Genome-Wide Identification and Expression Analysis of the COL Gene Family in Hemerocallis citrina Baroni
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Collection
2.2. Identification, Distribution, and Physicochemical Analysis of H. citrina COL Genes
2.3. Gene Structure, Conserved Domain Analyses, and Three-Dimensional Models of the B-Box
2.4. Sequence Alignment, Phylogenetic Analysis, and Collinearity Analysis of HcCOLs from H. citrina and Other Plants
2.5. Analysis of Cis-Acting Elements
2.6. Expression Analysis by qRT-PCR
3. Results
3.1. Identification of COL Genes in H. citrina
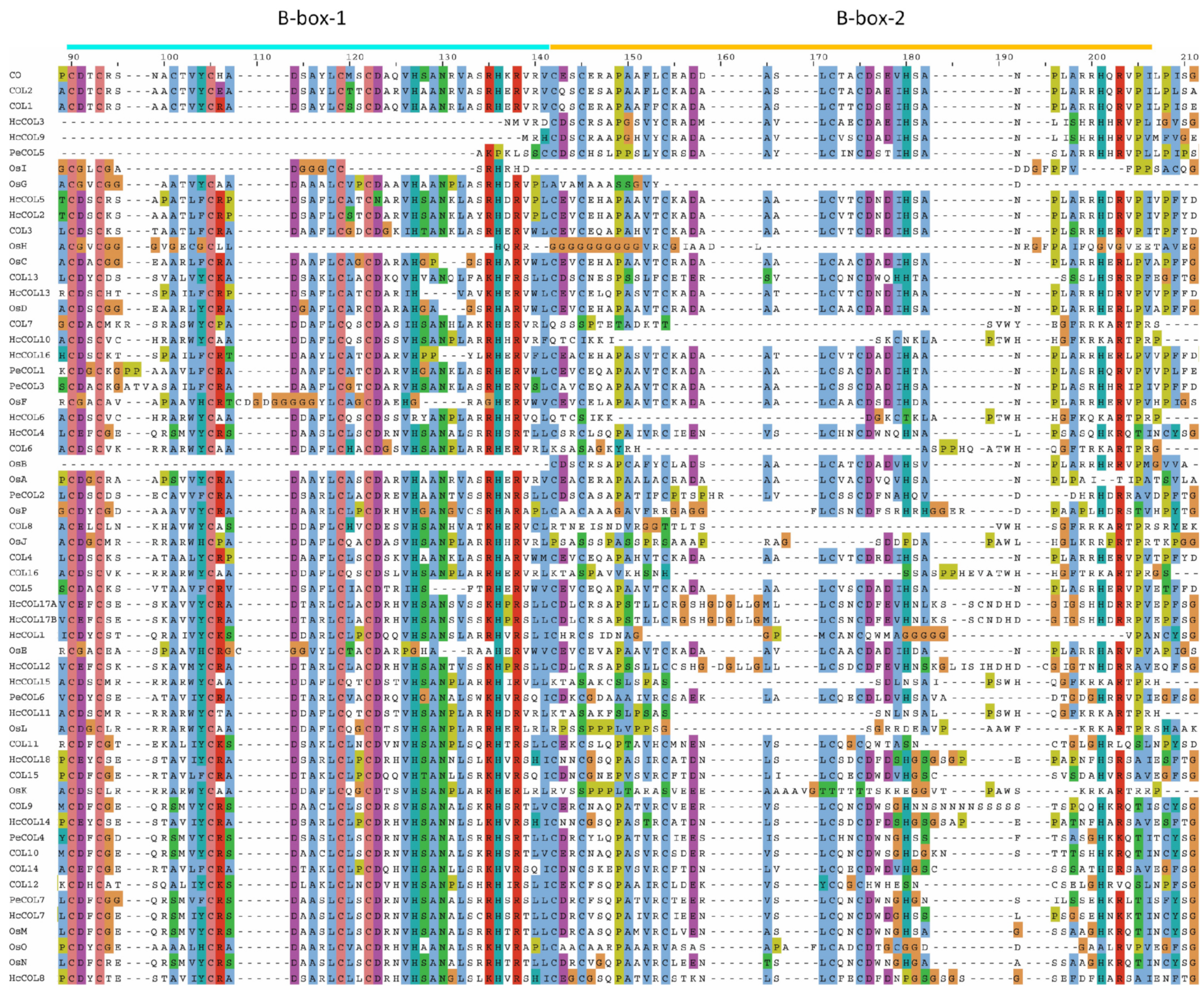
3.2. Gene Structure and Protein Conserved Domain Analyses
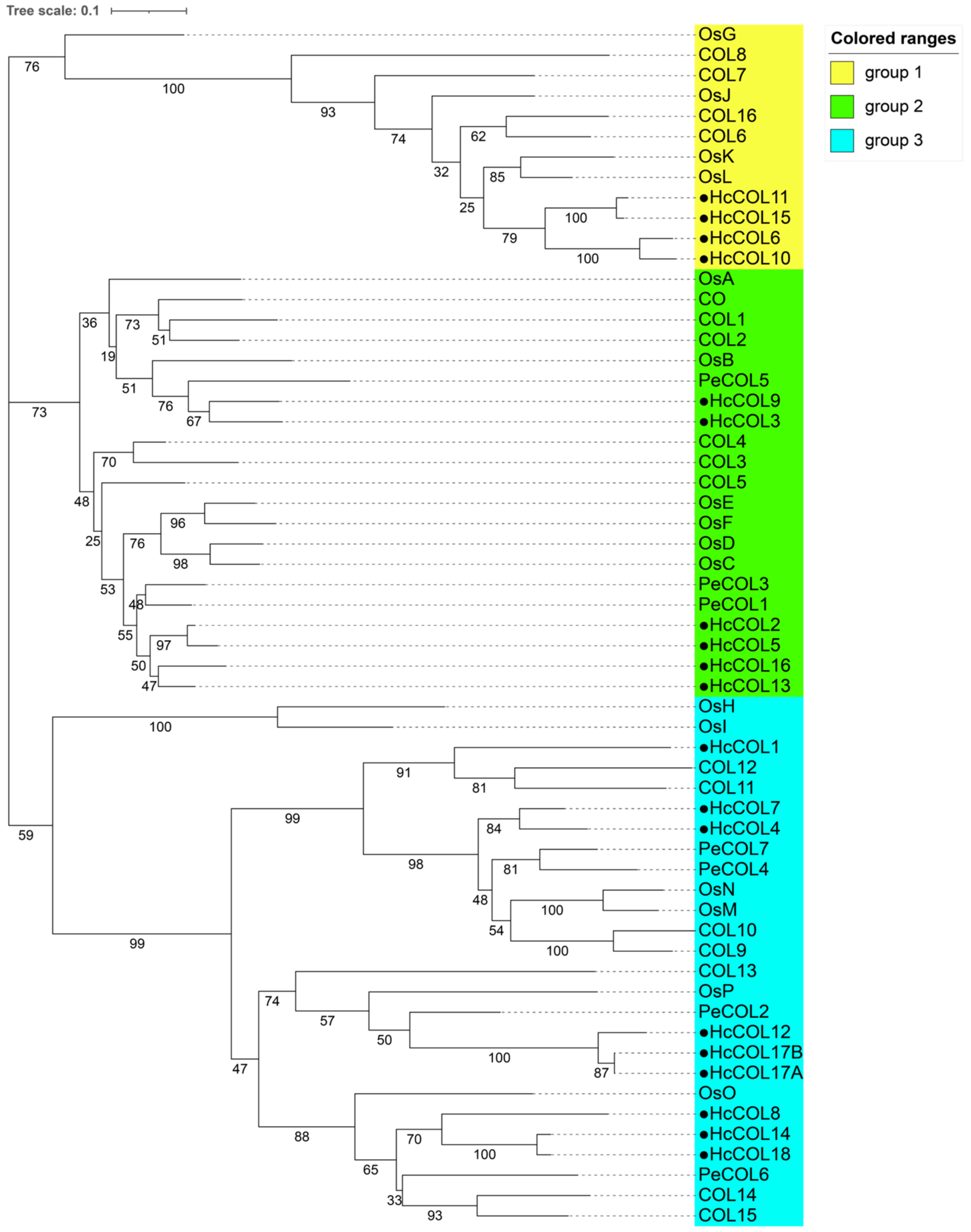
3.3. Sequence Alignment and Phylogenetic Analysis
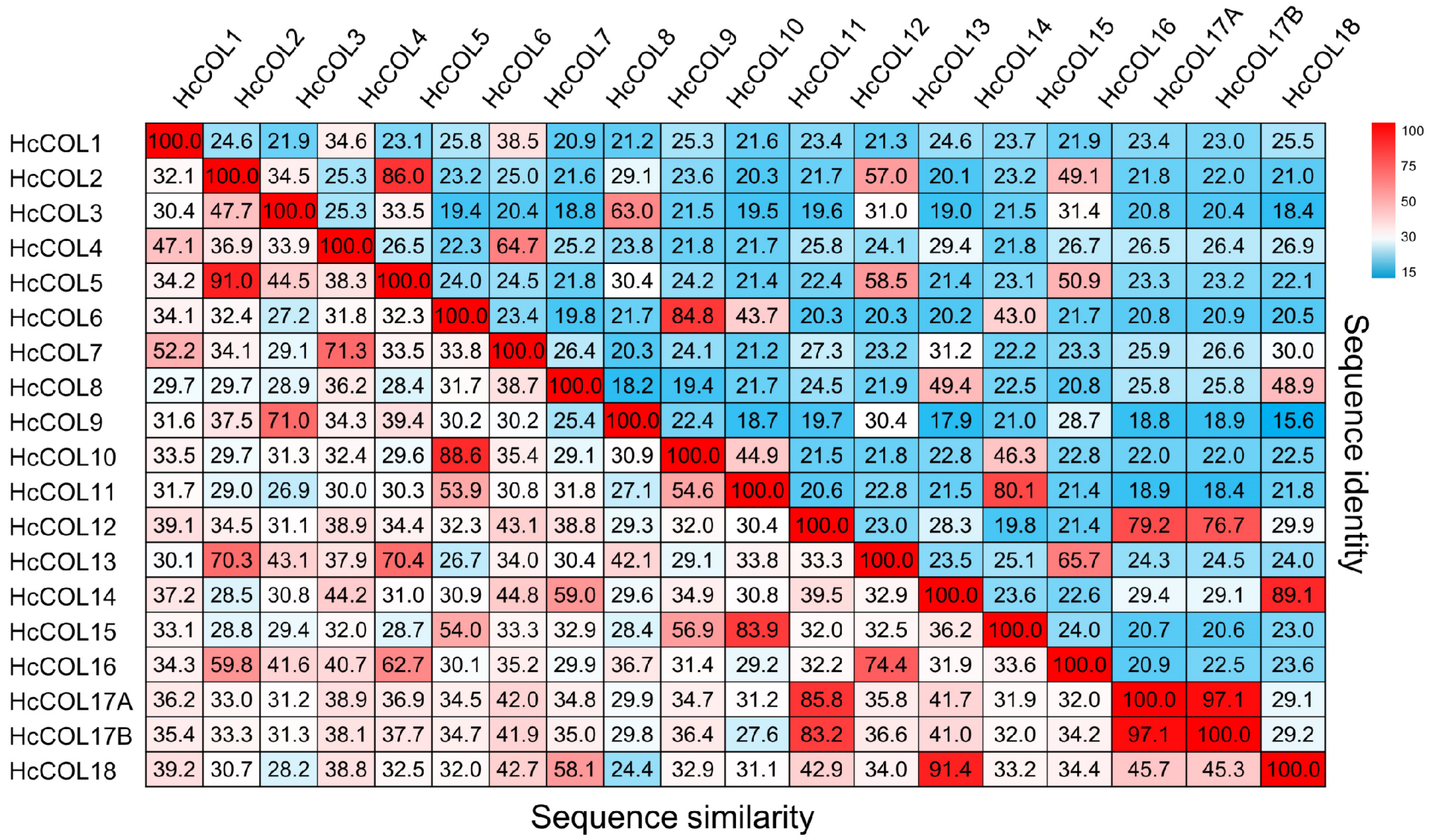
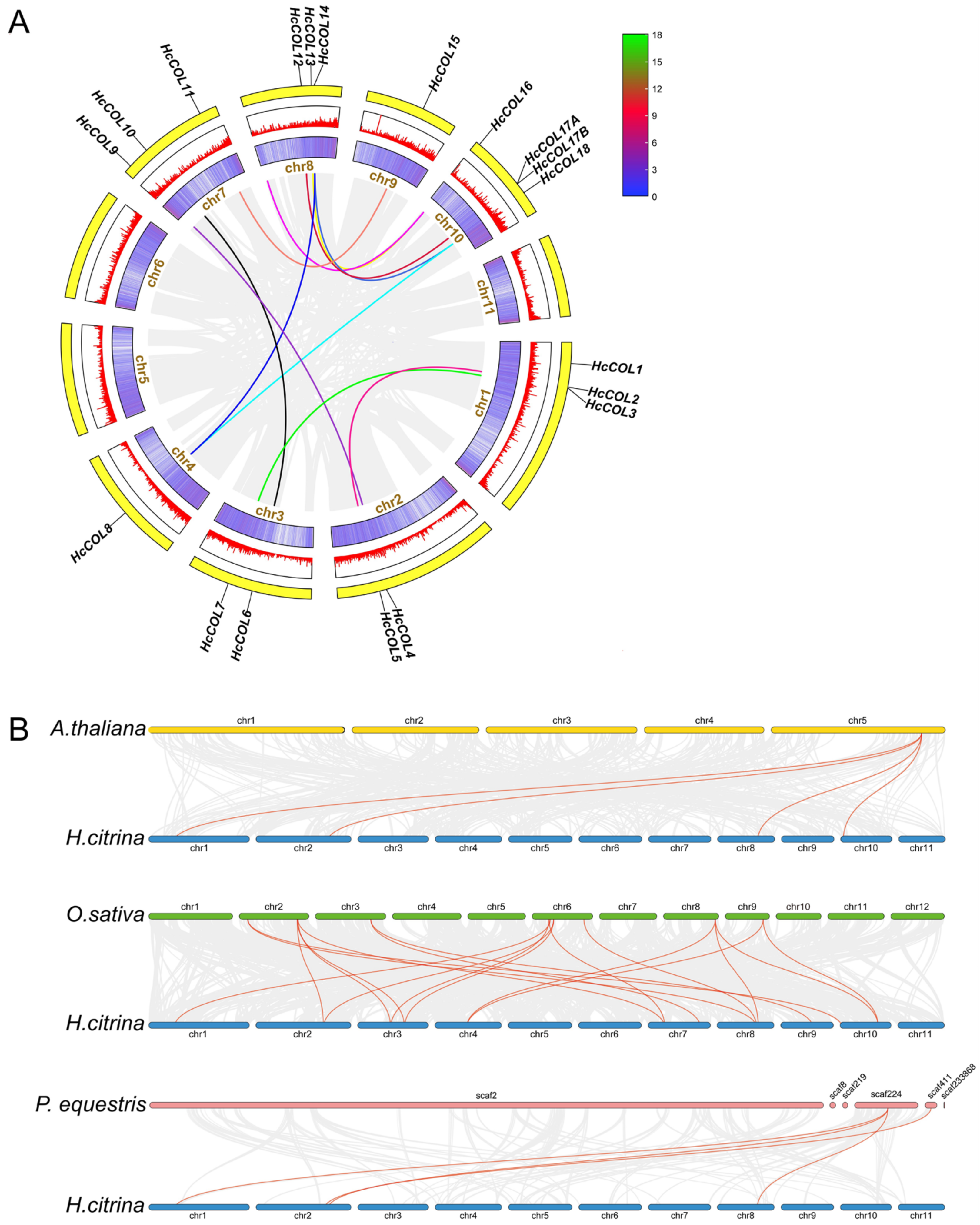
3.4. Collinearity Analysis within H. citrina and Among Different Species
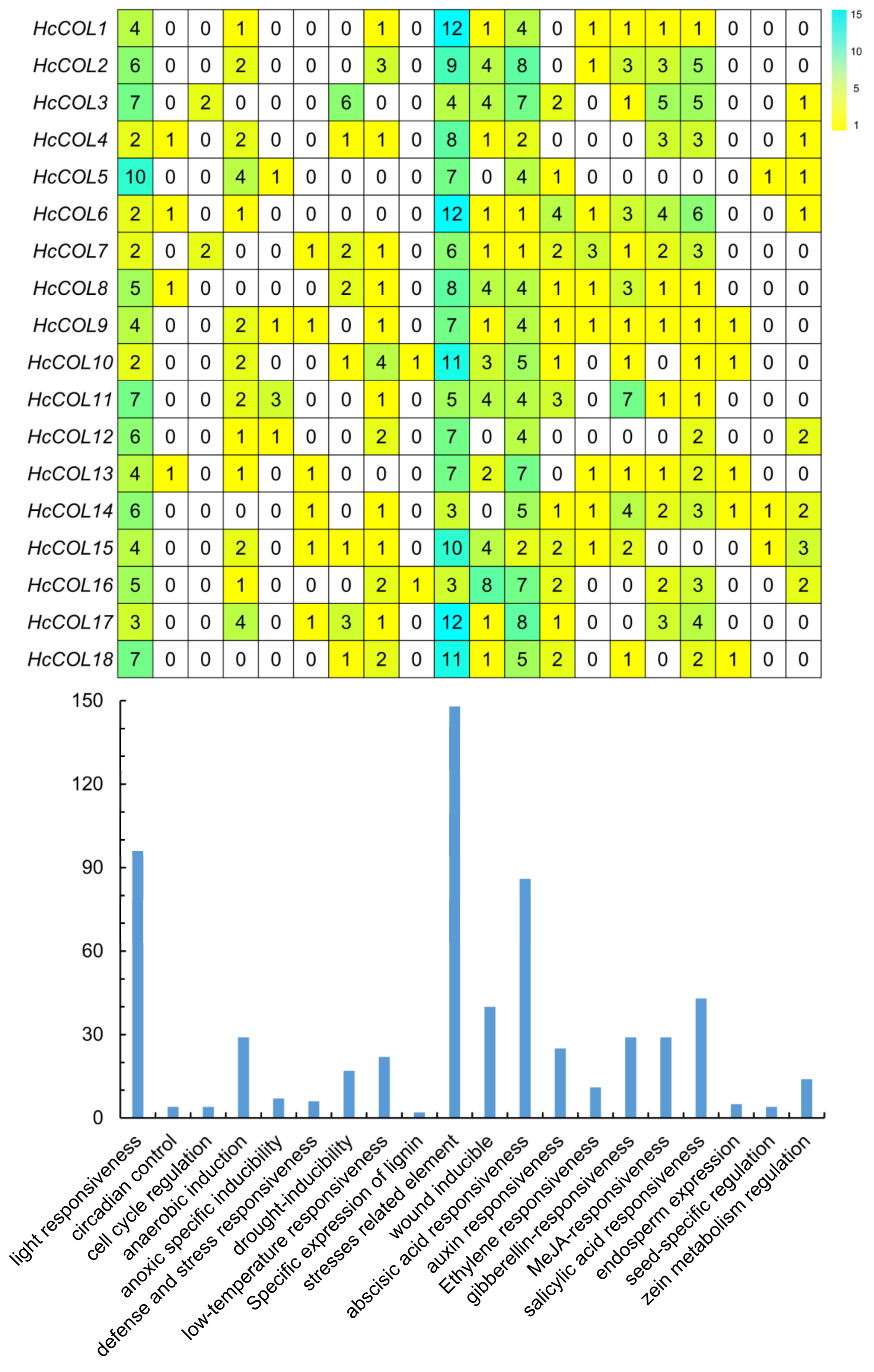
3.5. Putative Cis-Acting Element and Functional Annotation Analysis
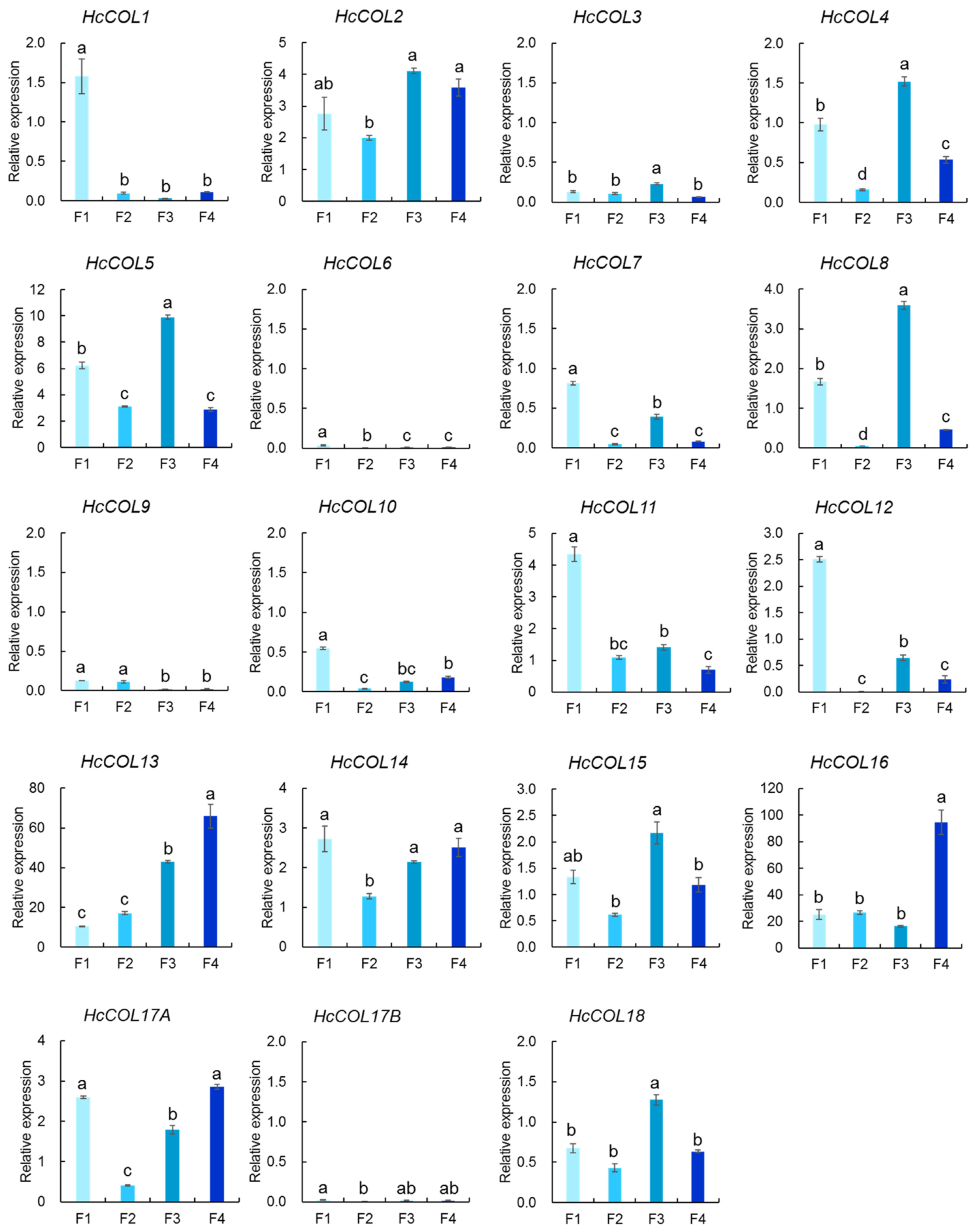
3.6. HcCOL Gene Expression Analysis of Four Phases of Flower Development in H. citrina
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550.e1–550.e2. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C.; et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rédei, G.P. Supervital Mutants of Arabidopsis. Genetics 1962, 47, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Hanhart, C.J.; van der Veen, J.H. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. MGG 1991, 229, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Robson, F.; Costa, M.M.; Hepworth, S.R.; Vizir, I.; Piñeiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Yoo, S.K.; Chung, K.S.; Kim, J.; Lee, J.H.; Hong, S.M.; Yoo, S.J.; Yoo, S.Y.; Lee, J.S.; Ahn, J.H. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar] [CrossRef]
- Zeng, X.; Lv, X.; Liu, R.; He, H.; Liang, S.; Chen, L.; Zhang, F.; Chen, L.; He, Y.; Du, J. Molecular basis of CONSTANS oligomerization in FLOWERING LOCUS T activation. J. Integr. Plant Biol. 2022, 64, 731–740. [Google Scholar] [CrossRef]
- Ledger, S.; Strayer, C.; Ashton, F.; Kay, S.A.; Putterill, J. Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J. 2001, 26, 15–22. [Google Scholar] [CrossRef]
- Hassidim, M.; Harir, Y.; Yakir, E.; Kron, I.; Green, R.M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009, 230, 481–491. [Google Scholar] [CrossRef]
- Takase, T.; Kakikubo, Y.; Nakasone, A.; Nishiyama, Y.; Yasuhara, M.; Yoko, T.O.; Kiyosue, T. Characterization and transgenic study of CONSTANS-LIKE8 (COL8) gene in Arabidopsis thaliana: Expression of 35S:COL8 delays flowering under long-day conditions. Plant Tissue Cult. Lett. 2012, 28, 439–446. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M.; Henschel, M.; Robers, L.; Kaufholdt, D.; Hänsch, R.; Adrian, J.; Ponnu, J.; Hoecker, U. The Transcription Factor COL12 Is a Substrate of the COP1/SPA E3 Ligase and Regulates Flowering Time and Plant Architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef]
