Immunomodulatory Effects of Anadenanthera colubrina Bark Extract in Experimental Autoimmune Encephalomyelitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Animals and Ethical Aspects
2.3. Induction of Experimental Autoimmune Encephalomyelitis
2.4. Treatment with Ethanolic Extract of Anadenanthera colubrina (EtAc) and Clinical Assessment
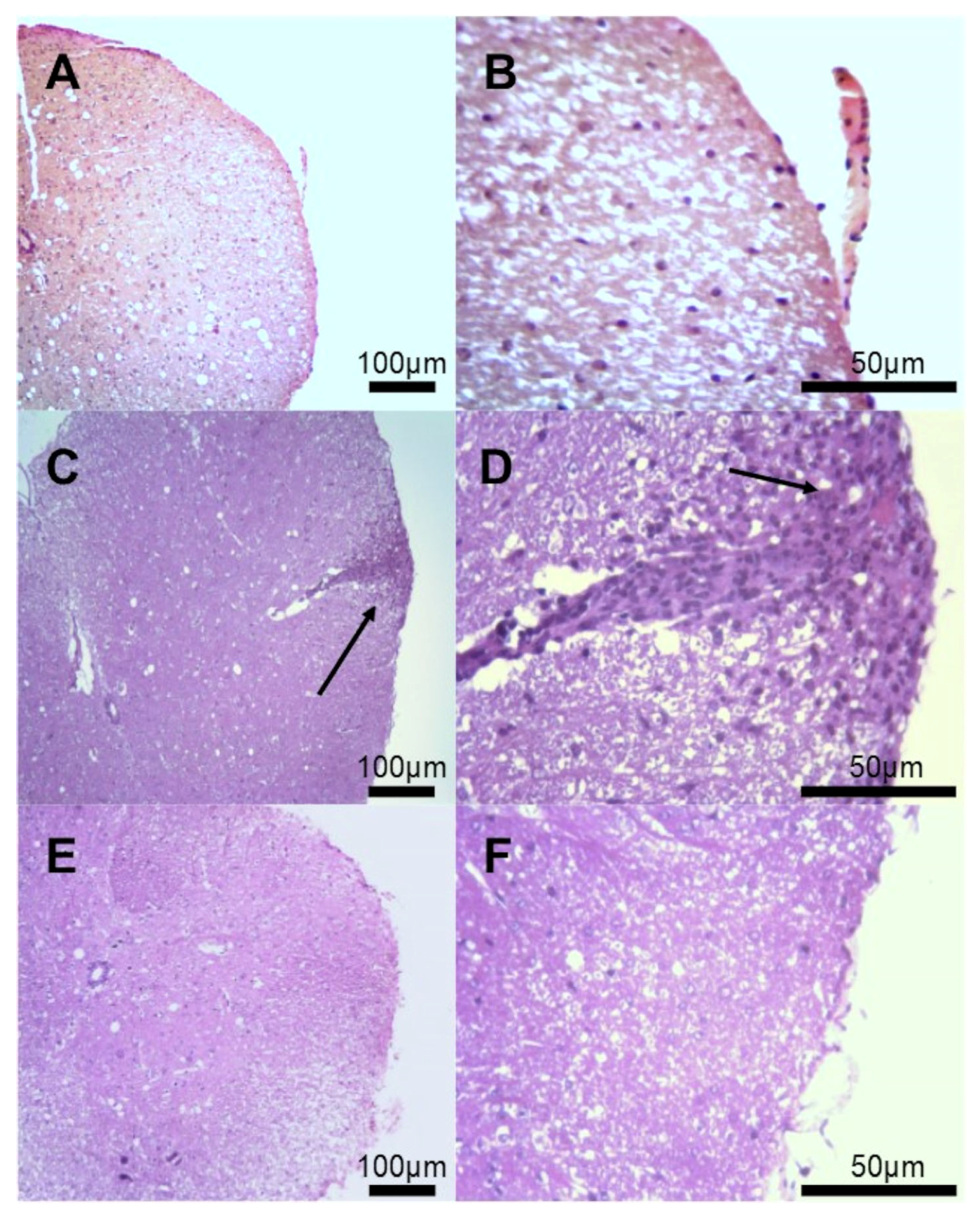
2.5. Histopathological Analysis
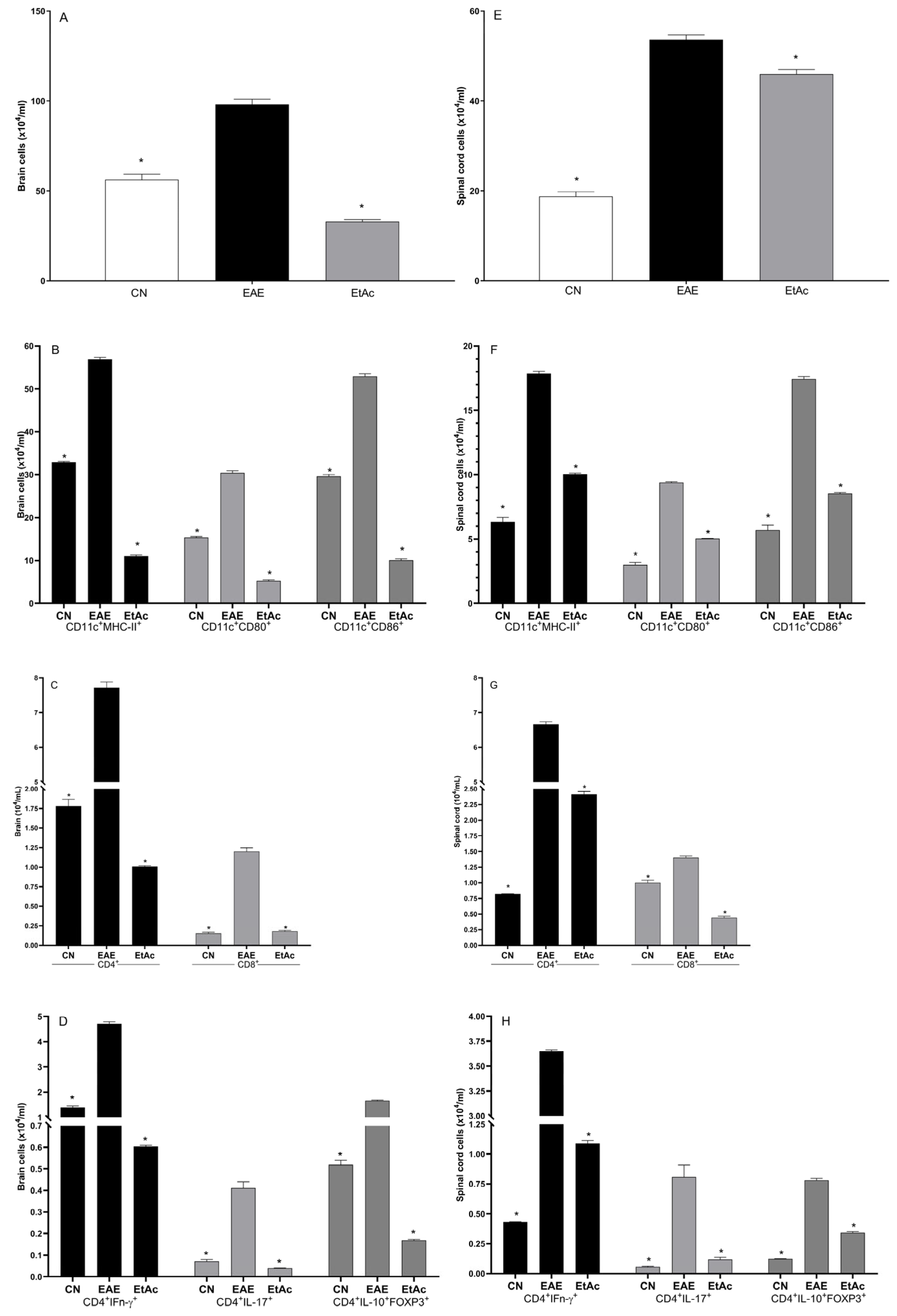
2.6. Brain and Spinal Cord Cells Isolation and Evaluation of the Cellular Profile
2.7. Cytokine Content
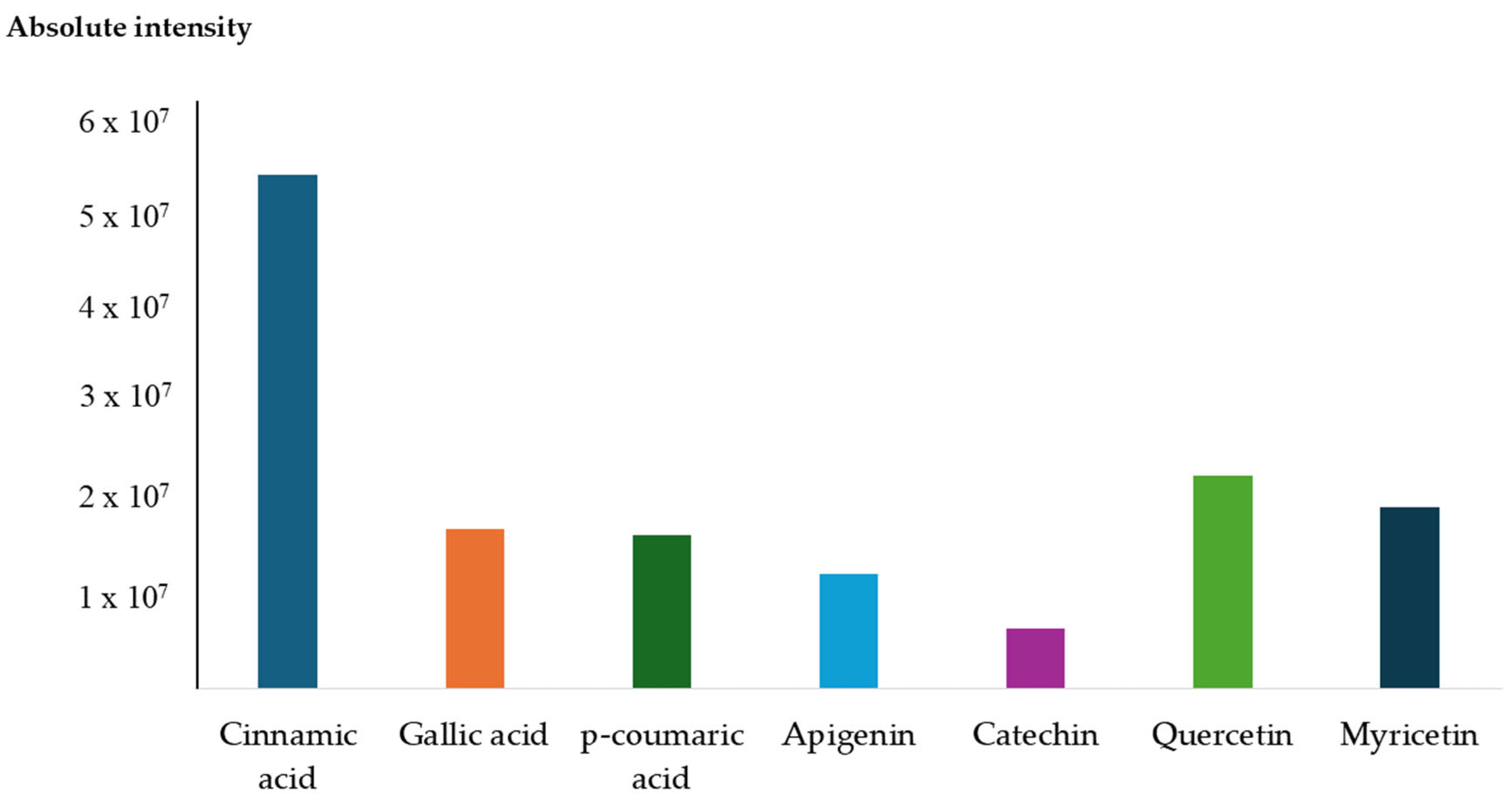
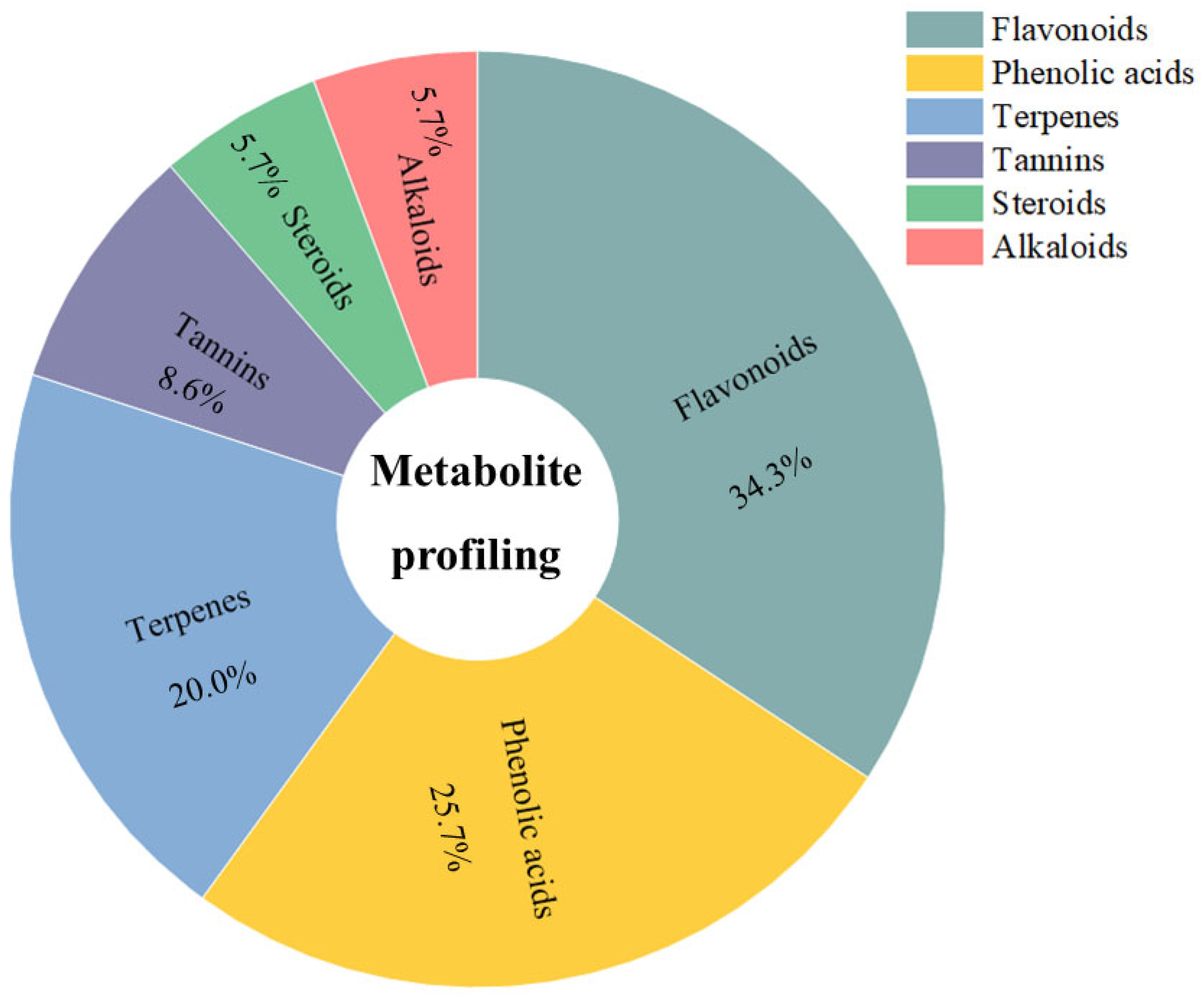
2.8. Compound Profiling
2.9. Statistical Analysis
3. Results and Discussion
3.1. EtAc Improves the Clinical Signs of Experimental Autoimmune Encephalomyelitis (EAE)
3.2. Histological Evaluation of Inflammatory Infiltrates
3.3. Histological Evaluation of Demyelination
3.4. Expression of Cellular Markers in the Central Nervous System
3.5. Cytokine Analyses
3.6. Compound Profiling of Anadenanthera colubrina Bark Ethanolic Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez, C.A.; Cuascut, F.X.; Hutton, G.J. Immunopathogenesis, diagnosis, and treatment of multiple sclerosis: A clinical update. Neurol. Clin. 2023, 41, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, C.; Pugliatti, M.; Vermersch, P.; Grigoriadis, N.; Alkhawajah, M.; Airas, L.; Oreja-Guevara, C. Diagnosis and treatment of progressive multiple sclerosis: A position paper. Eur. J. Neurol. 2023, 30, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; A Cohen, J.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Rosalen, P.L.; Freires, I.A.; Sardi, J.C.O.; Lima, R.F.; Lazarini, J.G.; Costa, T.K.V.L.D.; Pereira, J.V.; Godoy, G.P.; Costa, E.M.M.B. Anadenanthera Colubrina vell Brenan: Anti-Candida and antibiofilm activities, toxicity and therapeutical action. Braz. Oral Res. 2019, 33, e023. