ROS Chronicles in HIV Infection: Genesis of Oxidative Stress, Associated Pathologies, and Therapeutic Strategies
Abstract
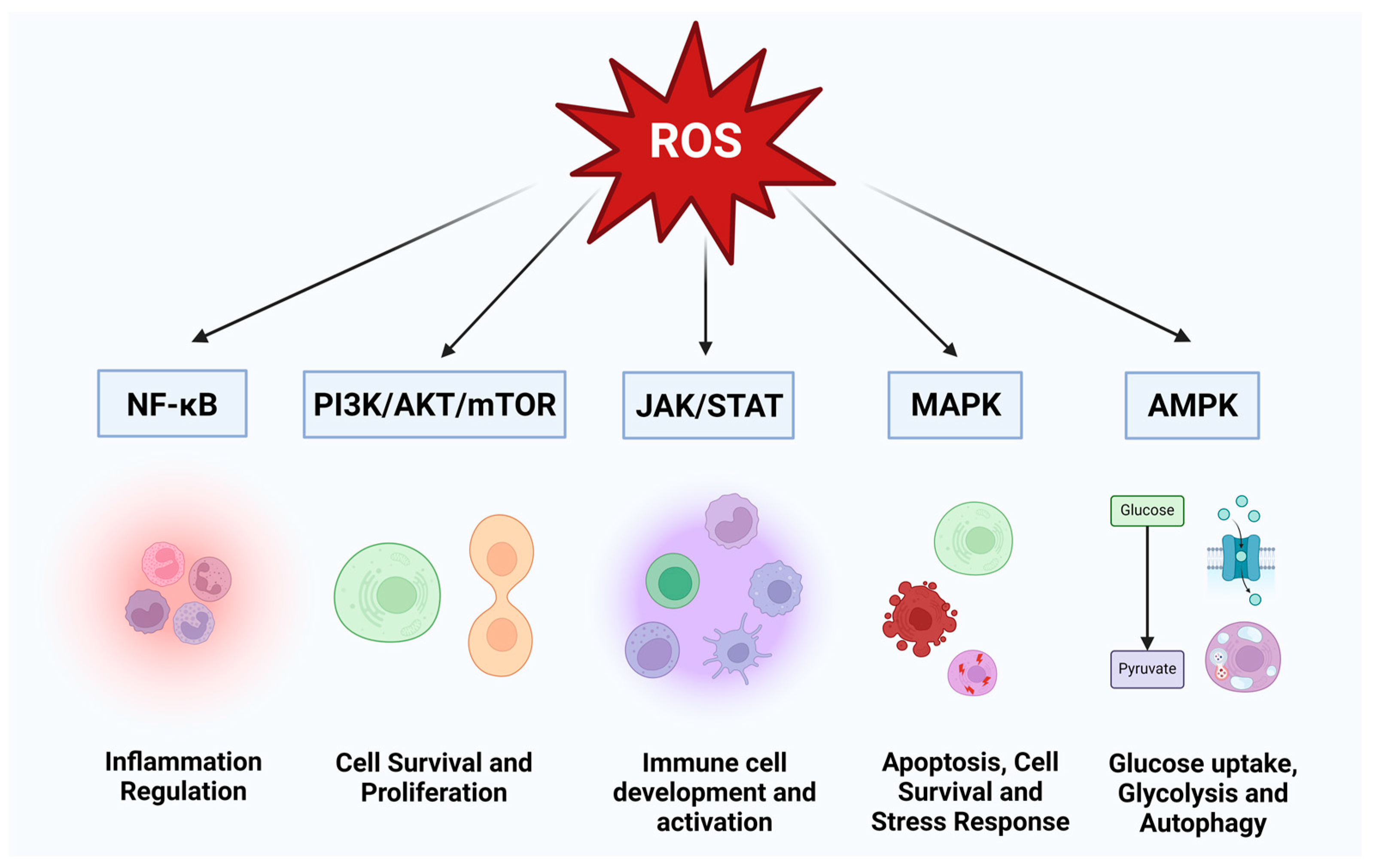
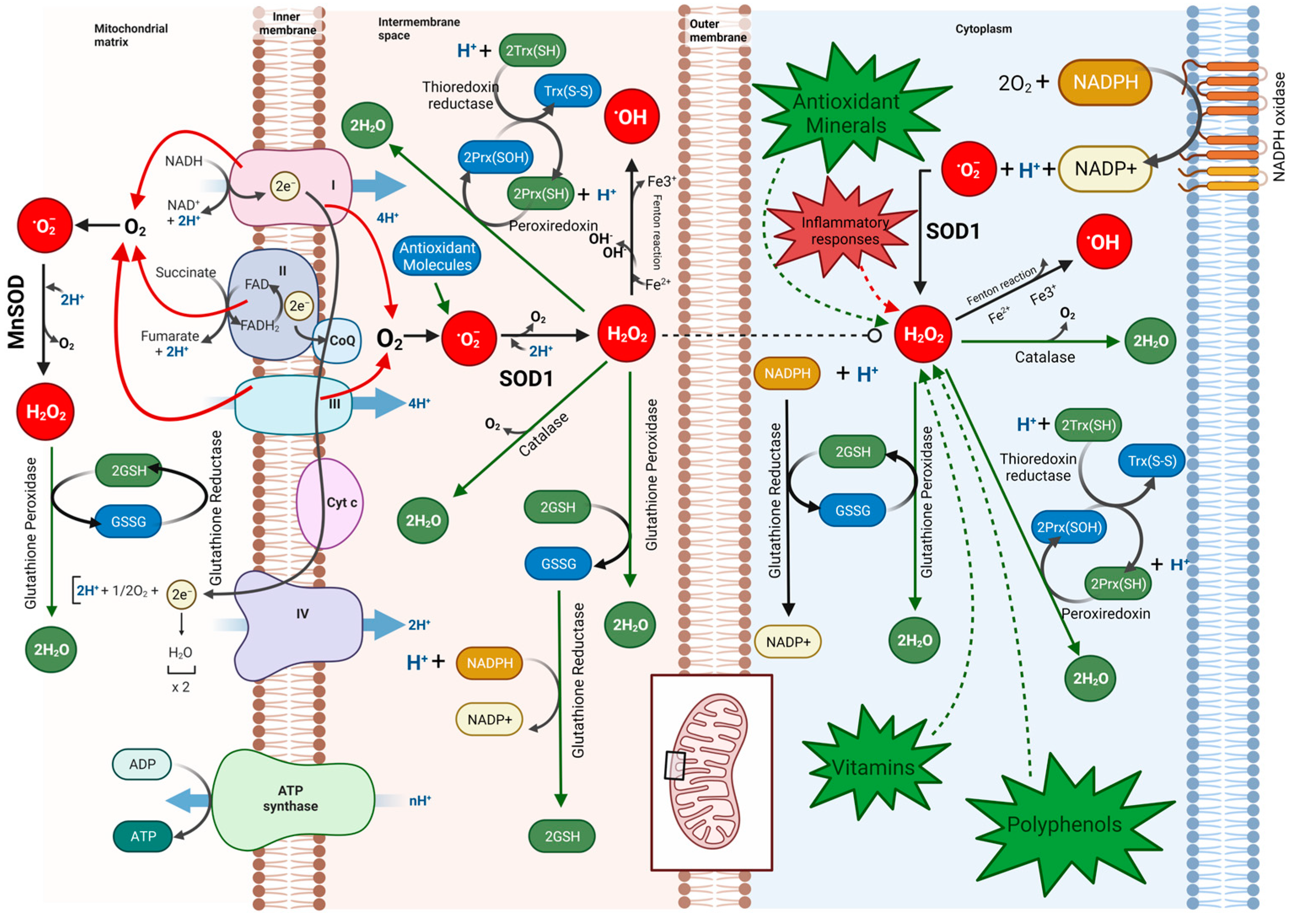
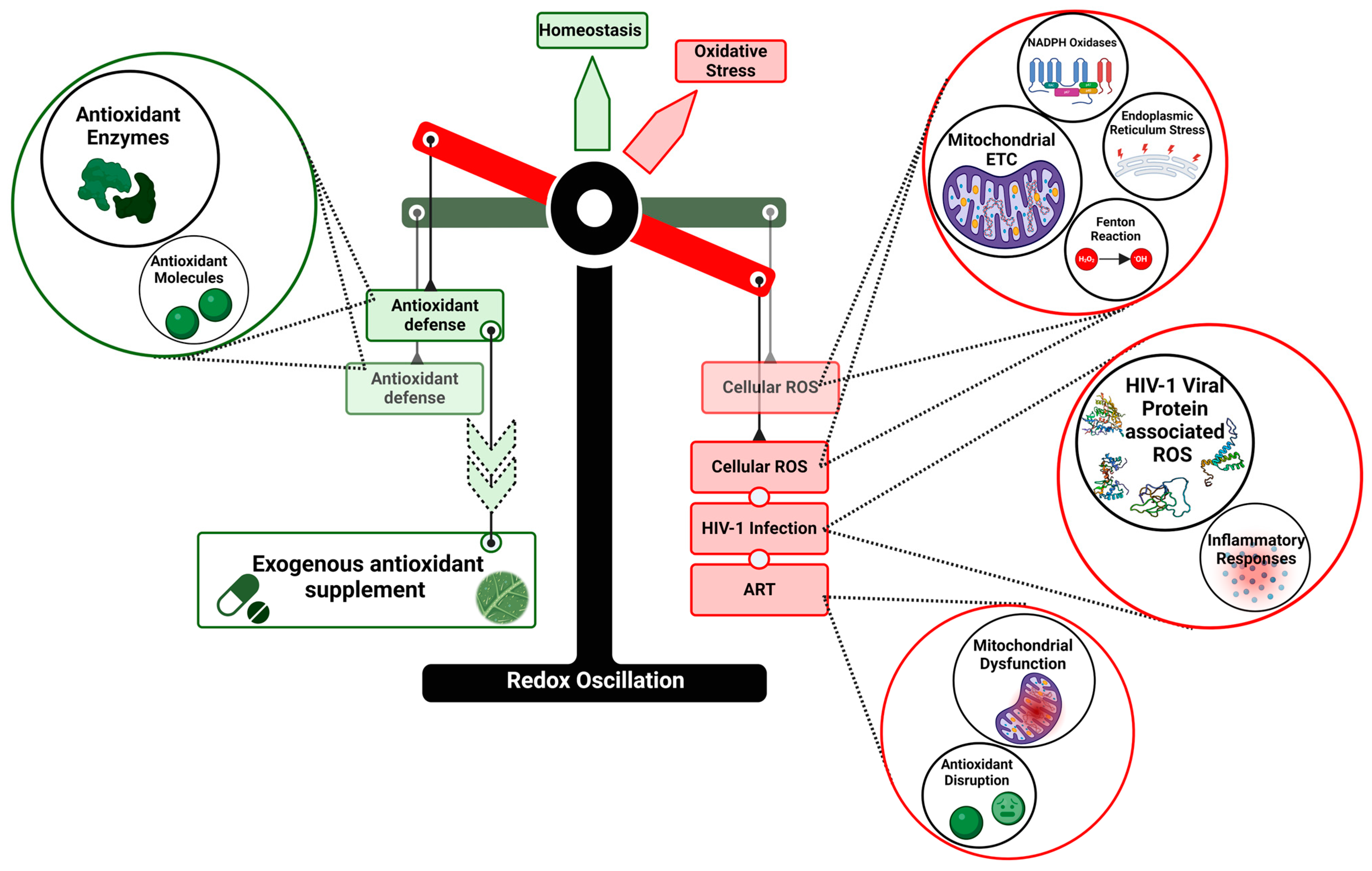
1. Introduction
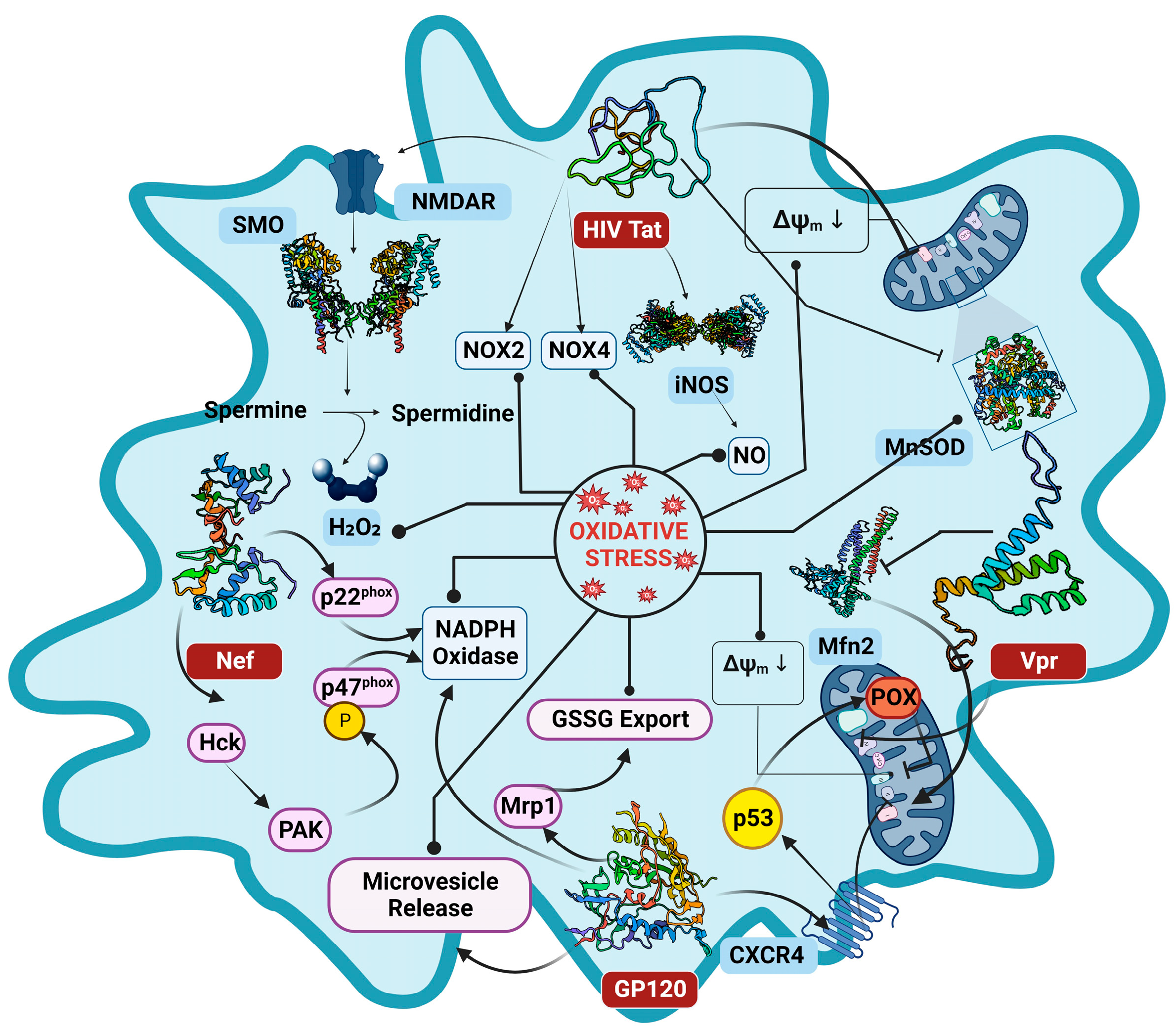
2. Molecular Interaction of HIV Proteins in ROS Generation
2.1. Tat (Trans-Activator of Transcription) Protein
2.2. The Envelope Protein GP120
2.3. Negative Regulatory Factor
2.4. Viral Protein R
2.5. Reverse Transcriptase
3. HIV ART-Associated OS
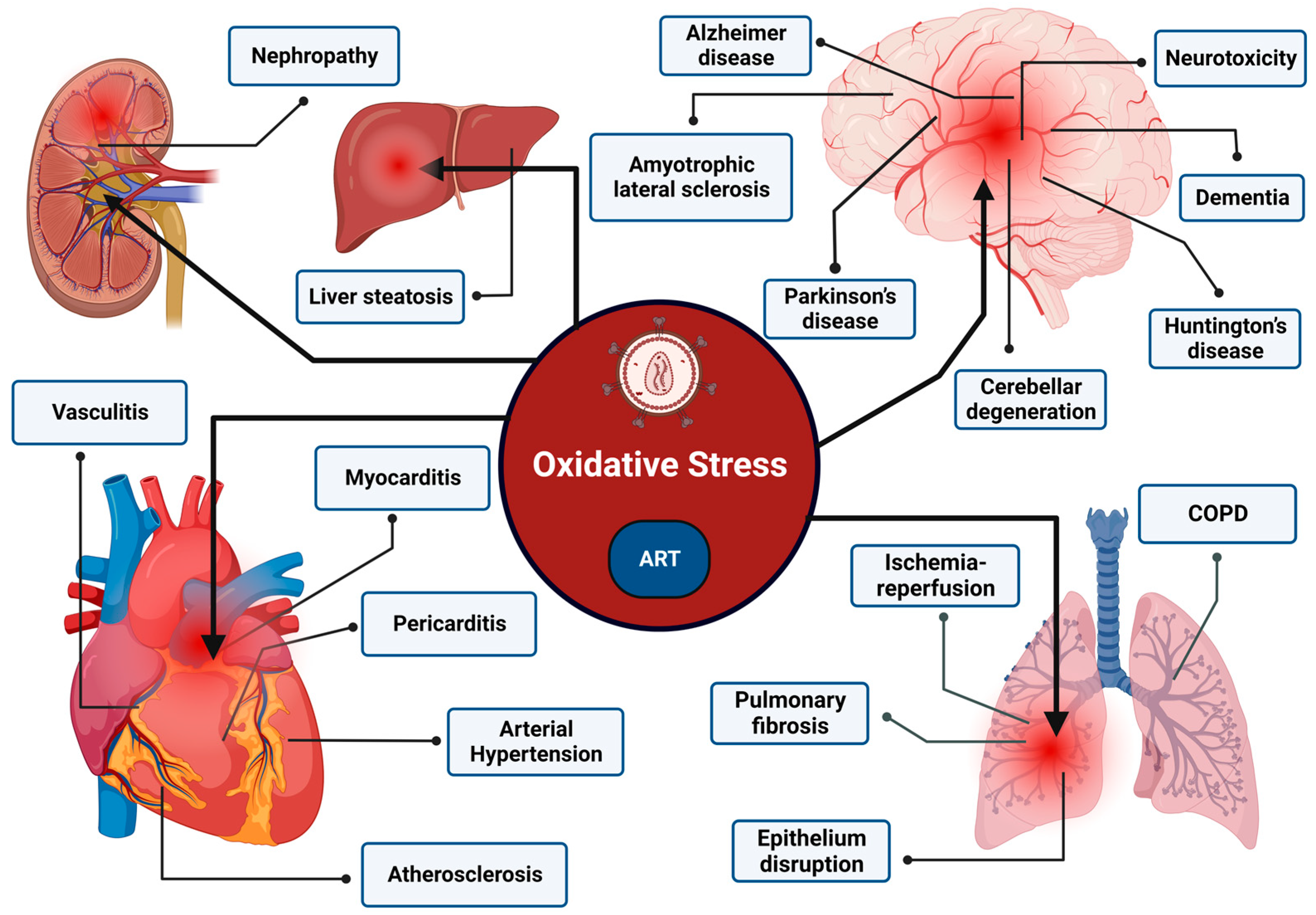
4. ROS-Associated Pathologies
5. Antioxidants in HIV Therapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, L.; Yu, F.; Du, L.-Y.; Xu, W.; Jiang, S.-B. Tactics Used by HIV-1 to Evade Host Innate, Adaptive, and Intrinsic Immunities. Chin. Med. J. (Engl.) 2013, 126, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV–Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef]
- Papadopulos-Eleopulos, E. Reappraisal of Aids—Is the Oxidation Induced by the Risk Factors the Primary Cause? Med. Hypotheses 1988, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive Oxygen Species: Role in the Development of Cancer and Various Chronic Conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB Activation by Reactive Oxygen Species: Fifteen Years Later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial Dynamics, ROS, and Cell Signaling: A Blended Overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wong, H.S. Are Mitochondria the Main Contributor of Reactive Oxygen Species in Cells? J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX Enzymes and the Biology of Reactive Oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Nasi, A.; McArdle, S.; Gaudernack, G.; Westman, G.; Melief, C.; Rockberg, J.; Arens, R.; Kouretas, D.; Sjölin, J.; Mangsbo, S. Reactive Oxygen Species as an Initiator of Toxic Innate Immune Responses in Retort to SARS-CoV-2 in an Ageing Population, Consider N-Acetylcysteine as Early Therapeutic Intervention. Toxicol. Rep. 2020, 7, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; De Carvalho, L.R.P.; Mustafá, Y.M.; Da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Sun, R.; Sun, S.; Zhang, Y.; Zhou, Y.; Shan, Y.; Li, X.; Fang, W. PCV2 Induces Reactive Oxygen Species To Promote Nucleocytoplasmic Translocation of the Viral DNA Binding Protein HMGB1 To Enhance Its Replication. J. Virol. 2020, 94, e00238-20. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Cheng, A.; Wen, X.; Ou, X.; Mao, S.; Gao, Q.; Sun, D.; Jia, R.; Yang, Q.; et al. Enterovirus Replication Organelles and Inhibitors of Their Formation. Front. Microbiol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Jareño, E.J.; Bosch-Morell, F.; Fernández-Delgado, R.; Donat, J.; Romero, F.J. Serum Malondialdehyde in HIV-Seropositive Children Negatively Correlates with CD4+ Lymphocytes Count. BioFactors 1998, 8, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Aukrust, P.; Svardal, A.M.; Müller, F.; Lunden, B.; Berge, R.K.; Ueland, P.M.; Frøland, S.S. Increased Levels of Oxidized Glutathione in CD4+ Lymphocytes Associated with Disturbed Intracellular Redox Balance in Human Immunodeficiency Virus Type 1 Infection. Blood 1995, 86, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Aukrust, P.; Svardal, A.M.; Müller, F.; Lunden, B.; Berge, R.K.; Frøland, S.S. Decreased Levels of Total and Reduced Glutathione in CD4+ Lymphocytes in Common Variable Immunodeficiency Are Associated with Activation of the Tumor Necrosis Factor System: Possible Immunopathogenic Role of Oxidative Stress. Blood 1995, 86, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxidative Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; Fortune, T.; Hegde, E.; Weinstein, M.P.; Keane, A.M.; Mangold, J.F.; Swartz, T.H. Oxidative Phosphorylation in HIV-1 Infection: Impacts on Cellular Metabolism and Immune Function. Front. Immunol. 2024, 15, 1360342. [Google Scholar] [CrossRef] [PubMed]
- Nabel, G.; Baltimore, D. An Inducible Transcription Factor Activates Expression of Human Immunodeficiency Virus in T Cells. Nature 1987, 326, 711–713. [Google Scholar] [CrossRef]
- Staal, F.J.; Roederer, M.; Herzenberg, L.A. Intracellular Thiols Regulate Activation of Nuclear Factor Kappa B and Transcription of Human Immunodeficiency Virus. Proc. Natl. Acad. Sci. USA 1990, 87, 9943–9947. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Wong, L.M.; Jiang, G. NF-κB Sub-Pathways and HIV Cure: A Revisit. EBioMedicine 2021, 63, 103159. [Google Scholar] [CrossRef]
- Gama, W.M.; Oliveira, L.B.; Chaves, Y.O.; Ribeiro, F.; Almeida, T.V.R.; Baptista, B.J.A.; Santana, M.F.; Ferreira, L.C.; Lacerda, M.V.G.; Nogueira, P.A. Increased Levels of Reactive Oxygen Species in Platelets and Platelet-Derived Microparticles and the Risk of Respiratory Failure in HIV/AIDS Patients. Memórias Do Inst. Oswaldo Cruz 2020, 115, e200082. [Google Scholar] [CrossRef]
- Mastrantonio, R.; Cervelli, M.; Pietropaoli, S.; Mariottini, P.; Colasanti, M.; Persichini, T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS ONE 2016, 11, e0149802. [Google Scholar] [CrossRef]
- Shah, A.; Kumar, S.; Simon, S.D.; Singh, D.P.; Kumar, A. HIV Gp120- and Methamphetamine-Mediated Oxidative Stress Induces Astrocyte Apoptosis via Cytochrome P450 2E1. Cell Death Dis. 2013, 4, e850. [Google Scholar] [CrossRef]