- Steinbach, Y. The Arabidopsis thaliana CONSTANS-LIKE 4 (COL4)—A Modulator of Flowering Time. Front. Plant Sci. 2019, 10, 651. [Google Scholar] [CrossRef]
- Serrano-Bueno, G.; de Los Reyes, P.; Chini, A.; Ferreras-Garrucho, G.; Sánchez de Medina-Hernández, V.; Boter, M.; Solano, R.; Valverde, F. Regulation of floral senescence in Arabidopsis by coordinated action of CONSTANS and jasmonate signaling. Mol. Plant 2022, 15, 1710–1724. [Google Scholar] [CrossRef]
- Du, J.; Zhu, X.; He, K.; Kui, M.; Zhang, J.; Han, X.; Fu, Q.; Jiang, Y.; Hu, Y. CONSTANS interacts with and antagonizes ABF transcription factors during salt stress under long-day conditions. Plant Physiol. 2023, 193, 1675–1694. [Google Scholar] [CrossRef]
- Yu, B.; He, X.; Tang, Y.; Chen, Z.; Zhou, L.; Li, X.; Zhang, C.; Huang, X.; Yang, Y.; Zhang, W.; et al. Photoperiod controls plant seed size in a CONSTANS-dependent manner. Nat. Plants 2023, 9, 343–354. [Google Scholar] [CrossRef]
- Min, J.H.; Chung, J.S.; Lee, K.H.; Kim, C.S. The CONSTANS-like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 313–324. [Google Scholar] [CrossRef]
- Datta, S.; Hettiarachchi, G.H.; Deng, X.W.; Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006, 18, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Li, H.; Zhao, X.; Liu, X.; Ortiz, M.; Lin, C.; Liu, B. CONSTANS-LIKE 7 regulates branching and shade avoidance response in Arabidopsis. J. Exp. Bot. 2013, 64, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Long, H.; Yan, J.; Ye, L.; Zhang, Q.; Chen, H.; Gao, S.; Wang, Y.; Wang, X.; Sun, S. A HY5-COL3-COL13 regulatory chain for controlling hypocotyl elongation in Arabidopsis. Plant Cell Environ. 