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Protocolo Clínico e Diretrizes Terapêuticas da Esclerose Múltipla. 2021. Available online: https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2022/portal_portaria-conjunta-no-1-pcdt_esclerose-multipla.pdf (accessed on 12 March 2023).
- Çakan, M.; Çakan, E.; Gümüş, Y.; Özen, P.A.; Tuncer, A.; Karabudak, R. Investigating the Side Effects of Treatments Among Different Age and Gender Groups of Multiple Sclerosis Patients (P2-6.015). Neurology 2024, 102, 6641. [Google Scholar] [CrossRef]
- Yuan, J.; Tao, Y.; Wang, M.; Huang, F.; Wu, X. Natural compounds as potential therapeutic candidates for multiple sclerosis: Emerging preclinical evidence. Phytomedicine 2023, 123, 155248. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, U.P.; De Medeiros, P.M.; De Almeida, A.L.S.; Monteiro, J.M.; Neto, E.M.D.F.L.; de Melo, J.G.; Dos Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Santos, F.R.; Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; Martins, D.T.O. Ethnopharmacology of medicinal plants of the Pantanal region (Mato Grosso, Brazil). Evid. Based Complement. Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef]
- Lewis, G.; Schrire, B.; MacKinder, B.; Lock, M. Legumes of the World, 1st ed.; Kew Pub: Richmond, UK, 2005. [Google Scholar]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 4th ed.; Instituto Plantarium: Nova Odessa, Brazil, 2002. [Google Scholar]
- Torres, C.M.; Repke, D.B. Anadenanthera. Visionary Plant of Ancient South America, 1st ed.; The Haworth Press: Binghamton, NY, USA, 2006. [Google Scholar]
- Magalhães, P.S.C.; Teixeira, M.C.S.A.; Mendes, M.R.A.; Lemos, J.R.; Rodrigues, B.J.S. Fruit and seed morphometry and methods for overcoming sleep of Anadenanthera colubrina (Vell.) Brenan (Fabaceae). Res. Soc. Dev. 2021, 10, e6010313034. [Google Scholar] [CrossRef]