- Banki, K.; Hutter, E.; Gonchoroff, N.J.; Perl, A. Molecular Ordering in HIV-Induced Apoptosis: Oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J. Biol. Chem. 1998, 273, 11944–11953. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Amini, S.; Sen, S.; Khalili, K.; Sawaya, B.E. Regulation of the HIV-1 Promoter by HIF-1α and Vpr Proteins. Virol. J. 2011, 8, 477. [Google Scholar] [CrossRef]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New Insights into Pathogenesis Point to HIV-1 Tat as a Key Vaccine Target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef]
- Ianiro, G.; D’Ezio, V.; Carpinelli, L.; Casella, C.; Bonaccorsi Di Patti, M.C.; Rosa, L.; Valenti, P.; Colasanti, M.; Musci, G.; Cutone, A.; et al. Iron Saturation Drives Lactoferrin Effects on Oxidative Stress and Neurotoxicity Induced by HIV-1 Tat. Int. J. Mol. Sci. 2023, 24, 7947. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat Is a Multifunctional Viral Protein That Modulates Cellular Gene Expression and Functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef]
- Ali, A.; Mishra, R.; Kaur, H.; Chandra Banerjea, A. HIV-1 Tat: An Update on Transcriptional and Non-Transcriptional Functions. Biochimie 2021, 190, 24–35. [Google Scholar] [CrossRef]
- Lecoeur, H.; Borgne-Sanchez, A.; Chaloin, O.; El-Khoury, R.; Brabant, M.; Langonné, A.; Porceddu, M.; Brière, J.-J.; Buron, N.; Rebouillat, D.; et al. HIV-1 Tat Protein Directly Induces Mitochondrial Membrane Permeabilization and Inactivates Cytochrome c Oxidase. Cell Death Dis. 2012, 3, e282. [Google Scholar] [CrossRef]
- Macho, A.; Calzado, M.A.; Jiménez-Reina, L.; Ceballos, E.; León, J.; Muñoz, E. Susceptibility of HIV-1-TAT Transfected Cells to Undergo Apoptosis. Biochemical Mechanisms. Oncogene 1999, 18, 7543–7551. [Google Scholar] [CrossRef]
- Flores, S.C.; Marecki, J.C.; Harper, K.P.; Bose, S.K.; Nelson, S.K.; McCord, J.M. Tat Protein of Human Immunodeficiency Virus Type 1 Represses Expression of Manganese Superoxide Dismutase in HeLa Cells. Proc. Natl. Acad. Sci. USA 1993, 90, 7632–7636. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.S.; Krause, K.-H.; Singh, S.K. HIV-1 Tat C Modulates NOX2 and NOX4 Expressions through miR-17 in a Human Microglial Cell Line. J. Neurochem. 2014, 131, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-S.; Sang, W.-W.; Ruan, Z.; Wang, Y.-O. Akt/Nox2/NF-κB Signaling Pathway Is Involved in Tat-Induced HIV-1 Long Terminal Repeat (LTR) Transactivation. Arch. Biochem. Biophys. 2011, 505, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, M.O.; Shatrov, V.A.; Schulze-Osthoff, K.; Frank, R.; Kraft, M.; Los, M.; Krammer, P.H.; Dröge, W.; Lehmann, V. HIV-1 Tat Potentiates TNF-Induced NF-Kappa B Activation and Cytotoxicity by Altering the Cellular Redox State. EMBO J. 1995, 14, 546–554. [Google Scholar] [CrossRef]
- Seve, M.; Favier, A.; Osman, M.; Hernandez, D.; Vaitaitis, G.; Flores, N.C.; McCord, J.M.; Flores, S.C. The Human Immunodeficiency Virus-1 Tat Protein Increases Cell Proliferation, Alters Sensitivity to Zinc Chelator-Induced Apoptosis, and Changes Sp1 DNA Binding in HeLa Cells. Arch. Biochem. Biophys. 1999, 361, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.J.; Marin, M.C.; Nehete, P.N.; McConnell, K.; el-Naggar, A.K.; McDonnell, T.J. Expression of Human Immunodeficiency Virus Type I Tat Results in Down-Regulation of Bcl-2 and Induction of Apoptosis in Hematopoietic Cells. Oncogene 1996, 13, 487–493. [Google Scholar]
- Steiner, J.; Haughey, N.; Li, W.; Venkatesan, A.; Anderson, C.; Reid, R.; Malpica, T.; Pocernich, C.; Butterfield, D.A.; Nath, A. Oxidative Stress and Therapeutic Approaches in HIV Dementia. Antioxid. Redox Signal. 2006, 8, 2089–2100. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Barger, S.W.; Moss, N.I.; Pham, J.T.; Keller, J.N.; Nath, A. Pro-Inflammatory and pro-Oxidant Properties of the HIV Protein Tat in a Microglial Cell Line: Attenuation by 17 Beta-Estradiol. J. Neurochem. 2001, 78, 1315–1324. [Google Scholar] [CrossRef]
- Richard, M.J.; Guiraud, P.; Didier, C.; Seve, M.; Flores, S.C.; Favier, A. Human Immunodeficiency Virus Type 1 Tat Protein Impairs Selenoglutathione Peroxidase Expression and Activity by a Mechanism Independent of Cellular Selenium Uptake: Consequences on Cellular Resistance to UV-A Radiation. Arch. Biochem. Biophys. 2001, 386, 213–220. [Google Scholar] [CrossRef]
- Smith, L.K.; Babcock, I.W.; Minamide, L.S.; Shaw, A.E.; Bamburg, J.R.; Kuhn, T.B. Direct Interaction of HIV Gp120 with Neuronal CXCR4 and CCR5 Receptors Induces Cofilin-Actin Rod Pathology via a Cellular Prion Protein- And NOX-Dependent Mechanism. PLoS ONE 2021, 16, e0248309. [Google Scholar] [CrossRef]
- Omeragic, A.; Hoque, M.T.; Choi, U.-Y.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-Gamma: Potential Molecular Therapeutic Target for HIV-1-Associated Brain Inflammation. J. Neuroinflamm. 2017, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, C.; Lv, S.; Chang, L.; Hua, W.; Weng, W.; Wu, H.; Dai, L. Role of Microglia in HIV-1 Infection. AIDS Res. Ther. 2023, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Frouard, J.; Xie, G.; Corley, M.J.; Helmy, E.; Zhang, G.; Schwarzer, R.; Montano, M.; Sohn, P.; Roan, N.R.; et al. Neuroinflammation Generated by HIV-Infected Microglia Promotes Dysfunction and Death of Neurons in Human Brain Organoids. PNAS Nexus 2024, 3, pgae179. [Google Scholar] [CrossRef] [PubMed]
- Roggero, R.; Robert-Hebmann, V.; Harrington, S.; Roland, J.; Vergne, L.; Jaleco, S.; Devaux, C.; Biard-Piechaczyk, M. Binding of Human Immunodeficiency Virus Type 1 Gp120 to CXCR4 Induces Mitochondrial Transmembrane Depolarization and Cytochrome c -Mediated Apoptosis Independently of Fas Signaling. J. Virol. 2001, 75, 7637–7650. [Google Scholar] [CrossRef]
