid Antibodies. Toxicol. Pathol. 2023, 51, 1926233231202995. [Google Scholar] [CrossRef]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2021, 7, e10258. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Ou, L. AAV Engineering for Improving Tropism to the Central Nervous System. Biology 2023, 12, 186. [Google Scholar] [CrossRef]
- Minskaia, E.; Galieva, A.; Egorov, A.D.; Ivanov, R.; Karabelsky, A. Viral Vectors in Gene Replacement Therapy. Biochemistry 2023, 88, 2157–2178. [Google Scholar] [CrossRef]
- Ruggiero, R.; Balzano, N.; Nicoletti, M.M.; di Mauro, G.; Fraenza, F.; Campitiello, M.R.; Rossi, F.; Capuano, A. Real-World Safety Data of the Orphan Drug Onasemnogene Abeparvovec (Zolgensma()) for the SMA Rare Disease: A Pharmacovigilance Study Based on the EMA Adverse Event Reporting System. Pharmaceuticals 2024, 17, 394. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Hemophilia B and gene therapy: A new chapter with etranacogene dezaparvovec. Blood Adv. 2024, 8, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, C.M.; Goedeker, N.L.; Aqul, A.A.; Butterfield, R.J.; Connolly, A.M.; Crystal, R.G.; Godwin, K.E.; Hor, K.N.; Mathews, K.D.; Proud, C.M.; et al. Management of Select Adverse Events Following Delandistrogene Moxeparvovec Gene Therapy for Patients with Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2024, 11, 687–699. [Google Scholar] [CrossRef]
- Long, B.R.; Robinson, T.M.; Day, J.R.S.; Yu, H.; Lau, K.; Imtiaz, U.; Patton, K.S.; de Hart, G.; Henshaw, J.; Agarwal, S.; et al. Clinical immunogenicity outcomes from GENEr8-1, a phase 3 study of valoctocogene roxaparvovec, an AAV5-vectored gene therapy for hemophilia A. Mol. Ther. 2024, in press. [Google Scholar] [CrossRef]
- Fonck, C.; Su, C.; Arens, J.; Koziol, E.; Srimani, J.; Henshaw, J.; Van Tuyl, A.; Chandra, S.; Vettermann, C.; O’Neill, C.A. Lack of germline transmission in male mice following a single intravenous administration of AAV5-hFVIII-SQ gene therapy. Gene Ther. 2023, 30, 581–586. [Google Scholar] [CrossRef]
- Kaczmarek, R. Gene therapy—Are we ready now? Haemophilia 2022, 28 (Suppl. S4), 35–43. [Google Scholar] [CrossRef]
- Fox, T.A.; Booth, C. Improving access to gene therapy for rare diseases. Dis. Model. Mech. 2024, 17, dmm050623. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Petrini, C. A few ethical issues in translational research for gene and cell therapy. J. Transl. Med. 2019, 17, 395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.X.; Wang, S.M.; Bai, Y.H.; Luo, T.T.; Wang, J.Q.; Dai, C.Q.; Guo, B.L.; Luo, S.C.; Wang, D.H.; Yang, Y.L.; et al. Lentiviral Vectors and Adeno-Associated Virus Vectors: Useful Tools for Gene Transfer in Pain Research. Anat. Rec. 2018, 301, 825–836. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, J.; Xu, J.P.; Wang, J.; Yang, X. Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Mil. Med. Res. 2023, 10, 12. [Google Scholar] [CrossRef]
- Rahman, M.U.; Bilal, M.; Shah, J.A.; Kaushik, A.; Teissedre, P.L.; Kujawska, M. CRISPR-Cas9-Based Technology and Its Relevance to Gene Editing in Parkinson’s Disease. Pharmaceutics 2022, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
| Serotypes | Origin | Primary Receptor | Secondary Receptor | Natural Tropism | Clinical Trials |
|---|---|---|---|---|---|
| AAV1 | NHP | Sialic acid | AAVR | Muscle, CNS, heart, liver, lung | None |
| AAV2 | Human | HSPG | Integrin, FGFR, HGFR, LamR | Heart, CNS, liver, lung, retina | Pomoe disease, Parkinson’s disease, hemophilia |
| AAV3 | NHP | HSPG | LamR, FGFR, HGFR, AAVR | Liver | None |
| AAV4 | NHP | Sialic acid | Unknown | Retina, lungs, kidney | None |
| AAV5 | Human | Sialic acid | PDGFR, AAVR | Retina, CNS, liver | Hemophilia |
| AAV6 | Human | HSPG, sialic acid | EGFR, AAVR | Heart, liver, muscle, retina | Hemophilia, mucopolysaccharidosis |
| AAV7 | NHP | Unknown | Unknown | Liver | None |
| AAV8 | NHP | Unknown | LamR, AAVR | Muscle, heart, CNS, liver | Eye disease, hemophilia, myopathy |
| AAV9 | Human | Galactose | LamR, AAVR | Heart, CNS, liver | Muscular diseases, pompe disease, Danon disease |
| AAV10 | NHP | Unknown | Unknown | Muscle, myoblast tissue | None |
| AAV11 | NHP | Unknown | Unknown | Muscle, myoblast tissue | None |
| AAV12 | NHP | Unknown | Unknown | Salivary glands, muscle | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, Y.; Yashiro, R. Therapeutic Application and Structural Features of Adeno-Associated Virus Vector. Curr. Issues Mol. Biol. 2024, 46, 8464-8498. https://doi.org/10.3390/cimb46080499
Matsuzaka Y, Yashiro R. Therapeutic Application and Structural Features of Adeno-Associated Virus Vector. Current Issues in Molecular Biology. 2024; 46(8):8464-8498. https://doi.org/10.3390/cimb46080499
Chicago/Turabian StyleMatsuzaka, Yasunari, and Ryu Yashiro. 2024. "Therapeutic Application and Structural Features of Adeno-Associated Virus Vector" Current Issues in Molecular Biology 46, no. 8: 8464-8498. https://doi.org/10.3390/cimb46080499
APA StyleMatsuzaka, Y., & Yashiro, R. (2024). Therapeutic Application and Structural Features of Adeno-Associated Virus Vector. Current Issues in Molecular Biology, 46(8), 8464-8498. https://doi.org/10.3390/cimb46080499