2021, 44, 130–142. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2484. [Google Scholar] [CrossRef]
- Zong, W.; Ren, D.; Huang, M.; Sun, K.; Feng, J.; Zhao, J.; Xiao, D.; Xie, W.; Liu, S.; Zhang, H.; et al. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol. 2021, 229, 1635–1649. [Google Scholar] [CrossRef]
- Kim, S.K.; Yun, C.H.; Lee, J.H.; Jang, Y.H.; Park, H.Y.; Kim, J.K. OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 2008, 228, 355–365. [Google Scholar] [CrossRef]
- Tan, J.; Wu, F.; Wan, J. Flowering time regulation by the CONSTANS-Like gene OsCOL10. Plant Signal Behav. 2017, 12, e1267893. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.; Xu, Y.; Li, X.; Xiao, J.; Xiong, L. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Zhang, M.; Zhan, X.; Shen, X.; Yu, P.; Chen, D.; Liu, Q.; Sinumporn, S.; Hussain, K.; et al. The rice CONSTANS-like protein OsCOL15 suppresses flowering by promoting Ghd7 and repressing RID1. Biochem. Biophys. Res. Commun. 2018, 495, 1349–1355. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, X.M.; Chen, D.; Zhang, Y.; Ma, W.; Zhang, H.; Sun, L.; Yang, Z.; Zhao, C.; Zhan, X.; et al. OsCOL16, encoding a CONSTANS-like protein, represses flowering by up-regulating Ghd7 expression in rice. Plant Sci. 2017, 260, 60–69. [Google Scholar] [CrossRef]