- Pandhare, J.; Dash, S.; Jones, B.; Villalta, F.; Dash, C. A Novel Role of Proline Oxidase in HIV-1 Envelope Glycoprotein-Induced Neuronal Autophagy. J. Biol. Chem. 2015, 290, 25439–25451. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Bendayan, R. HIV-1 Viral Envelope Glycoprotein Gp120 Produces Oxidative Stress and Regulates the Functional Expression of Multidrug Resistance Protein-1 (Mrp1) in Glial Cells. J. Neurochem. 2008, 106, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.F.H.; Widder, J.D.; McNally, J.S.; McCann, L.; Jones, D.P.; Harrison, D.G. The Role of the Multidrug Resistance Protein-1 in Modulation of Endothelial Cell Oxidative Stress. Circ. Res. 2005, 97, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, J.G.; Stockelman, K.; Levy, M.; Madden Brewster, L.; Bammert, T.D.; Greiner, J.J.; Connick, E.; DeSouza, C.A. Effects of HIV-1 Gp120 and TAT-Derived Microvesicles on Endothelial Cell Function. J. Appl. Physiol. 2019, 126, 1242–1249. [Google Scholar] [CrossRef]
- Espert, L.; Denizot, M.; Grimaldi, M.; Robert-Hebmann, V.; Gay, B.; Varbanov, M.; Codogno, P.; Biard-Piechaczyk, M. Autophagy Is Involved in T Cell Death after Binding of HIV-1 Envelope Proteins to CXCR4. J. Clin. Investig. 2006, 116, 2161–2172. [Google Scholar] [CrossRef]
- Cutler, R.G.; Haughey, N.J.; Tammara, A.; McArthur, J.C.; Nath, A.; Reid, R.; Vargas, D.L.; Pardo, C.A.; Mattson, M.P. Dysregulation of Sphingolipid and Sterol Metabolism by ApoE4 in HIV Dementia. Neurology 2004, 63, 626–630. [Google Scholar] [CrossRef]
- Basmaciogullari, S.; Pizzato, M. The Activity of Nef on HIV-1 Infectivity. Front. Microbiol. 2014, 5, 232. [Google Scholar] [CrossRef] [PubMed]
- Buffalo, C.Z.; Iwamoto, Y.; Hurley, J.H.; Ren, X. How HIV Nef Proteins Hijack Membrane Traffic To Promote Infection. J. Virol. 2019, 93, e01322-19. [Google Scholar] [CrossRef] [PubMed]
- Olivetta, E.; Mallozzi, C.; Ruggieri, V.; Pietraforte, D.; Federico, M.; Sanchez, M. HIV-1 Nef Induces P47phox Phosphorylation Leading to a Rapid Superoxide Anion Release from the U937 Human Monoblastic Cell Line. J. Cell. Biochem. 2009, 106, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Salmen, S.; Colmenares, M.; Peterson, D.L.; Reyes, E.; Rosales, J.D.; Berrueta, L. HIV-1 Nef Associates with P22-Phox, a Component of the NADPH Oxidase Protein Complex. Cell. Immunol. 2010, 263, 166–171. [Google Scholar] [CrossRef]
- Vilhardt, F.; Plastre, O.; Sawada, M.; Suzuki, K.; Wiznerowicz, M.; Kiyokawa, E.; Trono, D.; Krause, K.H. The HIV-1 Nef Protein and Phagocyte NADPH Oxidase Activation. J. Biol. Chem. 2002, 277, 42136–42143. [Google Scholar] [CrossRef]
- Masanetz, S.; Lehmann, M.H. HIV-1 Nef Increases Astrocyte Sensitivity towards Exogenous Hydrogen Peroxide. Virol. J. 2011, 8, 35. [Google Scholar] [CrossRef]
- Ranki, A.; Nyberg, M.; Ovod, V.; Haltia, M.; Elovaara, I.; Raininko, R.; Haapasalo, H.; Krohn, K. Abundant Expression of HIV Nef and Rev Proteins in Brain Astrocytes in Vivo Is Associated with Dementia. AIDS 1995, 9, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Planelles, V. The Role of Vpr in HIV-1 Pathogenesis. Curr. HIV Res. 2005, 3, 43–51. [Google Scholar] [CrossRef]
- Hrimech, M.; Yao, X.-J.; Bachand, F.; Rougeau, N.; Cohen, É.A. Human Immunodeficiency Virus Type 1 (HIV-1) Vpr Functions as an Immediate-Early Protein during HIV-1 Infection. J. Virol. 1999, 73, 4101–4109. [Google Scholar] [CrossRef]
- Kitayama, H.; Miura, Y.; Ando, Y.; Hoshino, S.; Ishizaka, Y.; Koyanagi, Y. Human Immunodeficiency Virus Type 1 Vpr Inhibits Axonal Outgrowth through Induction of Mitochondrial Dysfunction. J. Virol. 2008, 82, 2528–2542. [Google Scholar] [CrossRef]
- Jacotot, E.; Ferri, K.F.; El Hamel, C.; Brenner, C.; Druillennec, S.; Hoebeke, J.; Rustin, P.; Métivier, D.; Lenoir, C.; Geuskens, M.; et al. Control of Mitochondrial Membrane Permeabilization by Adenine Nucleotide Translocator Interacting with HIV-1 Viral Protein R and Bcl-2. J. Exp. Med. 2001, 193, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chiang, S.F.; Lin, T.Y.; Chiou, S.H.; Chow, K.C. HIV-1 Vpr Triggers Mitochondrial Destruction by Impairing Mfn2-Mediated ER-Mitochondria Interaction. PLoS ONE 2012, 7, e33657. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Mukerjee, R.; Fan, S.; Del Valle, L.; Michiels, C.; Sweet, T.; Rom, I.; Khalili, K.; Rappaport, J.; Amini, S.; et al. Activation of the Oxidative Stress Pathway by HIV-1 Vpr Leads to Induction of Hypoxia-Inducible Factor 1α Expression. J. Biol. Chem. 2009, 284, 11364–11373. [Google Scholar] [CrossRef] [PubMed]
- Huard, S.; Chen, M.; Burdette, K.E.; Fenyvuesvolgyi, C.; Yu, M.; Elder, R.T.; Zhao, R.Y. HIV-1 Vpr-Induced Cell Death in Schizosaccharomyces Pombe Is Reminiscent of Apoptosis. Cell Res. 2008, 18, 961–973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaguliants, M.; Smirnova, O.; Ivanov, A.V.; Kilpelainen, A.; Kuzmenko, Y.; Petkov, S.; Latanova, A.; Krotova, O.; Engström, G.; Karpov, V.; et al. Oxidative Stress Induced by HIV-1 Reverse Transcriptase Modulates the Enzyme’s Performance in Gene Immunization. Human Vaccines Immunother. 2013, 9, 2111–2119. [Google Scholar] [CrossRef]
- Bayurova, E.; Jansons, J.; Skrastina, D.; Smirnova, O.; Mezale, D.; Kostyusheva, A.; Kostyushev, D.; Petkov, S.; Podschwadt, P.; Valuev-Elliston, V.; et al. HIV-1 Reverse Transcriptase Promotes Tumor Growth and Metastasis Formation via ROS-Dependent Upregulation of Twist. Oxidative Med. Cell. Longev. 2019, 2019, 6016278. [Google Scholar] [CrossRef] [PubMed]
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef]