- de Viana, M.L.; Giamminola, E.; Russo, R.; Ciaccio, M. Morphology and genetics of Anadenanthera colubrina var. cebil (Fabaceae) tree from Salta (Northwestern Argentina). Rev. Biol. Trop. 2014, 62, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; de Almeida, C.D.F.C.R.; de Albuquerque, U.P.; de Lucena, R.F.; Florentino, A.T.; de Oliveira, R.L. Use and traditional management of Anadenanthera colubrina (Vell.) Brenan in the semi-arid region of northeastern Brazil. J. Ethnobiol. Ethnomed. 2006, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, U.P.; de Oliveira, R.F. Is the use-impact on native caatinga species in Brazil reduced by the high species richness of medicinal plants? J. Ethnopharmacol. 2007, 113, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Silva, B. Estudo etnobotânico de plantas medicinais da mata ciliar do submédio São Francisco, NORDESTE DO BRASIL. Rev. Ouricuri 2020, 10, 11–26. [Google Scholar] [CrossRef]
- Pereira Sousa, E.A.; Mendonça, A.C.A.M.; Garcia, I.R.; Nobre Lisboa, M.A.; Kamdem, J.P.; Cruz, G.V.; Silva, M.A.P.; Fernandes, G.P.; Calixto Júnior, J.T. Ethnoknowledge of medicinal and mystical plants used by healers in Juazeiro do Norte, Ceara, Northeast Brazil. Indian J. Tradit. Knowl. 2021, 20, 154–166. [Google Scholar] [CrossRef]
- Delices, M.; Muller, J.A.I.; Arunachalam, K.; Martins, D.T.O. Anadenanthera colubrina (Vell) Brenan: Ethnobotanical, phytochemical, pharmacological and toxicological aspects. J. Ethnopharmacol. 2023, 300, 115745. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, D.R.C.; da Silva, L.C.N.; da Silva, A.G.; de Araújo, J.M.; Machado, A.J.; Correia, M.T.S.; da Silva, M.V. Comparative analysis of anti-Staphylococcus aureus action of leaves and fruits of Anadenanthera colubrina var. cebil (Griseb.) Altschul. Afr. J. Microbiol. Res. 2014, 8, 2690–2696. [Google Scholar] [CrossRef]