- Xu, C.; Shan, J.; Liu, T.; Wang, Q.; Ji, Y.; Zhang, Y.; Wang, M.; Xia, N.; Zhao, L. CONSTANS-LIKE 1a positively regulates salt and drought tolerance in soybean. Plant Physiol. 2023, 191, 2427–2446. [Google Scholar] [CrossRef]
- González-Schain, N.D.; Díaz-Mendoza, M.; Zurczak, M.; Suárez-López, P. Potato CONSTANS is involved in photoperiodic tuberization in a graft-transmissible manner. Plant J. 2012, 70, 678–690. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Liang, R.; Lan, M.; Yu, H.; Guo, Y.; Chen, S.; Lu, T.; Mo, X.; He, X. Genome-wide identification of the mango CONSTANS (CO) family and functional analysis of two MiCOL9 genes in transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 1028987. [Google Scholar] [CrossRef]
- Liang, R.Z.; Luo, C.; Liu, Y.; Hu, W.L.; Guo, Y.H.; Yu, H.X.; Lu, T.T.; Chen, S.Q.; Zhang, X.J.; He, X.H. Overexpression of two CONSTANS-like 2 (MiCOL2) genes from mango delays flowering and enhances tolerance to abiotic stress in transgenic Arabidopsis. Plant Sci. 2023, 327, 111541. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.Y.; Wang, J.N.; Kuang, J.F.; Shan, W.; Lu, W.J. Molecular characterization and expression profiles of MaCOL1, a CONSTANS-like gene in banana fruit. Gene 2012, 496, 110–117. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Z.; Li, X.; Xie, L.; Bai, Y.; Peng, L.; Li, J.; Gao, J. Expression Analysis and Regulation Network Identification of the CONSTANS-Like Gene Family in Moso Bamboo (Phyllostachys edulis) Under Photoperiod Treatments. DNA Cell Biol. 2019, 38, 607–626. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- An, H.; Roussot, C.; Suárez-López, P.; Corbesier, L.; Vincent, C.; Piñeiro, M.; Hepworth, S.; Mouradov, A.; Justin, S.; Turnbull, C.; et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 2004, 131, 3615–3626. [Google Scholar] [CrossRef]
- Ayre, B.G.; Turgeon, R. Graft transmission of a floral stimulant derived from CONSTANS. Plant Physiol. 2004, 135, 2271–2278. [Google Scholar] [CrossRef]
- Du, W.; Li, S.; Gong, F.F.; Liu, J.; Gao, Y.; Xiong, X.; Wang, J.Y.; Kang, X.P. Effects of light treatment on the flowering rhythm of Hemerocallis citrina cv. ‘DatongHuanghua’and the expression characteristics of photoperiod key gene HcCOL14. J. Agric. Univ. Hebei 2020, 43, 8. [Google Scholar] [CrossRef]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian Clock and Photoperiodic Flowering in Arabidopsis: CONSTANS Is a Hub for Signal Integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef]
- Qing, Z.; Liu, J.; Yi, X.; Liu, X.; Hu, G.; Lao, J.; He, W.; Yang, Z.; Zou, X.; Sun, M.; et al. The chromosome-level Hemerocallis citrina Borani genome provides new insights into the rutin biosynthesis and the lack of colchicine. Hortic. Res. 2021, 8, 89. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, J.; Liu, C.; Cai, C.; Zhou, B.; Zhang, T.; Guo, W. Molecular evolution and phylogenetic analysis of eight COL superfamily genes in group I related to photoperiodic regulation of flowering time in wild and domesticated cotton (Gossypium) species. PLoS ONE 2015, 10, e0118669. [Google Scholar] [CrossRef]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef]