- Peng, Y.; Zong, Y.; Wang, D.; Chen, J.; Chen, Z.-S.; Peng, F.; Liu, Z. Current Drugs for HIV-1: From Challenges to Potential in HIV/AIDS. Front. Pharmacol. 2023, 14, 1294966. [Google Scholar] [CrossRef]
- Ngondi, J.L.; Oben, J.; Forkah, D.M.; Etame, L.H.; Mbanya, D. The Effect of Different Combination Therapies on Oxidative Stress Markers in HIV Infected Patients in Cameroon. AIDS Res. Ther. 2006, 3, 19. [Google Scholar] [CrossRef]
- Masiá, M.; Padilla, S.; Bernal, E.; Almenar, M.V.; Molina, J.; Hernández, I.; Graells, M.L.; Gutiérrez, F. Influence of Antiretroviral Therapy on Oxidative Stress and Cardiovascular Risk: A Prospective Cross-Sectional Study in HIV-Infected Patients. Clin. Ther. 2007, 29, 1448–1455. [Google Scholar] [CrossRef]
- Atiba, A.S.; Oparinde, D.P.; Adelekan, A.; Jimoh, A.K.; Temitope, A.; Niran, A. Oxidative Stress and Serum Selenium in HIV Patients on Different Antiretroviral Regimen. Greener J. Med. Sci. 2012, 2, 163–167. [Google Scholar] [CrossRef][Green Version]
- Schank, M.; Zhao, J.; Moorman, J.P.; Yao, Z.Q. The Impact of Hiv-and Art-Induced Mitochondrial Dysfunction in Cellular Senescence and Aging. Cells 2021, 10, 174. [Google Scholar] [CrossRef]
- Day, B.J.; Lewis, W. Oxidative Stress in NRTI-Induced Toxicity: Evidence from Clinical Experience and Experiments in Vitro and in Vivo. Cardiovasc. Toxicol. 2004, 4, 207–216. [Google Scholar] [CrossRef]
- Lund, K.C.; Wallace, K.B. Direct, DNA Pol-Gamma-Independent Effects of Nucleoside Reverse Transcriptase Inhibitors on Mitochondrial Bioenergetics. Cardiovasc. Toxicol. 2004, 4, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Sato, T.; Sato, Y.; Medin, J.A.; Kushimoto, S.; Yanagisawa, T. Azidothymidine-Triphosphate Impairs Mitochondrial Dynamics by Disrupting the Quality Control System. Redox Biol. 2017, 13, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Iorjiim, W.M.; Omale, S.; Etuh, M.A.; Ubani, A.; Alemika, E.T.; Gyang, S.S. Senescence and Oxidative Stress Toxicities Induced by Lamivudine and Tenofovir in Drosophila Melanogaster. Ann. Pharm. Fr. 2022, 80, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the Cellular Antioxidant System Contributes to Tenofovir Disoproxil Fumarate—Induced Mitochondrial Damage and Increased Oxido-Nitrosative Stress in the Kidney. J. Biomed. Sci. 2013, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Blas-García, A.; Martí-Rodrigo, A.; Víctor, V.M.; Polo, M.; Alegre, F.; Funes, H.A.; Apostolova, N.; Esplugues, J.V. The Purine Analogues Abacavir and Didanosine Increase Acetaminophen-Induced Hepatotoxicity by Enhancing Mitochondrial Dysfunction. J. Antimicrob. Chemother. 2016, 71, 916–926. [Google Scholar] [CrossRef]
- Apostolova, N.; Gomez-Sucerquia, L.J.; Moran, A.; Alvarez, A.; Blas-Garcia, A.; Esplugues, J.V. Enhanced Oxidative Stress and Increased Mitochondrial Mass during Efavirenz-Induced Apoptosis in Human Hepatic Cells. Br. J. Pharmacol. 2010, 160, 2069–2084. [Google Scholar] [CrossRef]
- Weiß, M.; Kost, B.; Renner-Müller, I.; Wolf, E.; Mylonas, I.; Brüning, A. Efavirenz Causes Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy in Endothelial Cells. Cardiovasc. Toxicol. 2016, 16, 90–99. [Google Scholar] [CrossRef]
- Maandi, S.C.; Maandi, M.T.; Patel, A.; Manville, R.W.; Mabley, J.G. Divergent Effects of HIV Reverse Transcriptase Inhibitors on Pancreatic Beta-Cell Function and Survival: Potential Role of Oxidative Stress and Mitochondrial Dysfunction. Life Sci. 2022, 294, 120329. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Mondal, D.; Agrawal, K.C. HIV-1 Protease Inhibitor Induced Oxidative Stress Suppresses Glucose Stimulated Insulin Release: Protection with Thymoquinone. Exp. Biol. Med. 2009, 234, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, R.K.; Petruschke, R.A. Pharmacological and Therapeutic Properties of Ritonavir-Boosted Protease Inhibitor Therapy in HIV-Infected Patients. J. Antimicrob. Chemother. 2004, 53, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.W.; Montaner, J.S.G. Ritonavir-Boosted Protease Inhibitors in HIV Therapy. Ann. Med. 2011, 43, 375–388. [Google Scholar] [CrossRef]
- Chen, X.; Mak, I.T. Mg Supplementation Protects against Ritonavir-Mediated Endothelial Oxidative Stress and Hepatic eNOS Downregulation. Free Radic. Biol. Med. 2014, 69, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.L.; Lee, R.N.; Panvelker, N.; Li, J.; Harowitz, J.; Jordan-Sciutto, K.L.; Akay-Espinoza, C. Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response. J. Neuroimmune Pharmacol. 2018, 13, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Taura, M.; Kariya, R.; Kudo, E.; Goto, H.; Iwawaki, T.; Amano, M.; Suico, M.A.; Kai, H.; Mitsuya, H.; Okada, S. Comparative Analysis of ER Stress Response into HIV Protease Inhibitors: Lopinavir but Not Darunavir Induces Potent ER Stress Response via ROS/JNK Pathway. Free Radic. Biol. Med. 2013, 65, 778–788. [Google Scholar] [CrossRef]
- Estrada, V.; Monge, S.; Gómez-Garre, D.; Sobrino, P.; Berenguer, J.; Ignacio Bernardino, J.; Santos, J.; Moreno Zamora, A.; Martínez, E.; Ramón Blanco, J. Comparison of Oxidative Stress Markers in HIV-Infected Patients on Efavirenz or Atazanavir/Ritonavir-Based Therapy. J. Int. AIDS Soc. 2014, 17, 19544. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Zhao, X.Z.; Passos, D.O.; Lyumkis, D.; Burke, T.R.; Hughes, S.H. Integrase Strand Transfer Inhibitors Are Effective Anti-HIV Drugs. Viruses 2021, 13, 205. [Google Scholar] [CrossRef]