- Lima, R.F.; Alves, E.P.; Rosalen, P.L.; Ruiz, A.L.; Teixeira Duarte, M.C.; Góes, V.F.; de Medeiros, A.C.; Pereira, J.V.; Godoy, G.P.; Melo de Brito Costa, E.M. Antimicrobial and Antiproliferative Potential of Anadenanthera colubrina (Vell.) Brenan. Evid. Based Complement. Altern. Med. 2014, 2014, 802696. [Google Scholar] [CrossRef]
- Guarneire, G.J.; Cardoso Junior, O.; Lima, N.M.; Santos, E.A.; Schulze, C.A.; Silva, W.P.; Pedro Oliveira Batista, J.; de Paula Carli, G.; Castro, S.B.; Alves, C.C.S.; et al. Effect of Anadenanthera colubrina protease inhibitors as an anti-inflammatory mediator. Nat. Prod. Res. 2021, 35, 1690–1695. [Google Scholar] [CrossRef]
- Junior, O.C.; Lima, N.M.; Silva, M.G.A.; Aguiar, V.B.; Carli, G.P.; Scherrer, E.C.; Castro, S.B.R.; Alves, C.C.S.; Oliveira, M.A.L.; Carli, A.P. In vitro and in vivo evaluation of anti-inflammatory activity and free radical scavenging potential of leaves extract from Anadenanthera colubrina. Nat. Prod. Res. 2021, 35, 4819–4823. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.A.; Pasetto, S.; Silva, J.P.R.E.; Tavares, J.F.; Costa, E.M.M.B.; Murata, R.M. Anandenanthera colubrina (Vell.) Brenan as an inhibitor of HIV-1 BaL infection. Nat. Prod. Res. 2022, 36, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Mota, G.S.; Sartori, C.J.; Miranda, I.; Quilhó, T.; Mori, F.A.; Pereira, H. Bark anatomy, chemical composition and ethanol-water extract composition of Anadenanthera peregrina and Anadenanthera colubrina. PLoS ONE 2017, 12, e0189263. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.A.; Pasetto, S.; Nonaka, C.F.W.; Costa, E.M.M.B.; Murata, R.M. Yeast-Host Interactions: Anadenanthera colubrina Modulates Virulence Factors of C. albicans and Inflammatory Response in vitro. Front. Pharmacol. 