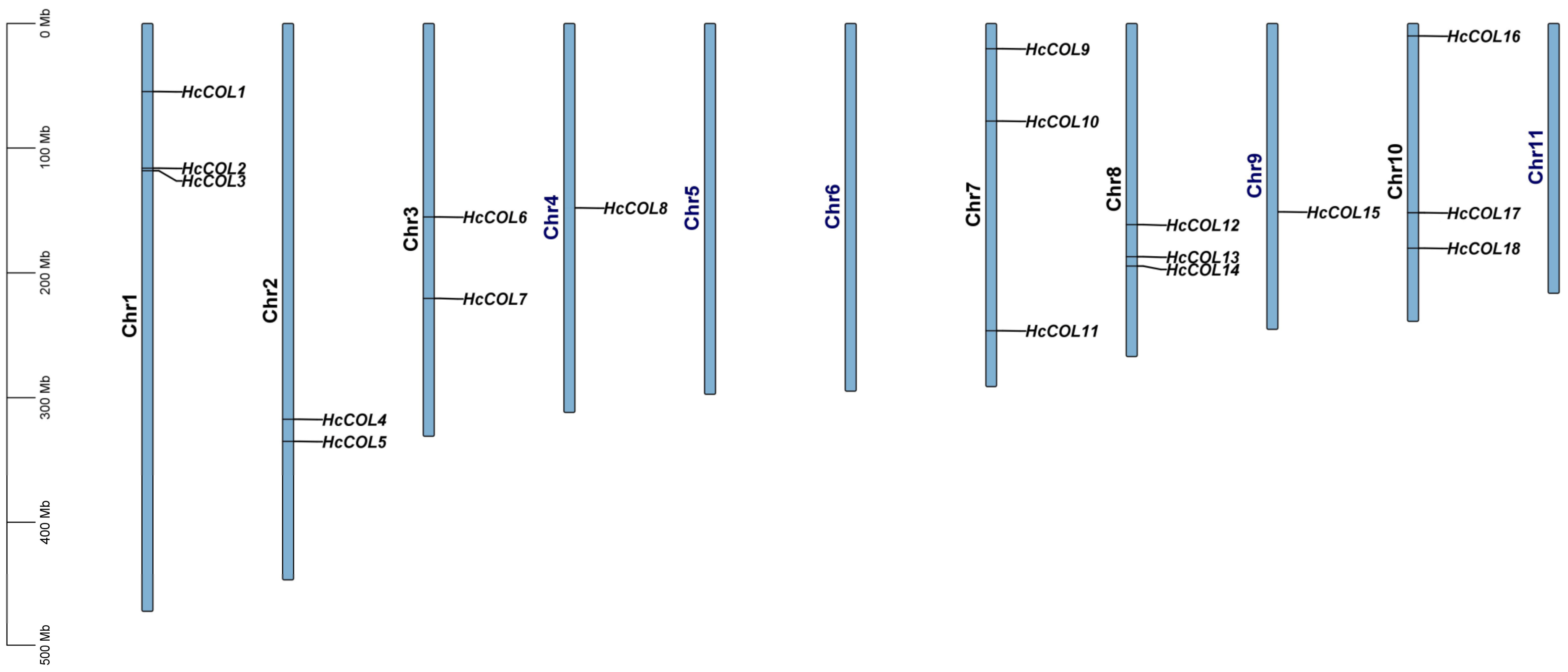



| Gene | Transcript ID | Length of ORF (bp) | Length of cDNA (bp) | AA | Chromosome Position | MW (kDa) | pI | Predicted Location |
|---|---|---|---|---|---|---|---|---|
| HcCOL1 | HHC001023.1 | 5639 | 1119 | 372 | Chr1 | 40,466.48 | 5.24 | Nucleus |
| HcCOL2 | HHC002209.1 | 1193 | 888 | 295 | Chr1 | 32,518.03 | 6.02 | Nucleus |
| HcCOL3 | HHC002254.1 | 1791 | 783 | 260 | Chr1 | 29,533.84 | 5.14 | Nucleus |
| HcCOL4 | HHC009483.1 | 16,688 | 1020 | 339 | Chr2 | 37,039.32 | 8.87 | Nucleus |
| HcCOL5 | HHC009754.1 | 1282 | 900 | 299 | Chr2 | 32,795.27 | 6.33 | Nucleus |
| HcCOL6 | HHC012875.1 | 1405 | 1152 | 383 | Chr3 | 43,535.87 | 5.77 | Nucleus |
| HcCOL7 | HHC013899.1 | 18,306 | 1242 | 413 | Chr3 | 45,350.19 | 5.07 | Nucleus |
| HcCOL8 | HHC019369.2 | 14,562 | 1545 | 514 | Chr4 | 57,358.38 | 7.02 | Nucleus |
| HcCOL9 | HHC030027.1 | 1040 | 735 | 244 | Chr7 | 27,408.75 | 6.00 | Nucleus |
| HcCOL10 | HHC030937.1 | 1520 | 1152 | 383 | Chr7 | 43,026.35 | 5.67 | Nucleus |
| HcCOL11 | HHC032260.1 | 2090 | 1341 | 446 | Chr7 | 49,662.55 | 5.00 | Nucleus |
| HcCOL12 | HHC034570.1 | 2966 | 1260 | 419 | Chr8 | 47,005.43 | 4.85 | Nucleus |
| HcCOL13 | HHC034944.1 | 1383 | 939 | 312 | Chr8 | 34,296.95 | 5.84 | Nucleus |
| HcCOL14 | HHC035073.1 | 7230 | 1290 | 429 | Chr8 | 46,963.08 | 5.58 | Nucleus |