- Latronico, T.; Pati, I.; Ciavarella, R.; Fasano, A.; Mengoni, F.; Lichtner, M.; Vullo, V.; Mastroianni, C.M.; Liuzzi, G.M. In Vitro Effect of Antiretroviral Drugs on Cultured Primary Astrocytes: Analysis of Neurotoxicity and Matrix Metalloproteinase Inhibition. J. Neurochem. 2018, 144, 271–284. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.A.M.; Signoretto, E.; Bissinger, R.; Lang, F. Enhanced Eryptosis Following Exposure to Dolutegravir. Cell. Physiol. Biochem. 2016, 39, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Burke, J.R.; Woldstad, C.J.; Fang, M.; Bade, A.N.; McMillan, J.; Edagwa, B.; Boska, M.D.; Gendelman, H.E.; Siuzdak, G. Nanoformulated Antiretroviral Therapy Attenuates Brain Metabolic Oxidative Stress. Mol. Neurobiol. 2019, 56, 2896–2907. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Hsu, W.-L.; Wu, C.-Y.; Lai, Y.-C.; Chen, T.-C. Dolutegravir-Induced Growth and Lifespan Effects in Caenorhabditis Elegans. BMC Pharmacol. Toxicol. 2023, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qu, Q.R.; Hoque, M.T.; Bendayan, R. Dolutegravir Disrupts Mouse Blood-Brain Barrier by Inducing Endoplasmic Reticulum Stress. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Sillman, B.; Bade, A.N.; Dash, P.K.; Bhargavan, B.; Kocher, T.; Mathews, S.; Su, H.; Kanmogne, G.D.; Poluektova, L.Y.; Gorantla, S.; et al. Creation of a Long-Acting Nanoformulated Dolutegravir. Nat. Commun. 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-Associated Neurocognitive Disorder--Pathogenesis and Prospects for Treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global Prevalence and Burden of HIV-Associated Neurocognitive Disorder: A Meta-Analysis. Neurology 2020, 95, E2610–E2621. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, J.P.; Agrawal, L.; Reyes, B.A.S.; Van Bockstaele, E.J.; Strayer, D.S. Oxidative Stress Is Associated with Neuroinflammation in Animal Models of HIV-1 Tat Neurotoxicity. Antioxidants 2014, 3, 414–438. [Google Scholar] [CrossRef]
- Shi, B.; Raina, J.; Lorenzo, A.; Busciglio, J.; Gabuzda, D. Neuronal Apoptosis Induced by HIV-1 Tat Protein and TNF-α: Potentiation of Neurotoxicity Mediated by Oxidative Stress and Implications for HIV-1 Dementia. J. Neuro Virol. 1998, 4, 281–290. [Google Scholar] [CrossRef]
- Kallianpur, K.J.; Gerschenson, M.; Mitchell, B.I.; LiButti, D.E.; Umaki, T.M.; Ndhlovu, L.C.; Nakamoto, B.K.; Chow, D.C.; Shikuma, C.M. Oxidative Mitochondrial DNA Damage in Peripheral Blood Mononuclear Cells Is Associated with Reduced Volumes of Hippocampus and Subcortical Gray Matter in Chronically HIV-Infected Patients. Mitochondrion 2016, 28, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kopstein, M.; Mohlman, D.J. HIV-1 Encephalopathy And Aids Dementia Complex. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Anthony, I.C.; Ramage, S.N.; Carnie, F.W.; Simmonds, P.; Bell, J.E. Accelerated Tau Deposition in the Brains of Individuals Infected with Human Immunodeficiency Virus-1 before and after the Advent of Highly Active Anti-Retroviral Therapy. Acta Neuropathol. 2006, 111, 529–538. [Google Scholar] [CrossRef]
- Cho, Y.E.; Lee, M.H.; Song, B.J. Neuronal Cell Death and Degeneration through Increased Nitroxidative Stress and Tau Phosphorylation in HIV-1 Transgenic Rats. PLoS ONE 2017, 12, e0169945. [Google Scholar] [CrossRef]
- Haque, M.M.; Murale, D.P.; Kim, Y.K.; Lee, J.S. Crosstalk between Oxidative Stress and Tauopathy. Int. J. Mol. Sci. 2019, 20, 1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Na, R.; Gu, M.; Richardson, A.; Ran, Q. Lipid Peroxidation Up-Regulates BACE1 Expression in Vivo: A Possible Early Event of Amyloidogenesis in Alzheimer’s Disease. J. Neurochem. 2008, 107, 197–207. [Google Scholar] [CrossRef]
- Murakami, K.; Murata, N.; Noda, Y.; Tahara, S.; Kaneko, T.; Kinoshita, N.; Hatsuta, H.; Murayama, S.; Barnham, K.J.; Irie, K.; et al. SOD1 (Copper/Zinc Superoxide Dismutase) Deficiency Drives Amyloid β Protein Oligomerization and Memory Loss in Mouse Model of Alzheimer Disease. J. Biol. Chem. 2011, 286, 44557–44568. [Google Scholar] [CrossRef]
- DeVaughn, S.; Müller-Oehring, E.M.; Markey, B.; Brontë-Stewart, H.M.; Schulte, T. Aging with HIV-1 Infection: Motor Functions, Cognition, and Attention—A Comparison with Parkinson’s Disease. Neuropsychol. Rev. 2015, 25, 424–438. [Google Scholar] [CrossRef]
- Moradi Vastegani, S.; Nasrolahi, A.; Ghaderi, S.; Belali, R.; Rashno, M.; Farzaneh, M.; Khoshnam, S.E. Mitochondrial Dysfunction and Parkinson’s Disease: Pathogenesis and Therapeutic Strategies. Neurochem. Res. 2023, 48, 2285–2308. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Liberman, A.; Stopa, E.G. Neural Heme Oxygenase-1 Expression in Idiopathic Parkinson’s Disease. Exp. Neurol. 1998, 150, 60–68. [Google Scholar] [CrossRef]
- Paxinou, E.; Chen, Q.; Weisse, M.; Giasson, B.I.; Norris, E.H.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.Y.; Ischiropoulos, H. Induction of α-Synuclein Aggregation by Intracellular Nitrative Insult. J. Neurosci. 2001, 21, 8053–8061. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Beckman, J.S. Oxidative Stress and Nitration in Neurodegeneration: Cause, Effect, or Association? J. Clin. Investig. 2003, 111, 163–169. [Google Scholar] [CrossRef]
- Hodara, R.; Norris, E.H.; Giasson, B.I.; Mishizen-Eberz, A.J.; Lynch, D.R.; Lee, V.M.Y.; Ischiropoulos, H. Functional Consequences of α-Synuclein Tyrosine Nitration: Diminished Binding to Lipid Vesicles and Increased Fibril Formation. J. Biol. Chem. 2004, 279, 47746–47753. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Satin, Z.A.; Bayat, E. ALS-Like Disorder in Three HIV-Positive Patients: Case Series. Case Rep. Neurol. 2021, 13, 59–64. [Google Scholar] [CrossRef]