2021, 12, 629778. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Marinho, R.R.; Ekundi-Valentim, E.; Rodrigues, L.; Yamamoto, M.H.; Teixeira, S.A.; Muscara, M.N.; Costa, S.K.; Thomazzi, S.M. Beneficial effects of Anadenanthera colubrina (Vell.) Brenan extract on the inflammatory and nociceptive responses in rodent models. J. Ethnopharmacol. 2013, 148, 218–222. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (-)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Miljković, D.; Dekanski, D.; Miljković, Ž.; Momčilović, M.; Mostarica-Stojkovic, M. Dry olive leaf extract ameliorates experimental autoimmune encephalomyelitis. Clin. Nutr. 2009, 28, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Linington, C.; Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006, 129, 1953–1971. [Google Scholar] [CrossRef]
- Becher, B.; Bechmann, I.; Greter, M. Antigen presentation in autoimmunity and CNS inflammation: How T lymphocytes recognize the brain. J. Mol. Med. 2006, 84, 532–543. [Google Scholar] [CrossRef]
- De Paula, M.L.; Rodrigues, D.H.; Teixeira, H.C.; Barsante, M.M.; Souza, M.A.; Ferreira, A.P. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008, 8, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.F.; Lima, N.M.; Lima, G.S.; Roque, J.V.; Preet, G.; Oppong-Danquah, E.; Andrade, T.J.A.S.; Jaspars, M.; Vaz, B.G. Mass spectrometry-based untargeted metabolomics approaches for comprehensive structural annotation of bioactive metabolites from bushy cashew (Anacardium humile) fruits. S. Afr. J. Bot. 2023, 163, 121–134. [Google Scholar] [CrossRef]
- Piao, W.H.; Wong, R.; Bai, X.F.; Huang, J.; Campagnolo, D.I.; Dorr, R.T.; Vollmer, T.L.; Shi, F.D. Therapeutic effect of anthracene-based anticancer agent ethonafide in an animal model of multiple sclerosis. J. Immunol. 2007, 179, 7415–7423. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, R.; van Tol, E.A.; van Noort, J.M. Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem. Pharmacol. 2005, 70, 220–228. [Google Scholar] [CrossRef]