| HcCOL15 | HHC038535.1 | 2279 | 1266 | 421 | Chr9 | 46,404.69 | 5.07 | Nucleus |
| HcCOL16 | HHC040621.1 | 1391 | 1005 | 334 | Chr10 | 36,251.84 | 7.98 | Nucleus |
| HcCOL17a | HHC041944.1 | 2697 | 1215 | 404 | Chr10 | 44,434.74 | 5.47 | Nucleus |
| HcCOL17b | HHC041944.2 | 2697 | 1209 | 402 | Chr10 | 44,224.5 | 5.40 | Nucleus |
| HcCOL18 | HHC042453.1 | 5721 | 1269 | 422 | Chr10 | 46,607.94 | 5.32 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Z.; Ma, G.; Xie, L.; Yao, X.; Zhan, S.; Zhou, Y. Genome-Wide Identification and Expression Analysis of the COL Gene Family in Hemerocallis citrina Baroni. Curr. Issues Mol. Biol. 2024, 46, 8550-8566. https://doi.org/10.3390/cimb46080503
Zuo Z, Ma G, Xie L, Yao X, Zhan S, Zhou Y. Genome-Wide Identification and Expression Analysis of the COL Gene Family in Hemerocallis citrina Baroni. Current Issues in Molecular Biology. 2024; 46(8):8550-8566. https://doi.org/10.3390/cimb46080503
Chicago/Turabian StyleZuo, Ziwei, Guangying Ma, Lupeng Xie, Xingda Yao, Shuxia Zhan, and Yuan Zhou. 2024. "Genome-Wide Identification and Expression Analysis of the COL Gene Family in Hemerocallis citrina Baroni" Current Issues in Molecular Biology 46, no. 8: 8550-8566. https://doi.org/10.3390/cimb46080503
APA StyleZuo, Z., Ma, G., Xie, L., Yao, X., Zhan, S., & Zhou, Y. (2024). Genome-Wide Identification and Expression Analysis of the COL Gene Family in Hemerocallis citrina Baroni. Current Issues in Molecular Biology, 46(8), 8550-8566. https://doi.org/10.3390/cimb46080503