- Orsini, M.; Oliveira, A.B.; Nascimento, O.J.M.; Reis, C.H.M.; Leite, M.A.A.; de Souza, J.A.; Pupe, C.; de Souza, O.G.; Bastos, V.H.; de Freitas, M.R.G.; et al. Amyotrophic Lateral Sclerosis: New Perpectives and Update. Neurol. Int. 2015, 7, 39–47. [Google Scholar] [CrossRef]
- Pedersen, W.A.; Fu, W.; Keller, J.N.; Markesbery, W.R.; Appel, S.; Smith, R.G.; Kasarskis, E.; Mattson, M.P. Protein Modification by the Lipid Peroxidation Product 4-Hydroxynonenal in the Spinal Cords of Amyotrophic Lateral Sclerosis Patients. Ann. Neurol. 1998, 44, 819–824. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of Als. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, the Bad, and the Ugly. J. Huntington’s Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Akay, C.; Cooper, M.; Odeleye, A.; Jensen, B.K.; White, M.G.; Vassoler, F.; Gannon, P.J.; Mankowski, J.; Dorsey, J.L.; Buch, A.M.; et al. Antiretroviral Drugs Induce Oxidative Stress and Neuronal Damage in the Central Nervous System. J. NeuroVirology 2014, 20, 39–53. [Google Scholar] [CrossRef]
- Kolson, D.L. Developments in Neuroprotection for HIV-Associated Neurocognitive Disorders (HAND). Curr. HIV/AIDS Rep. 2022, 19, 344–357. [Google Scholar] [CrossRef]
- Wallace, D.R. HIV-Associated Neurotoxicity and Cognitive Decline: Therapeutic Implications. Pharmacol. Ther. 2022, 234, 108047. [Google Scholar] [CrossRef]
- Caniglia, E.C.; Cain, L.E.; Justice, A.; Tate, J.; Logan, R.; Sabin, C.; Winston, A.; Van Sighem, A.; Miro, J.M.; Podzamczer, D.; et al. Antiretroviral Penetration into the CNS and Incidence of AIDS-Defining Neurologic Conditions. Neurology 2014, 83, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zareba, K.M.; Miller, T.L.; Lipshultz, S.E. Cardiovascular Disease and Toxicities Related to HIV Infection and Its Therapies. Expert Opin. Drug Saf. 2005, 4, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- García De La Asunción, J.; Del Olmo, M.L.; Gómez-Cambronero, L.G.; Sastre, J.; Pallardó, F.V.; Viña, J. AZT Induces Oxidative Damage to Cardiac Mitochondria: Protective Effect of Vitamins C and E. Life Sci. 2004, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying Causes and Long-Term Survival in Patients with Initially Unexplained Cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Chariot, P.; Perchet, H.; Monnet, I. Dilated Cardiomyopathy in HIV-Infected Patients. N. Engl. J. Med. 1999, 340, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.; Isnardi, D.; Saini, A.; Scarabelli, T.; Raddino, R.; Torre, D. Impact of Highly Active Antiretroviral Therapy in HIV-Positive Patients with Cardiac Involvement. J. Infect. 2000, 40, 282–284. [Google Scholar] [CrossRef]
- Kohler, J.J.; Hosseini, S.H.; Lewis, W. Mitochondrial DNA Impairment in Nucleoside Reverse Transcriptase Inhibitor-Associated Cardiomyopathy. Chem. Res. Toxicol. 2008, 21, 990–996. [Google Scholar] [CrossRef]
- Vernochet, C.; Damilano, F.; Mourier, A.; Bezy, O.; Mori, M.A.; Smyth, G.; Rosenzweig, A.; Larsson, N.G.; Kahn, C.R. Adipose Tissue Mitochondrial Dysfunction Triggers a Lipodystrophic Syndrome with Insulin Resistance, Hepatosteatosis, and Cardiovascular Complications. FASEB J. 2014, 28, 4408–4419. [Google Scholar] [CrossRef]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccalà, P.; Vullo, V. Molecular Mechanisms of Liver Fibrosis in HIV/HCV Coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Husain, M.; Meggs, L.G.; Vashistha, H.; Simoes, S.; Griffiths, K.O.; Kumar, D.; Mikulak, J.; Mathieson, P.W.; Saleem, M.A.; Del Valle, L.; et al. Inhibition of p66ShcA Longevity Gene Rescues Podocytes from HIV-1-Induced Oxidative Stress and Apoptosis. J. Biol. Chem. 2009, 284, 16648–16658. [Google Scholar] [CrossRef]
- Salhan, D.; Husain, M.; Subrati, A.; Goyal, R.; Singh, T.; Rai, P.; Malhotra, A.; Singhal, P.C. HIV-Induced Kidney Cell Injury: Role of ROS-Induced Downregulated Vitamin D Receptor. Am. J. Physiol.—Ren. Physiol. 2012, 303, F503–F514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salhan, D.; Pathak, S.; Husain, M.; Tandon, P.; Kumar, D.; Malhotra, A.; Meggs, L.G.; Singhal, P.C. HIV Gene Expression Deactivates Redox-Sensitive Stress Response Program in Mouse Tubular Cells Both in Vitro and in Vivo. Am. J. Physiol.—Ren. Physiol. 2012, 302, F129–F140. [Google Scholar] [CrossRef][Green Version]
- Kapasi, A.A.; Fan, S.; Singhal, P.C. P300 Modulates HIV-1 Gp120-Induced Apoptosis in Human Proximal Tubular Cells: Associated with Alteration of TGF-β and Smad Signaling. Nephron—Exp. Nephrol. 2006, 102, e30–e38. [Google Scholar] [CrossRef]
- Snyder, A.; Alsauskas, Z.C.; Leventhal, J.S.; Rosenstiel, P.E.; Gong, P.; Chan, J.J.; Barley, K.; He, J.C.; Klotman, M.E.; Ross, M.J.; et al. HIV-1 Viral Protein r Induces ERK and Caspase-8-Dependent Apoptosis in Renal Tubular Epithelial Cells. AIDS 2010, 24, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Plagov, A.; Lan, X.; Chandel, N.; Singh, T.; Lederman, R.; Ayasolla, K.R.; Mathieson, P.W.; Saleem, M.A.; Husain, M.; et al. mTOR Plays a Critical Role in P53-Induced Oxidative Kidney Cell Injury in HIVAN. Am. J. Physiol.—Ren. Physiol. 2013, 305, F343–F354. [Google Scholar] [CrossRef]