- Yin, L.L.; Lin, L.L.; Zhang, L.; Li, L. Epimedium flavonoids ameliorate experimental autoimmune encephalomyelitis in rats by modulating neuroinflammatory and neurotrophic responses. Neuropharmacology 2012, 63, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Y.; Niu, X.; Tang, H.; Meydani, S.N.; Wu, D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2018, 54, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Zhang, Y.; Gao, Y.H.; Deng, S.Y.; Wang, L.M.; Li, C.Q.; Li, X. Role of Plant-Derived Natural Compounds in Experimental Autoimmune Encephalomyelitis: A Review of the Treatment Potential and Development Strategy. Front. Pharmacol. 2021, 12, 639651. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Li, S.; Panny, L.; Lin, C.C.; Lin, S.C. Galangin ameliorates experimental autoimmune encephalomyelitis in mice via modulation of cellular immunity. J. Immunotoxicol. 2021, 18, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, X.; Xing, F.; Wu, H.; Shi, H.; Huang, F.; Xu, Q.; Wu, X. Total flavonoids of astragalus attenuates experimental autoimmune encephalomyelitis by suppressing the activation and inflammatory responses of microglia via JNK/AKT/NFκB signaling pathway. Phytomedicine 2021, 80, 153385. [Google Scholar] [CrossRef]
- Marik, C.; Felts, P.A.; Bauer, J.; Lassmann, H.; Smith, K.J. Lesion genesis in a subset of patients with multiple sclerosis: A role for innate immunity? Brain 2007, 130, 2800–2815. [Google Scholar] [CrossRef]
- Henderson, A.P.; Barnett, M.H.; Parratt, J.D.; Prineas, J.W. Multiple sclerosis: Distribution of inflammatory cells in newly forming lesions. Ann. Neurol. 2009, 66, 739–753. [Google Scholar] [CrossRef]
- Dong, Y.; Yong, V.W. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat. Rev. Neurol. 2019, 15, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Løbner, M.; Cédile, O.; Owens, T. Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflammation 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- Giovanni, F.; Domenico, P.; Alessandro, M.; Raffaele, I.; Viviana, N.; Katia, P.A.; Paola, B.A. Circulating CD8+CD56-perforin+ T cells are increased in multiple sclerosis patients. J. Neuroimmunol. 2021, 240, 137–141. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, G. Chemokines and chemokine receptors in multiple sclerosis. Mediat. Inflamm. 2014, Volume 1, 659206. [Google Scholar] [CrossRef]
- Miller, C.H.; Maher, S.G.; Young, H.A. Clinical Use of Interferon-gamma. Ann. N. Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef]
- Liblau, R.S.; Singer, S.M.; McDevitt, H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 1995, 16, 34–38. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. Molnetenhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed]


| Cytokines | Groups Mean ± Standard Error | p * Value | ||
|---|---|---|---|---|
| CN | EAE | EtAc | ||
| IL-1β | 1410 ± 60.55 | 1691± 27.05 | 2238± 67.89 | 0.02 |
| IL-6 | 667.3 ± 9.10 | 691.5 ± 7.05 | 868 ± 55.16 | 0.016 |
| IL-12p70 | 2397 ± 39.84 | 1543 ± 194.2 | 698.1 ± 45.95 | 0.005 |
| IFN-γ | 62.45 ± 6.84 | 91.75 ± 6.75 | 192.40 ± 11.68 | 0.028 |
| TNF | 484.0 ± 43.46 | 636.8 ± 17.55 | 435.5 ± 45.87 | 0.003 |
| IL-10 | 3788 ± 158.5 | 4143 ± 194.8 | 5554 ± 319.4 | 0.004 |
| Cytokines | Groups Mean ± Standard Error | p * Value | ||
|---|---|---|---|---|
| CN | EAE | EtAc | ||
| IL-1β | 1290 ± 46.95 | 1685± 69.05 | 1021± 36.78 | 0.008 |
| IL-6 | 822.5 ± 35.57 | 1354 ± 160.5 | 805.4 ± 149.5 | 0.056 |
| IL-12p70 | 3557 ± 212.9 | 4403 ± 497.7 | 2766 ± 775.4 | 0.191 |