- Kalyesubula, R.; Perazella, M.A. Nephrotoxicity of HAART. AIDS Res. Treat. 2011, 2011, 562790. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.; Perazella, M. Nephrotoxicity Associated with Antiretroviral Therapy in HIV-Infected Patients. Curr. Drug Saf. 2008, 2, 147–154. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Wilson, G.J.; Gois, P.H.F.; Zhang, A.; Wang, X.; Law, B.M.P.; Kassianos, A.J.; Healy, H.G. The Role of Oxidative Stress and Inflammation in Acute Oxalate Nephropathy Associated With Ethylene Glycol Intoxication. Kidney Int. Rep. 2018, 3, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Is Oxidative Stress, a Link between Nephrolithiasis and Obesity, Hypertension, Diabetes, Chronic Kidney Disease, Metabolic Syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Kirk, G.D. HIV-Associated Obstructive Lung Diseases: Insights and Implications for the Clinician. Lancet Respir. Med. 2014, 2, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Fois, A.G.; Calia, G.M.; Babudieri, S.; Soddu, V.; Becciu, F.; Fiori, M.L.; Spada, V.; Lovigu, C.; Mannazzu, M.; et al. Chronic Obstructive Pulmonary Disease: An Emerging Comorbidity in HIV-Infected Patients in the HAART Era? Infection 2013, 41, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lipman, M.; Brown, J. HIV-Related Chronic Obstructive Pulmonary Disease: Are Lung CD4 T Cells Bothered? Am. J. Respir. Crit. Care Med. 2014, 190, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J. Pulmonary Complications of HIV Infection. Respirology 2008, 13, 181–190. [Google Scholar] [CrossRef]
- Otoupalova, E.; Smith, S.; Cheng, G.; Thannickal, V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020, 10, 509–547. [Google Scholar] [CrossRef]
- Fan, X.; Staitieh, B.S.; Jensen, J.S.; Mould, K.J.; Greenberg, J.A.; Joshi, P.C.; Koval, M.; Guidot, D.M. Activating the Nrf2-Mediated Antioxidant Response Element Restores Barrier Function in the Alveolar Epithelium of HIV-1 Transgenic Rats. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2013, 305, L267–L277. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.-W.; Chan, D.P.; Singhal, A.; Zhang, Y.; Lee, S.-S. Does Oxidative Stress Contribute to Adverse Outcomes in HIV-Associated TB? J. Antimicrob. Chemother. 2018, 73, 1117–1120. [Google Scholar] [CrossRef]
- Herbert, C.; Luies, L.; Loots, D.T.; Williams, A.A. The Metabolic Consequences of HIV/TB Co-Infection. BMC Infect. Dis. 2023, 23, 536. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Marquis, G.S.; Douglas, S.D.; Kruzich, L.A.; Wilson, C.M. Glutathione, Glutathione Peroxidase, and Selenium Status in HIV-Positive and HIV-Negative Adolescents and Young Adults. Am. J. Clin. Nutr. 2007, 85, 173–181. [Google Scholar] [CrossRef]
- Quaye, O.; Kuleape, J.A.; Bonney, E.Y.; Puplampu, P.; Tagoe, E.A. Imbalance of Antioxidant Enzymes Activities and Trace Elements Levels in Ghanaian HIV-Infected Patients. PLoS ONE 2019, 14, e0220181. [Google Scholar] [CrossRef]
- Stewart, T.; Campa, A.; Shin, D.; Martinez, S.S.; Li, Y.; Hatsu, I.; Baum, M.K. Antioxidant Supplementation in HIV+ Persons on Antiretroviral Therapy (ART): A Pilot Study. FASEB J. 2011, 25, 981. [Google Scholar] [CrossRef]
- Underwood, B.R.; Imarisio, S.; Fleming, A.; Rose, C.; Krishna, G.; Heard, P.; Quick, M.; Korolchuk, V.I.; Renna, M.; Sarkar, S.; et al. Antioxidants Can Inhibit Basal Autophagy and Enhance Neurodegeneration in Models of Polyglutamine Disease. Hum. Mol. Genet. 2010, 19, 3413–3429. [Google Scholar] [CrossRef]
- Jaruga, P.; Jaruga, B.; Gackowski, D.; Olczak, A.; Halota, W.; Pawlowska, M.; Olinski, R. Supplementation with Antioxidant Vitamins Prevents Oxidative Modification of DNA in Lymphocytes of HIV-Infected Patients. Free Radic. Biol. Med. 2002, 32, 414–420. [Google Scholar] [CrossRef]
- Kpewou, D.E.; Mensah, F.O.; Appiah, C.A.; Alidu, H.W.; Badii, V.S. Serum Vitamin E Deficiency among People Living with HIV and Undergoing Antiretroviral Therapy at Ho Teaching Hospital, Ghana. Heliyon 2021, 7, e07339. [Google Scholar] [CrossRef]
- Allard, J.P.; Aghdassi, E.; Chau, J.; Tam, C.; Kovacs, C.M.; Salit, I.E.; Walmsley, S.L. Effects of Vitamin E and C Supplementation on Oxidative Stress and Viral Load in HIV-Infected Subjects. AIDS 1998, 12, 1653–1659. [Google Scholar] [CrossRef]
- Spada, C.; Treitinger, A.; Reis, M.; Masokawa, I.Y.; Verdi, J.C.; Luiz, M.C.; Silveira, M.V.S.; Oliveira, O.V.; Michelon, C.M.; Ávila-Júnior, S.; et al. An Evaluation of Antiretroviral Therapy Associated with α-Tocopherol Supplementation in HIV-Infected Patients. Clin. Chem. Lab. Med. 2002, 40, 456–459. [Google Scholar] [CrossRef]
- Singhal, N.; Austin, J. A Clinical Review of Micronutrients in HIV Infection. J. Int. Assoc. Physicians AIDS Care 2002, 1, 63–75. [Google Scholar] [CrossRef]
- Chiu, H.; Fischman, D.A.; Hammerling, U. Vitamin A Depletion Causes Oxidative Stress, Mitochondrial Dysfunction, and PARP-1-dependent Energy Deprivation. FASEB J. 2008, 22, 3878–3887. [Google Scholar] [CrossRef] [PubMed]
- Petiz, L.L.; Girardi, C.S.; Bortolin, R.C.; Kunzler, A.; Gasparotto, J.; Rabelo, T.K.; Matté, C.; Moreira, J.C.F.; Gelain, D.P. Vitamin A Oral Supplementation Induces Oxidative Stress and Suppresses IL-10 and HSP70 in Skeletal Muscle of Trained Rats. Nutrients 2017, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Petiz, L.L.; Kunzler, A.; Bortolin, R.C.; Gasparotto, J.; Matté, C.; Moreira, J.C.F.; Gelain, D.P. Role of Vitamin A Oral Supplementation on Oxidative Stress and Inflammatory Response in the Liver of Trained Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Teto, G.; Kanmogne, G.D.; Torimiro, J.N.; Alemnji, G.; Nguemaim, F.N.; Takou, D.; Nanfack, A.; Tazoacha, A. Lipid Peroxidation and Total Cholesterol in HAART-Naïve Patients Infected with Circulating Recombinant Forms of Human Immunodeficiency Virus Type-1 in Cameroon. PLoS ONE 2013, 8, e65126. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.D.; Campa, A.M.; Ondercin, J.P.; Leoung, G.S.; Pless, R.F.; Baum, M.K. Micronutrient Supplementation Increases CD4 Count in HIV-Infected Individuals on Highly Active Antiretroviral Therapy: A Prospective, Double-Blinded, Placebo-Controlled Trial. J. Acquir. Immune Defic. Syndr. 