| IFN-γ | 158.5 ± 10.70 | 232.5 ± 25.41 | 101.1 ± 3.59 | 0.007 |
| TNF | 1960 ± 20.57 | 2076 ± 67.64 | 1417 ± 83.48 | 0.008 |
| IL-10 | 748.6 ± 74.69 | 300.6 ± 7.21 | 118.8 ± 24.60 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, K.A.; Soares, I.G.M.; Oliveira, L.M.A.; Braga, M.A.; Soares, P.P.C.; Guarneire, G.J.; Scherrer, E.C.; Silva, F.S.; Lima, N.M.; La Porta, F.A.; et al. Immunomodulatory Effects of Anadenanthera colubrina Bark Extract in Experimental Autoimmune Encephalomyelitis. Curr. Issues Mol. Biol. 2024, 46, 8726-8740. https://doi.org/10.3390/cimb46080515
Ramos KA, Soares IGM, Oliveira LMA, Braga MA, Soares PPC, Guarneire GJ, Scherrer EC, Silva FS, Lima NM, La Porta FA, et al. Immunomodulatory Effects of Anadenanthera colubrina Bark Extract in Experimental Autoimmune Encephalomyelitis. Current Issues in Molecular Biology. 2024; 46(8):8726-8740. https://doi.org/10.3390/cimb46080515
Chicago/Turabian StyleRamos, Karla A., Igor G. M. Soares, Larissa M. A. Oliveira, Mariana A. Braga, Pietra P. C. Soares, Gracimerio J. Guarneire, Elaine C. Scherrer, Fernando S. Silva, Nerilson M. Lima, Felipe A. La Porta, and et al. 2024. "Immunomodulatory Effects of Anadenanthera colubrina Bark Extract in Experimental Autoimmune Encephalomyelitis" Current Issues in Molecular Biology 46, no. 8: 8726-8740. https://doi.org/10.3390/cimb46080515
APA StyleRamos, K. A., Soares, I. G. M., Oliveira, L. M. A., Braga, M. A., Soares, P. P. C., Guarneire, G. J., Scherrer, E. C., Silva, F. S., Lima, N. M., La Porta, F. A., de Jesus A. S. Andrade, T., Preet, G., Castro, S. B. R., Alves, C. C. S., & Carli, A. P. (2024). Immunomodulatory Effects of Anadenanthera colubrina Bark Extract in Experimental Autoimmune Encephalomyelitis. Current Issues in Molecular Biology, 46(8), 8726-8740. https://doi.org/10.3390/cimb46080515