2006, 42, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Kamwesiga, J.; Mutabazi, V.; Kayumba, J.; Tayari, J.C.K.; Smyth, R.; Fay, H.; Umurerwa, A.; Baziruwiha, M.; Ntizimira, C.; Murebwayire, A.; et al. Effect of Selenium Supplementation on CD4 T-Cell Recovery, Viral Suppression, Morbidity and Quality of Life of HIV-Infected Patients in Rwanda: Study Protocol for a Randomized Controlled Trial. Trials 2011, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Thangaraj, A.; Chivero, E.T.; Periyasamy, P.; Burkovetskaya, M.E.; Niu, F.; Guo, M.L.; Buch, S. N-Acetylcysteine Reverses Antiretroviral-Mediated Microglial Activation by Attenuating Autophagy-Lysosomal Dysfunction. Front. Neurol. 2020, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Visalli, V.; Muscoli, C.; Sacco, I.; Sculco, F.; Palma, E.; Costa, N.; Colica, C.; Rotiroti, D.; Mollace, V. N-Acetylcysteine Prevents HIV Gp 120-Related Damage of Human Cultured Astrocytes: Correlation with Glutamine Synthase Dysfunction. BMC Neurosci. 2007, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Price, T.O.; Uras, F.; Banks, W.A.; Ercal, N. A Novel Antioxidant N-Acetylcysteine Amide Prevents Gp120- and Tat-Induced Oxidative Stress in Brain Endothelial Cells. Exp. Neurol. 2006, 201, 193–202. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, S.; Pal, V.K.; Munshi, M.; Shekar, P.; Narasimha Murthy, D.T.; Mugesh, G.; Singh, A. Antioxidant Nanozyme Counteracts HIV-1 by Modulating Intracellular Redox Potential. EMBO Mol. Med. 2021, 13, e13314. [Google Scholar] [CrossRef]
- Song, S.; Satta, S.; Sharma, M.B.; Hugo, C.; Kossyvakis, A.; Sen Roy, S.; Kelesidis, T. Mitoquinone Mesylate and Mitochondrial DNA in End Organs in Humanized Mouse Model of Chronic Treated Human Immunodeficiency Virus Infection. J. Infect. Dis. 2023, 228, 59–63. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- de Mello Andrade, J.M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 253–265. ISBN 978-0-12-398456-2. [Google Scholar]
- Jaiswal, J.; Doharey, P.K.; Singh, R.; Tiwari, P.; Singh, N.; Kumar, A.; Gupta, V.K.; Siddiqui, A.J.; Sharma, B. Biochemical Characterization of Different Chemical Components of Parthenium Hysterophorus and Their Therapeutic Potential against HIV-1 RT and Microbial Growth. BioMed Res. Int. 2022, 2022, 3892352. [Google Scholar] [CrossRef] [PubMed]
- Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Moungkote, N.; Chuchawankul, S. Hiv-1 Protease and Reverse Transcriptase Inhibitory Activities of Curcuma Aeruginosa Roxb. Rhizome Extracts and the Phytochemical Profile Analysis: In Vitro and in Silico Screening. Pharmaceuticals 2021, 14, 1115. [Google Scholar] [CrossRef] [PubMed]
- Bektaş, E.; Sahin, H.; Beldüz, A.O.; Güler, H.İ. HIV-1-RT Inhibition Activity of Satureja Spicigera (C.KOCH) BOISS. Aqueous Extract and Docking Studies of Phenolic Compounds Identified by RP-HPLC-DAD. J. Food Biochem. 2022, 46, e13921. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, B.E.; Bradley, G.; Afolayan, A.J. Antioxidant and Phytochemical Properties of Carpobrotus edulis (L.) Bolus Leaf Used for the Management of Common Infections in HIV/AIDS Patients in Eastern Cape Province. BMC Complement. Altern. Med. 2012, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, P.; Shah, P.; Harshithkumar, R.; Said, M.S.; Bhoite, S.P.; Bokuri, S.; Ravindran, S.; Mishra, N.; Mukherjee, A. Antiviral and ROS Scavenging Potential of Carica Papaya Linn and Psidium Guajava Leaves Extract against HIV-1 Infection. BMC Complement. Med. Ther. 2023, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sinha, N.; Kodidela, S.; Godse, S.; Singla, B.; Singh, U.P.; Bhat, H.K. Resveratrol and Its Analogs Suppress HIV Replication, Oxidative Stress, and Inflammation in Macrophages. NeuroImmune Pharmacol. Ther. 2023, 2, 365–374. [Google Scholar] [CrossRef]
- Subramaniam, D.; Hanna, L.E.; Maheshkumar, K.; Ponmurugan, K.; Al-Dhabi, N.A.; Murugan, P. Immune Stimulatory and Anti-HIV-1 Potential of Extracts Derived from Marine Brown Algae Padina Tetrastromatica. J. Complement. Integr. Med. 2020, 17, 20190071. [Google Scholar] [CrossRef]
- Jadaun, P.; Seniya, C.; Pal, S.K.; Kumar, S.; Kumar, P.; Nema, V.; Kulkarni, S.S.; Mukherjee, A. Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches. Antioxidants 2022, 11, 1942. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harshithkumar, R.; Shah, P.; Jadaun, P.; Mukherjee, A. ROS Chronicles in HIV Infection: Genesis of Oxidative Stress, Associated Pathologies, and Therapeutic Strategies. Curr. Issues Mol. Biol. 2024, 46, 8852-8873. https://doi.org/10.3390/cimb46080523
Harshithkumar R, Shah P, Jadaun P, Mukherjee A. ROS Chronicles in HIV Infection: Genesis of Oxidative Stress, Associated Pathologies, and Therapeutic Strategies. Current Issues in Molecular Biology. 2024; 46(8):8852-8873. https://doi.org/10.3390/cimb46080523
Chicago/Turabian StyleHarshithkumar, R, Prachibahen Shah, Pratiksha Jadaun, and Anupam Mukherjee. 2024. "ROS Chronicles in HIV Infection: Genesis of Oxidative Stress, Associated Pathologies, and Therapeutic Strategies" Current Issues in Molecular Biology 46, no. 8: 8852-8873. https://doi.org/10.3390/cimb46080523
APA StyleHarshithkumar, R., Shah, P., Jadaun, P., & Mukherjee, A. (2024). ROS Chronicles in HIV Infection: Genesis of Oxidative Stress, Associated Pathologies, and Therapeutic Strategies. Current Issues in Molecular Biology, 46(8), 8852-8873. https://doi.org/10.3390/cimb46080523


_Kim.png)



